Abstract
Repeated stress can elicit symptoms of depression and anxiety. The amygdala is a significant contributor to the expression of emotion and the basolateral amygdala (BLA) is a major target for the effects of stress on emotion. The adolescent time period may be particularly susceptible to the effects of stress on emotion. While repeated stress has been demonstrated to modify the morphology of BLA neurons in adult rats, little is known about its effects on BLA neurons during adolescence. This study tests the effects of repeated stress during adolescence on BLA neuronal morphology, and whether these are similar to the effects of stress during adulthood. The BLA includes the basal (BA) and lateral (LAT) nuclei, which are differentially responsive to stress in adults. Therefore, effects of stress during adolescence were compared between the BA and LAT nuclei. Morphological features of reconstructed BLA neurons were examined using Golgi-Cox stained tissue from control or repeated restraint stress exposed rats. We found subtle dendritic growth coupled with loss of spines after repeated stress during adolescence. The magnitude and dendritic location of these differences varied between the BA and LAT nuclei in strong contrast to the stress-induced increases in spine number seen in adults. These results demonstrate that repeated stress during adolescence has markedly different effects on BLA neuronal morphology, and the extent of these changes are BLA nucleus-dependent. Moreover, altered neuroanatomy was associated with age-dependent effects of repeated stress on generalization of fear, and may point to the necessity for different approaches to target stress-induced changes in adolescents.
Keywords: Amygdala, restraint stress, golgi, morphology, spine, dendrite
1. Introduction
Stress often precipitates depression and anxiety disorders (Heim and Nemeroff, 2001; Lupien et al., 2009). A history of repeated or prolonged stress often leads to hyperactivity of the amygdala in humans (Armony et al., 2005; Bogdan et al., 2012; Dannlowski et al., 2012; Ganzel et al., 2007; Protopopescu et al., 2005; Shin et al., 1997; van Wingen et al., 2011). Depression and anxiety disorders in many patients are also associated with hyperactivity of the amygdala (Breiter et al., 1996; Davidson et al., 2003; Drevets et al., 1992; Sheline et al., 2001; Siegle et al., 2002; Thomas et al., 2001). In animal models, the basolateral complex of the amygdala (BLA) has emerged as a target for the effects of stress on a range of anxiety and depressive behaviors. Furthermore, these behavioral abnormalities after repeated stress are associated with hyperactivity of BLA neurons (Adamec et al., 2005; Correll et al., 2005; Mozhui et al., 2010; Rosenkranz et al., 2010; Zhang and Rosenkranz, 2012). A major drive of BLA neuronal activity derives from excitatory synaptic inputs to BLA neurons. Previous studies demonstrate increased excitatory drive to BLA neurons after repeated stress in adult rodents (Padival et al., 2013a; Hubert et al., 2014; Suvrathan et al., 2014; Mozhui et al., 2010), consistent with an increase of excitatory synaptic input to BLA neurons after repeated stress. Morphological correlates of increased excitatory synaptic inputs to BLA neurons have been observed in the form of increased dendritic spines and dendritic length after repeated stress (Adamec et al., 2012; Hill et al., 2011; Hill et al., 2012; Mitra et al., 2005; Vyas et al., 2002; Vyas et al., 2006).
The adolescent amygdala may be differentially vulnerable to the effects of stress compared to adults, resulting in a different cascade of changes in BLA physiology in adolescent rats (Hetzel and Rosenkranz, 2014; Zhang and Rosenkranz, 2012). Repeated stress increases the firing rate of BLA neurons in adult rats, but increases the number of active neurons in adolescent rats (Zhang and Rosenkranz, 2012). Moreover, increases in membrane excitability are seen across principal neurons of the BLA following stress exposure, but more selective effects are seen in the adult BLA (Hetzel and Rosenkranz, 2014). This parallels with distinct behavioral effects of stress across age (Eiland et al., 2012; Luine et al., 2007; Spear, 2000; Stone and Quartermain, 1997; Toledo-Rodriguez and Sandi, 2007; Zhang and Rosenkranz, 2013). One possible cause for this age-dependency is different effects of repeated stress on morphology of BLA neurons. The purpose of this study was to determine whether repeated stress causes morphological changes in the adolescent BLA that are distinct from changes in the adult BLA.
The BLA is composed of several nuclei, including the basal (BA) and lateral (LAT) nuclei. The BA and LAT have complementary, but distinct roles in affective behavior. Previous studies demonstrate spinogenesis and dendritic hypertrophy of BA and LAT neurons in adult rats exposed to repeated stress. However, the pattern of these morphological changes is different between the BA and LAT neurons (Nietzer et al., 2011; Padival et al., 2013b). Little is known about the effects of repeated stress on the morphology of BLA neurons in adolescence. Therefore, the current study examines and contrasts the pattern of changes in the BA and LAT nuclei of the BLA in adolescents. Changes in dendritic structure and spine number are associated with changes of excitatory drive, and were the focus of this study. Neuronal morphology was reconstructed after Golgi-Cox staining, and BA and LAT principal neuronal dendrites and spines were quantified. These morphological indices were compared between control and repeated restraint stress-exposed adolescent male rats. A change in the number of spines may impact the acuity of an afferent glutamatergic signal. Previous studies demonstrate that acuity of excitatory input to the BLA determines specificity of fear behavior (Shaban et al, 2006; Antunes and Moita, 2003). Therefore, the effects of stress on fear generalization were also compared between adolescent and adult male rats.
2. Experimental Procedures
All experiments were approved by the Institutional Animal Care and Use Committee of Rosalind Franklin University (protocol #11-47 and 13-10), and followed The Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Efforts were made to minimize animal suffering and to reduce the number of animals used. A portion of the data from adult rats from a previously published study (Padival et al., 2013b) were collected in a parallel manner with the current study, and were submitted to new analysis for comparison to adolescent rats in this study.
2.1 Subjects
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) were group housed (2-3/cage) in a controlled climate animal facility. The housing room had a 12:12 light:dark schedule and food and water were available ad libitum. After 1 week of habituation to the animal facility [postnatal day (P) 25 – 31], restraint stress was performed in a procedure room by placement of a rat in a restraint hemi-cylinder for 20 minutes per session. A control group was handled in the same manner as the restraint group, except they remained in a transparent transport cage with bedding in the housing room, instead of placement in a restraint cylinder. Rats were stressed or control handled for 7 out of 9 consecutive days (P32-40). This pattern has been shown to maximize the effects of repeated restraint on adrenal gland weight, anxiety-like behavior, and electrophysiological changes in the BLA (Zhang and Rosenkranz, 2012; Zhang et al., 2014). In addition, this pattern was chosen to conform to previous morphological studies in adult rats. One day after the final treatment (P41), rats were deeply anesthetized with chloral hydrate (400 mg/kg, i.p.) and decapitated. The brains were rapidly removed for Golgi-Cox staining. The adult rats in this study were exposed to control or repeated restraint procedures in the exact same manner as the adolescent rats, between P63-75.
2.2 Golgi-Cox stain
Golgi-Cox staining of brain tissue was performed using the FD Rapid GolgiStain Kit (FD NeuroTechnologies, Columbia, MD), following the protocol suggested by the manufacturer. After removal of the brain, the brain was blocked and placed immediately into Golgi impregnation solution in an opaque container, protected from light. The impregnation solution was changed after 24 hours and brains were stored in the dark for 15 – 18 days at room temperature. Brain tissue was then transferred to solution C of the FD Rapid GolgiStain kit for 24 hours at 4° C. After 24 hours the solution was replaced with fresh solution C, and the brain was stored at 4° C for 7 days. Brains were sectioned (100 μm thickness, Leica SM 2000 R microtome), and slices were collected in 20% sucrose in 0.1 M phosphate buffer (pH 7.4) at room temperature. Slices were mounted on gelatinized slides and air dried (25 minutes – 1 hour), then rinsed in double distilled H2O (2 times, 4 minutes each rinse). Slides were dehydrated in 50%, 75% and 95% ethanol for 4 minutes each, then in 100% ethanol four times for 3 minutes each. Slides were cleared with xylene (3 times, 4 minutes each), then coverslipped with Permount and were allowed to dry overnight.
2.3 Neuronal reconstruction
Golgi-stained neurons from LAT and BA were reconstructed using Neurolucida software (MBF Bioscience, Williston, VT) under bright field illumination with the 100x objective of a Nikon Eclipse E400 microscope. LAT and BA were defined based on previously established borders (Paxinos, 2004; Swanson and Petrovich, 1998). Some sections of Golgi-stained tissue were lightly counterstained with Nissl to determine LAT and BA borders based on fiber tracts and structural landmarks. Only neurons that appeared to be completely filled were utilized. Secondary and tertiary dendrites, as well as spines, had to be visible. In addition, there could be no breaks (> 5 μm) in the dendrites. Neurons were selected based on morphology consistent with BLA principal neurons (e.g. obvious primary dendrites and spines; McDonald, 1982). Reconstructions were performed by an individual that was blind to treatment conditions. Aspiny neurons that displayed small somata with few dendrites or large somata with bipolar primary dendrites were not included in this analysis. Dendritic branching was quantified as the number of intersections with concentric circles at increasing diameters (10 μm steps) in Sholl analysis (Sholl, 1953). Similarly, dendritic length and spine number were analyzed at 10 μm steps in Sholl analyses. To further measure these parameters in functional dendritic subdivisions, instead of only distance, the dendritic length and total number of spines in each dendritic branch order were quantified. Branch order was measured centrifugally, such that at each dendritic branch point the branch order number of both the parent dendrite and branch were increased. In addition, the normalized distribution of spines across the dendritic tree was quantified as [(Spine NumberBr)÷(Spine NumberPeak)] where Spine NumberBr = the number of spines at a specific branch order, and Spine NumberPeak = the number of spines on the branch with the largest number of spines in that neuron. This normalized for differences in spine number across groups and facilitated measurement of the shift of distributions across distance. Normalized dendritic length was calculated in the same manner. To compare the effects of stress across dendritic location and nucleus or age, the stress effect size was calculated across branches as , where Vstress= individual values at a specific branch from stress group and = mean value of that branch order in control group. Photographs were acquired at 10, 20 or 100X magnification from control and stress groups under similar light conditions. In the images displayed, the color was adjusted to grayscale and the size of the images was adjusted, and this was applied equally to all images.
2.4 Generalization of fear
To measure a behavior that can reflect the acuity of glutamatergic inputs to the BLA, generalization of fear-potentiated anxiety was measured (modified from Baldi et al, 2004; Korte et al, 1999 for testing in the open field). To accomplish this, individual rats were subjected to the same protocol as above, and then underwent behavioral testing instead of Golgi staining, beginning one day after the last restraint or control session.
Fear conditioning
Fear-potentiated anxiety was performed by first fear conditioning a CS+ tone in an operant chamber (10.6” × 10.6” × 14.1” height). Chambers were enclosed by sound-attenuating cabinets (UGO Basile, VA, Italy, 21” × 17.5” × 21.25” height). Mounted inside each cabinet were an audio speaker (UGO Basile, VA, Italy), a house light, an infrared LED light and a ceiling mounted digital camera that was sensitive to light in the IR range (Fire-i, Unibrain, San Ramon, CA) which was connected to a personal computer (Dell E6500) running video-tracking software (Any-Maze, Stoelting, WI). Conditioning consisted of 2 minutes habituation followed by 5 pairings of a tone (10s, 1 or 2 kHz, 85dB) with a co-terminating footshock (1s, threshold intensity; defined as the intensity that produced forepaw withdrawal determined from previous studies in adult and adolescent control and stress rats; Zhang and Rosenkranz, 2013).
Conditioning trials were presented at 60s intertrial intervals. Rats remained in the chamber for 1 minute after the end of last conditioning trial, and were then returned to their home cage. Freezing was defined as lack of all movement, except movement related to respiration, and quantified by the software based on a threshold of change in video image pixels.
Fear testing
After one day, anxiety-like behavior was measured in the open field. Rats were placed in an open field for 5 minutes. The open field (black opaque, 24 × 35 in) was in a novel room, under dim light (20-25 lux) with ambient noise produced by a white noise generator (65-70 dB). After testing, animals were returned to their home cage. After one additional day, fear-potentiated anxiety was measured. The rat was returned to the open field and a tone was presented after 30s (3 times, 20s, 60s interval) during 5 minute exploration. The tone was either the CS+ or a distinct tone (1 kHz or 2 kHz, counterbalanced). Rat behavior was monitored (IR-sensitive camera, Fire-i, Unibrain) and tracked (Any-Maze). Time spent in the center of the open field was quantified.
2.5 Statistical analysis
Neurons were excluded from analysis if they did not display morphological aspects of BLA principal neurons (as described above), or if their mean length or spine density was >2 standard deviations (SD) from the mean. A total of 8 adolescent rats/group were used to obtain 16 neurons/nucleus/group (range of 1-3 neurons/nucleus/rat). Due to potential non-independence of samples from the same rat, group analysis was performed using a nested ANOVA approach. Only after passing this test (p>0.05 for nesting factor) was data about main effects interpreted. However, in no instances was the F value obtained by using a nesting approach significant for the nesting factor (i.e. there was no significant impact of utilizing multiple measures from the same rat). Analysis was also performed to compare within and between subject variability of dendritic length and spine number to test whether evidence of non-independence could be observed as smaller within subject variability compared to between subject variability within the same treatment group. Comparisons across multiple factors were examined using a two-way ANOVA. An alpha level of 0.05 was considered significant. Significant effects and interactions were further analyzed by Holm-Sidak's post hoc multiple comparisons test. For planned comparisons of single parameters between two groups, two-tailed unpaired t-tests were used. Data were tested for normality of distribution (Kolmgorov and Smirnov test), and equal variance (Bartlett’s test). To test whether the population distribution of mean values across groups was significantly different, the cumulative probability distribution data were fit to a curve and the extra-sum-of-squares test was used to determine whether the best-fit parameters of the curves differed significantly or whether one curve could be explained as a nested sample of the other curve. To test whether the anatomical distribution of spines or length across the dendrites was shifted, the normalized distributions were best-fit to a function and the parameters of the best-fit were compared between groups. Chi-squared analysis was performed to determine if the branch order with peak number of spines or length differed between groups. Exploration in the open field was measured as time spent in the center area in the presence or absence of an auditory tone. This was compared between stress conditions and ages with a two-way repeated measures ANOVA. Statistical tests were performed using Prism 6 software (GraphPad Software, La Jolla, CA) or SPSS Statistics (v21, IBM, New York, NY). All values are expressed as the mean ± S.E.M., except where noted.
3. Results
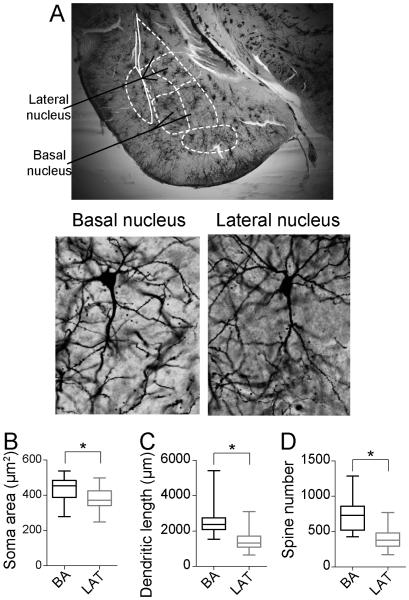
Neurons of the LAT and BA were reconstructed (Fig 1A). Only neurons that displayed a morphology consistent with BLA principal neurons were included for analysis (Methods). A total of 16 BA neurons and 16 LAT neurons per group satisfied the criteria and were included in the analysis (total of 64 neurons from adolescent rats). Similar to previous studies (McDonald, 1982), the soma size of BA neurons was significantly greater than LAT neurons (Fig 1B; BA soma area 430.7 ± 18.95 μm2, N=16; LAT soma area 375.7 ± 17.25 μm2, N=16; p=0.04, t=2.148, df=30, two-tailed unpaired t-test). The average total dendritic length of BA neurons (Fig1C; 2583 ± 229.4 μm, N=16) was significantly longer than LAT neurons (1473 ± 155.9 μm, N=16) in control adolescent rats (p=0.0004, t=4.0, df=30, two-tailed unpaired t-test). Similarly, there was a greater average number of spines in BA neurons (Fig 1D; 728.8 ± 56.15 spines, N=16) compared to LAT neurons (417.3 ± 42.56 spines, N=16) in control adolescent rats (p=0.0001, t=4.4, df=30, two-tailed unpaired t-test).
Figure 1. Morphological differences across nuclei in adolescent rats.
A) BA and LAT regions can be defined in Golgi-stained tissue with the aid of landmarks (top). Pyramidal-like neurons of the BA and LAT (bottom) were reconstructed after Golgi-Cox stain. The somatic area (B), average dendritic length (C) and average spine number (D) of principal neurons were significantly larger in the basal nucleus (BA) compared to the lateral nucleus (LAT) of adolescent rats. Average presented here as box plot with 5-95th percentile. * indicates p<0.05 in two-tailed t-test.
3.1 Effects of stress on LAT neurons
Dendritic length
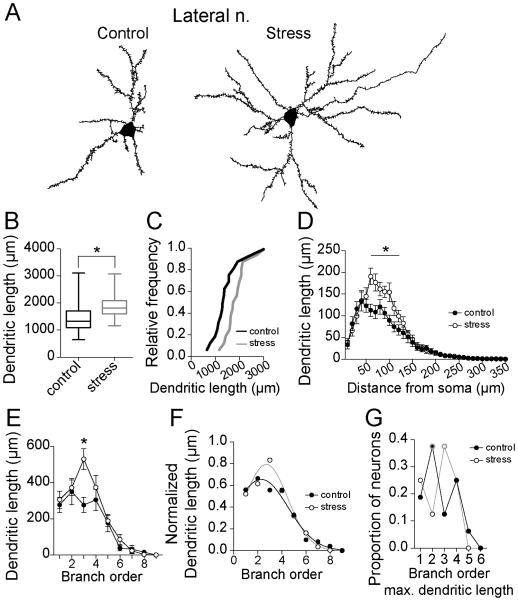
The vast majority of excitatory inputs form synapses at the dendritic region of amygdala neurons (Brinley-Reed et al., 1995; Farb et al., 1992; Farb and Ledoux, 1999; Muller et al., 2006; Rademacher et al., 2010; Radley et al., 2007; Smith and Pare, 1994). Dendritic length contributes to computational properties of neurons (Jaffe and Carnevale, 1999; Mainen and Sejnowski, 1996; Vetter et al., 2001), and often occurs in parallel with increased synaptic input. Repeated stress increased the average total dendritic length of LAT principle neurons in adolescent rats (Fig 2A,B; control 1473 ± 155.9 μm, N=16, stress 1884 ± 121.5 μm, N=16, p=0.046, t=2.1, df=30, two-tailed unpaired t-test; Variability due to within rat factors was not significantly different than variability due to between rat factors (sum of squares within rat=6080188.8, between rat=4644392.2, F=0.82, p=0.65). This difference was confirmed by comparison of the cumulative probability distribution of control and stress values. The distributions were significantly different (Fig 2C; p<0.0001, distributions best fit to different curves, F(3,26)=39.4, extra-sum-of-squares F test).
Figure 2. Repeated stress increases dendritic length of LAT neurons in adolescent rats.
A) Reconstruction of LAT principal neuron from control and stress group. B) Repeated stress increased the average dendritic length of principal neurons of the adolescent LAT (box plot with 5-95th percentile, * indicates p<0.05 in two-tailed t-test). C) Overall, there were more LAT neurons with higher average dendritic length after repeated stress than control, demonstrated by a shift in the cumulative frequency probability. D) There was a significant difference in the dendritic length at 60-120 μm from the soma (* indicates p<0.05 in post-hoc Holms-Sidak's tests). E) There was significantly greater dendritic length associated with the 3rd branch order after stress (* indicates p<0.05 in post-hoc Holms-Sidak's tests). F) When normalized by peak dendritic length, there was a significant shift in the distribution of dendrite across the entire dendritic tree towards 3rd order branches after stress. G) The branch that accounted for the most dendritic length shifted from the 2nd order to 3rd order after repeated stress (peak branch order is encircled with gray).
To test the dendritic locations at which the greatest effects of stress can be found, Sholl analysis of dendritic length was performed and compared between groups. Stress caused significant dendritic growth when measured over dendritic segments (Fig 2D; two-way repeated-measures ANOVA, stress × dendritic distance, main effect of distance p<0.0001, F(63, 1890)=77.4; main effect of stress p=0.046, F(1,30)=4.3; stress × distance interaction p<0.0001, F(63, 1890)=3.0). Stress significantly increased dendritic length at distances between 60-120 μm from the soma (p<0.05 post-hoc Holm-Sidak's multiple comparisons test).
Functionally, dendritic branch order may be more important than distance from the soma with regards to integration and propagation of synaptic signals. Therefore, we also analyzed dendritic length across branch order. Stress increased dendritic length across branch order (Fig 2E; two-way repeated-measures ANOVA, stress × dendritic branch, main effect of branch p<0.0001, F(11,330)=45.9; main effect of stress p=0.046, F(1,30)=4.3; stress × branch interaction p=0.01, F(11, 330)=2.3), with a significant difference in dendritic length at 3rd order branches (p<0.05 post-hoc Holm-Sidak's multiple comparisons test).
To determine whether dendritic growth led to a shift in the pattern across the dendritic tree, differences in dendritic length were normalized, and the relative contribution of each branch order to the total dendrite was measured. When normalized to peak dendritic length, stress shifted the anatomical distribution of dendritic length across branch order (Fig 2F; p=0.03, distributions best fit to different curves, F(3,18)=3.74, extra-sum-of-squares F test), with a shift towards a greater proportion of dendrite at higher order branches. The branch order where the peak dendritic length occurred shifted from the 2nd to the 3rd branch order (Fig 2G; Chi-square analysis of frequency of peak dendritic length at 1st-4th branch orders across population, chi-square=7.6, p=0.05).
Dendritic branching
Dendritic branching is a measure of dendritic complexity, and changes may indicate where dendritic growth is occurring. There was no significant effect of stress on overall dendritic branching in LAT neurons (two-way repeated-measures ANOVA, main effect of stress p=0.2, F(1,30)=1.7). However, there was a significant effect of stress across distance (two-way repeated-measures ANOVA, main effect of distance p<0.0001, F(34, 1020)=54.2; stress × distance interaction p=0.002, F(34,1020)=1.9). Specifically, stress increased dendritic branching at 60-70 μm and 90-100 μm from the soma. This is consistent with an increase in branching over a similar portion of dendrite that shows growth after stress (60 – 120 μm, above).
Spine number
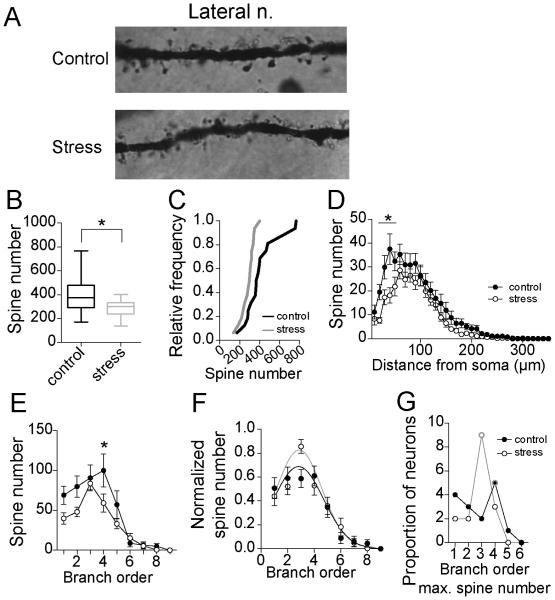
The majority of excitatory inputs forms synapses onto spines of BLA neurons. Therefore, spine number and distribution were quantified. Repeated stress decreased average spine number on LAT neurons of adolescent rats (Fig 3A,B; control 417.3 ± 42.6 spines, N=16, stress 285.4 ± 16.8 spines, N=16, p=0.007, t=2.88, df=30, two-tailed unpaired t-test; Variability due to within rat factors was not significantly different than variability due to between rat factors (sum of squares within rat=346688.5, between rat=294838.7, F=0.91, p=0.57). This difference was confirmed by comparison of the cumulative probability distributions of control and stress values across the populations (Fig 3C; p<0.0001, distributions best fit to different curves, F(3,26)=38.0, extra-sum-of-squares F test).
Figure 3. Repeated stress decreases spine number of LAT neurons in adolescent rats.
A) Examples of Golgi stain in secondary dendrites of LAT neuron from control and stress group (100x magnification). B) Repeated stress decreased the average spine number of principal neurons of the adolescent LAT (box plot with 5-95th percentile, * indicates p<0.05 in two-tailed t-test). C) Overall, there were fewer LAT neurons with higher average spine number after repeated stress than control, demonstrated by a shift in the cumulative frequency probability. D) There was a significant difference in the spine number at 20-60 μm from the soma (* indicates p<0.05 in post-hoc Holms-Sidak's tests). E) There was significantly fewer spines associated with the 4th branch order after stress (* indicates p<0.05 in post-hoc Holms-Sidak's tests). F) When normalized by peak spine number, there was a significant shift in the distribution of spine number across the entire dendritic tree towards 3rd order branches after stress. G) The branch that accounted for the most number of spines shifted from the 4th order to 3rd order after repeated stress (peak order is encircled with gray).
The anatomical distribution of spines across dendrites was examined with Sholl analysis. Stress caused a significant reduction of spines across the dendritic tree of LAT neurons (Fig 3D; two-way repeated-measures ANOVA, stress × dendritic distance, main effect of distance p<0.0001, F(63, 1890)=62.4; main effect of stress p=0.007, F(1,30)=8.3; stress × distance interaction p<0.0001, F(63, 1890)=2.3). When analyzed by segment, stress significantly decreased spine number at distances between 20-60 μm from the soma (p<0.05, post-hoc Holm-Sidak's multiple comparisons test).
The number of spines across branch order was also significantly decreased (Fig 3E; two-way repeated-measures ANOVA, stress × dendritic branch order, main effect of branch order p<0.0001, F(11,330)=33.7; main effect of stress p=0.007, F(1,30)=8.3; stress × branch order interaction p=0.05, F(11, 330)=1.9). This decrease reached significance at 2nd and 4th order branches (p<0.05, post-hoc Holm-Sidak's multiple comparisons test).
The relative balance of spines across branch order was measured as normalized spines/branch (normalized to peak spine number). Stress caused a shift in the relative anatomical distribution of spines across branch order (Fig 3F; p=0.04, distributions best fit to different curves, F(3,18)=3.14, extra-sum-of-squares F test). Furthermore, the shift in the branch order where the peak spine number occurred shifted significantly from the 4th to the 3rd branch order across populations (Fig 3G; Chi-square analysis of frequency of peak spine number at 1st-4th branch orders, chi-square=8.5, p=0.04). This is consistent with a shift in spine distribution across the dendritic tree due to decreased spines bracketing 3rd order dendritic branches.
Spine density
Repeated stress increased dendritic length with little effect on spine number at some branch orders, while repeated stress had little effect on dendritic length but decreased spine number at other branches. This complementary set of changes led to an overall decrease of spine density. Repeated stress significantly decreased average spine density in LAT neurons (Fig 4A; control 0.29 ± 0.02 spines/μm, N=16; stress 0.16 ± 0.01 spines/μm, N=16; p<0.0001, t=7.4, df=30, two-tailed unpaired t-test). When measured across branch order, repeated stress significantly decreased spine density across the dendritic tree (Fig 4B; two-way repeated-measures ANOVA, stress × dendritic branch order, main effect of branch order p<0.0001, F(5,150)=9.0; main effect of stress p<0.0001, F(1,30)=42.3; stress × branch order interaction p=0.02, F(5,150)=2.7), with significant differences in the 1st – 4th order branches (p<0.05, post-hoc Holm-Sidak's multiple comparisons test).
Figure 4. Repeated stress decreases spine density of LAT neurons in adolescent rats.
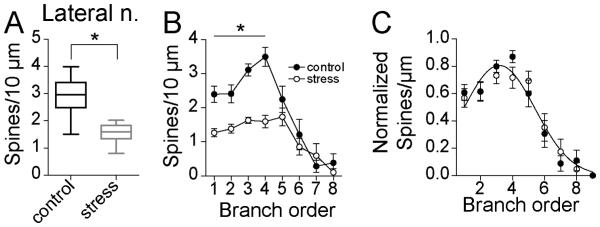
A) Repeated stress decreased the average spine density of principal neurons of the adolescent LAT (box plot with 5-95th percentile, * indicates p<0.05 in two-tailed t-test). B) There was significantly lower spine density in 1-4th branch order after stress (* indicates p<0.05 in post-hoc Holms-Sidak's tests). C) When normalized by peak spine density, there was no significant shift in the distribution of spine density across the entire dendritic tree.
3.2 Effects of stress on BA neurons
Dendritic length
Repeated stress had no significant effect on the average total dendritic length of BA principle neurons (Fig5A,B; control 2583 ± 229.4 μm, N=16, stress 3091 ± 190.0 μm, N=16, p=0.10, t=1.7, df=30, t-tailed unpaired t-test; Variability due to within rat factors was not significantly different than variability due to between rat factors (sum of squares within rat=802634.5, between rat=506417.9, F=0.67, p=0.78)). However, there was a small, but significant shift in the cumulative probability distribution of average control and stress values, perhaps indicative of a subpopulation of BA principal neurons that are responsive to stress in adolescents (Fig 5C; p<0.001, distributions best fit to different curves, F(3,26)=22.7, extra-sum-of-squares F test).
Figure 5. Repeated stress has weak effects on dendritic length of BA neurons in adolescent rats.
A) Reconstruction of a BA principal neuron from control and stress groups. B) Repeated stress had no significant effect on the average dendritic length of principal neurons of the adolescent BA (box plot with 5-95th percentile). C) Overall, there were statistically similar number of BA neurons with higher average dendritic length after repeated stress and control, indicated by no shift in the cumulative frequency probability. D) There was a significant decrease in the dendritic length after stress at 40-50 μm and 120-140 μm from the soma (* indicates p<0.05 in post-hoc Holms-Sidak's tests). E) There was no significant difference in dendritic length across branch order after stress. F) However, when normalized by peak dendritic length, there was a significant shift in the distribution of dendrite across the entire dendritic tree towards more distal dendrite after stress. G) The branch that accounted for the most dendritic length did not shift after repeated stress (peak order is encircled with gray).
Consistent with no overall effect of repeated stress on total dendritic length, there was no significant main effect of stress on dendritic length in Sholl analysis (Fig 5D; two-way repeated-measures ANOVA, stress × distance from soma, main effect of stress p=0.10, F(1,30)=2.9). However, there was a significant interaction between stress and distance from soma, consistent with local shifts of dendritic length (main effect of distance p<0.0001, F(63,1890)=64.6; stress × distance interaction p=0.0003, F(63,1890)=1.8). Significant local changes were found at 40 – 50 μm and 120 – 140 μm from the soma (p>0.05, post-hoc Holm-Sidak's multiple comparisons test).
Similarly, there was no significant effect of repeated stress on dendritic length across branch order (Fig 5E; two-way repeated-measures ANOVA, stress × dendritic branch order, main effect of branch order p<0.0001, F(11,330)=47.4; main effect of stress p=0.1, F(1,30)=2.9; stress × branch order interaction p=0.70, F(11,330)=0.74). Despite no significant main effect of stress, when values were normalized to measure the relative anatomical distribution, stress shifted the anatomical distribution of dendritic length across branch order (Fig 5F; p=0.05, distributions best fit to different curves, F(3,378)=2.7, extra-sum-of-squares F test) towards more dendrite in higher order branches, consistent with the Sholl analysis (above). However, there was no population shift in the branch order where the peak dendritic length occurred (Fig 5G; Chi-square analysis of frequency of peak dendritic length at 1st-4th branch orders, chi-square=1.1, p=0.77), indicating that the shift across the population was small.
Dendritic branching
While there was no significant main effect of stress on dendritic branching in Sholl analysis (number of intersections; two-way repeated-measures ANOVA, p=0.09, F(1,30)=3.1), there was a significant interaction between stress and distance (two-way repeated-measures ANOVA, main effect of distance, p<0.0001, F(34,1020)=34.5; distance × stress interaction p=0.0009, F(34,1020)=2.0). This is consistent with a distance-dependent effect of stress on dendritic branching, an anatomical shift in branching, and with small local changes of dendritic length in BA neurons (above).
Spine number
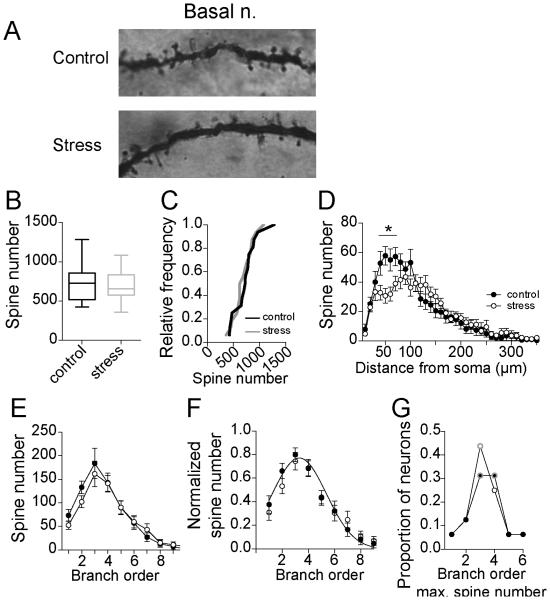
Repeated stress had no significant effect on total spine number of BA neurons (Fig 6A,B; control 728.8 ± 56.2, N=16, stress 688.6 ± 47.4, N=16, p=0.59, t=0.55, df=30, two-tailed unpaired t-test; Variability due to within rat factors was not significantly different than variability due to between rat factors (sum of squares within rat=691187.5, between rat=617864.9, F=0.95, p=0.54)), and had no significant effect on the cumulative probability distribution of average control and stress values (Fig 6C; p=0.23, distributions best fit to same curve, F(3,26)=1.5, extra-sum-of-squares F test).
Figure 6. Repeated stress has weak effects on spine number of BA neurons.
A) Examples of Golgi stain in secondary dendrites of a BA neuron from control and stress groups. B) Repeated stress had no significant effect on the average spine number of principal neurons of the adolescent BA (box plot with 5-95th percentile). C) Overall, there were statistically similar number of BA neurons with higher average spine number after repeated stress and control, indicated by no shift in the cumulative frequency probability. D) There was a significant decrease in the spine number after stress at 40-70 μm from the soma (* indicates p<0.05 in post-hoc Holms-Sidak's tests). E) There was no significant difference in spine number across branch order after stress. F) When normalized by peak dendritic length, there was no significant shift in the distribution of spines across the entire dendritic tree. G) The branch that accounted for the most spines did not shift after repeated stress (peak order is encircled with gray).
While there was no significant main effect of stress in Sholl analysis (two-way repeated-measures ANOVA, main effect of stress p=0.59, F(1,30)=0.3), there was a significant interaction between stress and distance from the soma (Fig 6D; main effect of distance p<0.0001, F(63,1890)=52.4; stress × distance interaction p<0.0001, F(63,1890)=2.6), with significant decreases in spine number at 40 – 70 μm from the soma (p>0.05 post-hoc Holm-Sidak's multiple comparisons test).
Consistent with minor effects of repeated stress on spine number in BA neurons, there was no significant effect of stress on spine number analyzed across branch order (Fig 6E; two-way repeated-measures ANOVA, stress × dendritic branch order, main effect of branch order p<0.0001, F(11,330)=41.1; main effect of stress p=0.58, F(1,30)=0.32; stress × branch order interaction p=0.87, F(11,330)=0.54). Even when normalized, there was no significant anatomical shift in the distribution of spines across branch order (Fig 6F; p=0.22, distributions best fit to same curve, F(3,18)=1.6, extra-sum-of-squares F test). There was also no population shift in the branch where the peak spine number occurred (Fig 6G; Chi-square analysis of frequency of peak dendritic length at 1st-4th branch orders, chi-square=0.38, p=0.95).
Spine density
The small, not significant increases of dendritic length across branches, combined with the small decreases of spine number led to a global decrease of spine density. Repeated stress significantly decreased average spine density in BA neurons (Fig 7A; control 0.29 ± 0.02 spines/μm, N=16; stress 0.23 ± 0.01 spines/μm, N=16; p=0.004, t=3.2, df=30, two-tailed unpaired t-test). When measured across branch order, repeated stress significantly decreased spine density across the dendritic tree (Fig 7B; two-way repeated-measures ANOVA, stress × dendritic branch order, main effect of branch order p<0.0001, F(7,210)=17.2; main effect of stress p=0.03, F(1,30)=5.3; stress × branch order interaction p=0.96, F(7,210)=0.3), without significant differences at any individual branch order (p>0.05, post-hoc Holm-Sidak's multiple comparisons test).
Figure 7. Repeated stress decreases spine density of BA neurons in adolescent rats.
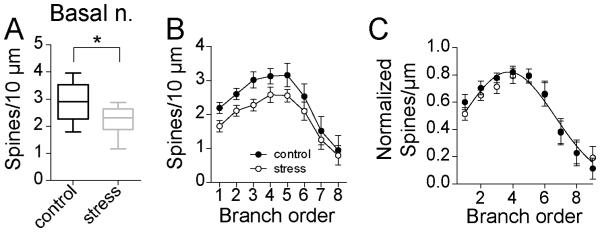
A) Repeated stress decreased the average spine density of principal neurons of the adolescent BA (box plot with 5-95th percentile, * indicates p<0.05 in two-tailed t-test). B) While there was significantly lower spine density, no individual branch reached statistically significantly lower spine density after stress. C) When normalized by peak spine density, there was no significant shift in the distribution of spine density across the entire dendritic tree.
3.3 Effect of stress across nuclei
Global spine number and dendritic length
To test whether stress had different effects in the LAT and BA nuclei, the average total dendritic length and total spine number were compared. There was a significant main effect of nucleus and a main effect of stress when the total dendritic length of BA and LAT neurons was compared (two-way ANOVA, stress × nucleus, main effect of stress p=0.04, F(1,60)=4.5; main effect of nucleus p<0.0001, F(1,60)=42.2). However, there was no significant interaction between stress and nuclei (interaction p=0.94, F(1,60)=0.01). Similarly, there were differences in total spine number between nuclei and an effect of stress on total spine number (two-way ANOVA, stress × nucleus, main effect of stress p=0.05, F(1,60)=4.0; main effect of nucleus p<0.0001, F(1,60)=68.2), but no differences in the effects of stress across nuclei (interaction p=0.29, F(1,60)=1.1). This indicates similar global changes of spine number and dendritic length between BA and LAT.
Localized effects of stress
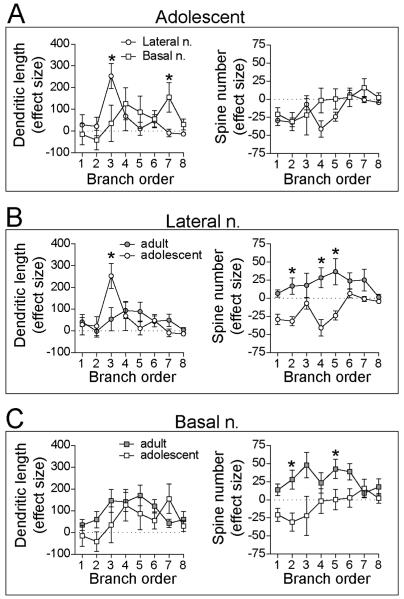
Despite small effects of stress on total dendritic length or spine number, there could be large differences between LAT and BA in effects of stress on specific subregions of the dendrite. To test whether stress had different effects at specific dendritic locations across nuclei, the stress effect size was compared across branches (Methods). There was a significant difference between BA and LAT in the effects of stress on dendritic length that depended on branch order (Fig 8A; two-way repeated-measures ANOVA, nucleus × branch order interaction p=0.01, F(7,210)=2.6; main effect of nucleus p=0.93, F(1,30)=0.01; main effect of branch order p=0.04, F(7,210)=2.1), with largest effects emerging at 3rd and 7th order branches (post-hoc Holm-Sidak's multiple comparisons test). In contrast, stress caused a similar decreases in spine number between BA and LAT (Fig 8A; two-way repeated-measures ANOVA, main effect of nucleus p=0.16, F(1,30)=2.1; nucleus × branch order interaction p=0.36, F(7,210)=1.1; main effect of branch order p=0.004, F(7,210)=3.1). Therefore, stress leads to localized effects on dendritic length, and these effects differ between BA and LAT. However, local effects of stress on spine number are similar between BA and LAT.
Figure 8. Repeated stress had different effects across nuclei and age.
The stress effect size across the dendritic tree was compared between nuclei and across age. A) Repeated stress in adolescents had different effects on LAT and BA dendritic length (left) particularly at 3rd order branches, but similar pattern of effects on spine number (right) . B) Repeated stress had significantly different pattern of local effects on dendritic length (left; particularly at 3rd order branches) and global differences in the effects on spine number (right) of LAT neurons in adult and adolescent rats. C) Repeated stress had a similar pattern of effects on dendritic length (left), but different pattern of global effect on spine number (right) of BA neurons in adult and adolescent rats. * indicates p<0.05 in post-hoc Holms-Sidak's tests after two-way repeated measures ANOVA.
Anatomical distribution pattern
To test whether there was a difference in the effects of stress on the relative anatomical distribution, normalized distribution across branch order was compared between LAT and BA (Methods). There was a significant difference between BA and LAT in the effects of stress on the distribution pattern of dendritic branch length (two-way repeated measures ANOVA, branch × nucleus interaction p=0.004, F(7,210)=3.1). Differences emerged between BA and LAT particularly at 3rd order branches (p<0.05 post-hoc Holm-Sidak's multiple comparisons test). Similarly, there was a significant difference between BA and LAT in the effects of stress on the distribution pattern of branch spine number (two-way repeated measures ANOVA, branch × nucleus interaction p=0.02, F(7,210)=2.4), with significant differences between the BA and LAT also emerging at 3rd order branches (p<0.05 post-hoc Holm-Sidak's multiple comparisons test). Both of these shifts are likely due to a shift in the spine and length distribution of LAT neurons towards 3rd order branches.
3.4 Effect of stress across age
Previous studies demonstrate developmental changes in BLA neuronal morphology (Koss et al., 2014; Bergstrom et al., 2010). In the current study, BA neurons from adult rats had significantly lower dendritic length compared to adolescent rats (two-way repeated measures ANOVA, main effect of age p<0.0001, F(1,42)=28.2; age × distance interaction p=0.004, F(32,1344)=1.8, with significant differences across 70-180 μm, p<0.05 post-hoc Holm-Sidak's multiple comparisons test). This was accompanied by a significant reduction in dendritic spine number in BA neurons from adult compared to adolescent rats (two-way repeated measures ANOVA, main effect of age p=0.0002, F(1,42)=16.5; age × distance interaction p=0.047, F(32,1344)=1.5, with significant differences at 30 and 100-110-180 μm, p<0.05 post-hoc Holm-Sidak's multiple comparisons test). In contrast, in LAT neurons there was no significant age difference between dendritic length (two-way repeated measures ANOVA, main effect of age p=0.57, F(1,42)=0.33; age × distance interaction p=0.25, F(32,1344)=1.1) or spine number (two-way repeated measures ANOVA, main effect of age p=0.44, F(1,42)=1.2; age × distance interaction p=0.28, F(32,1344)=1.0). To test whether repeated stress had age-specific effects across dendritic branches of BLA neurons, as observed in adults (Nietzer et al., 2011; Padival et al., 2013b), the effect size was compared.
Lateral amygdala
There was no significant difference across age in the effect of stress on LAT neuron dendritic length (Fig 8B; two-way repeated-measures ANOVA, main effect of age p=0.86, F(1,41)=0.03). However, there was a significant difference between adult and adolescent rats in the branch order most impacted by stress (age × branch order interaction p=0.007, F(7,287)=2.8; main effect of branch order p=0.0005, F(7,287)=3.9; 3rd order branches significant different, p<0.05, post-hoc Holm-Sidak's multiple comparisons test). There were even greater global age differences in the effects of stress on LAT spine number (Fig8B; two-way repeated-measures ANOVA, main effect of age p=0.002, F(1,41)=11.6) in addition to differences at specific branches (age × branch order interaction p=0.04, F(7,287)=2.1), with significant differences at 2nd, 4th and 5th order branches between adult and adolescent LAT neurons (p<0.05 post-hoc Holm-Sidak's multiple comparisons test). This indicates that the effects of stress on LAT dendrite morphology differed only in the location of changes between adults and adolescents, but effects of stress on LAT spine number differed globally (direction of changes) and locally (location of changes).
Basal amygdala
While there was a greater overall impact of stress on dendritic length in adult rats, this did not differ as a function of branch order (Fig 8C; two-way repeated-measures ANOVA, main effect of age p=0.04, F(1,42)=3.8; main effect of branch order p=0.02, F(7,294)=2.4; age × branch order interaction p=0.28, F(7,294)=1.2). There was a greater effect of stress on spine number of BA neurons in adult rats (Fig 8C; two-way repeated-measures ANOVA, main effect of age p<0.0001, F(1,42)=12.2), and this effect differed across branches (main effect of branch order p=0.46, F(7,294)=0.97; age × branch order interaction p=0.04, F(7,294)=2.0), with significant differences at 2nd and 5th order branches (p<0.05, post-hoc Holm-Sidak's multiple comparisons test). This indicates that there were broad age differences in the magnitude of the effects of stress on BA dendrite morphology and spines across the dendritic tree, combined with an additional localized difference in the effects of stress on spine number between adult and adolescents.
Anatomical distribution pattern
To test whether the relative pattern of change differs between adult and adolescent rats, the normalized changes in dendritic length and spine distribution were compared across age. There were substantial differences in the pattern of the effects of stress in LAT between adult and adolescent rats. While repeated stress caused dendritic growth and spinogenesis across a range of dendritic branches in adult rats leading to small shifts of the distribution, dendritic growth and spinogenesis occurred only in the 3rd branch order in LAT of adolescent rats leading to a more narrow distribution and significant differences between adults and adolescents (significant difference between adult and adolescent; p<0.05 post-hoc Holm-Sidak's multiple comparisons test). In BA neurons, differences in dendritic and spine growth were also apparent between adult and adolescent rats. There was little dendritic growth or spinogenesis in the BA of adolescent rats, while adult rats displayed substantial dendritic growth and spinogenesis in BA neurons, with a distal shift in the distribution, leading to significant differences in 5th and 6th order branches (significant difference between adult and adolescent; p<0.05 post-hoc Holm-Sidak's multiple comparisons test). Thus, the effects of stress on the relative anatomical distribution of spines and length across the dendritic tree differed between adult and adolescent BA neurons.
3.6. Effects of stress on fear-potentiated anxiety.
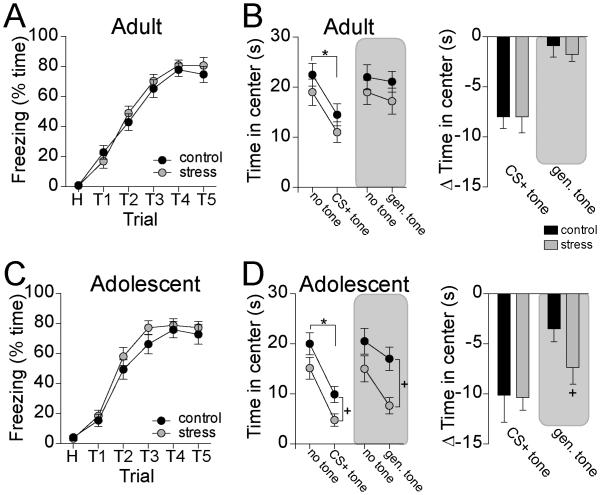
Previous studies did not find substantial differences in the effects of stress on anxiety-like behavior when comparing adult and adolescent rats (Zhang and Rosenkranz, 2012), perhaps because a change in anxiety may be driven by factors other than spine number. However, reduced number of spines after repeated stress may lead to weaker selectivity of conditioned fear responses, or generalization in the effects of conditioned fear on anxiety-like behavior. This was tested by measuring generalization of the effects of a CS+ on anxiety-like behavior in the open field (n=8-9 rats/group). A CS+ tone presented during open field exploration reduced time spent in the center of the open field compared to a no-tone condition (Fig 9B,D; two-way repeated-measures ANOVA, main effect of tone p<0.0001, F(1,14)=38.3; significant decrease in center time in adult and in adolescent rats, p<0.05 post-hoc Holm-Sidak's multiple comparisons test). A non-CS+ tone reduced center exploration only in adolescent rats (Fig 9B,D; two-way repeated-measures ANOVA, main effect of age p=0.02, F(1,15)=6.4; significant decrease in center time in adolescent rats only, p<0.05 post-hoc Holm-Sidak's multiple comparisons test). Repeated stress did not significantly enhance suppression of center exploration in adult rats upon presentation of the CS+ (Fig 9B; two-way repeated-measures ANOVA, main effect of tone p<0.0001, F(1,14)=64.6; main effect of stress p=0.28, F(1,14)=1.3). Similarly, repeated stress did not significantly enhance suppression of center exploration upon presentation of the non-CS+ tone in adult rats (Fig 9B; two-way repeated-measures ANOVA, main effect of tone p=0.07, F(1,16)=3.9, main effect of stress p=0.32, F(1,16)=1.1). However, repeated stress did enhance suppression of center exploration in adolescent rats upon presentation of the CS+ (Fig 9D; two-way repeated-measures ANOVA, main effect of tone p<0.0001, F(1,14)=47.6; main effect of stress p=0.04, F(1,14)=5.4). Repeated stress also significantly enhanced suppression of center exploration in adolescent rats upon presentation of the non-CS+ tone (Fig 9D; two-way repeated-measures ANOVA, main effect of tone p=0.0001, F(1,14)=26.7; main effect of stress p=0.03, F(1,14)=5.8; significant difference between control and stress during tone presentation, p<0.05 post-hoc Holm-Sidak's multiple comparisons test). To help exclude the possibility that generally increased unlearned sensitivity to tones in adolescent rats could account for these results, the effects of a tone (1 or 2 kHz) on open field exploration were tested in rats that were not exposed to CS conditioning. Adult and adolescent rats that were not exposed to CS conditioning did not display significant reduction of exploration in response to the tone (two-way repeated-measures ANOVA, main effect of age p=0.28, F(1,14)=1.3, N=8-9 rats/group). The effects of stress do not appear to be due to facilitation of acquisition, as stress did not facilitate freezing during conditioning in adult rats (Fig 9A; (two-way repeated-measures ANOVA, main effect of stress p=0.58, F(1,30)=0.32, stress × trial interaction p=0.56, F(5,150)=0.79) or adolescent rats (Fig 9C; two-way repeated-measures ANOVA, main effect of stress p=0.25, F(1,30)=1.4, stress × trial interaction p=0.78, F(5,150)=0.50). These data demonstrate that repeated stress leads to generalization of the effects of a fear conditioned stimulus on anxiety-like behavior in adolescent rats.
Figure 9. Repeated stress increases fear generalization in adolescent rats.
A) Fear conditioning in adult rats led to increased freezing to the CS+ tone. Stress did not increase this conditioned freezing. B) In adult rats, presentation of the CS+ tone caused suppression of exploration in the open field, while presentation of a distinct tone did not (left). Stress did not facilitate the effects of either tone (right). C) Fear conditioning in adolescent rats led to increased freezing to the CS+ tone. Stress did not increase this conditioned freezing. D) In adolescent rats, presentation of the CS+ tone caused suppression of exploration in the open field (left). Stress facilitated the effects of the distinct tone in adolescent rats (right). Δ Time in center = the difference in center time between the no-tone and tone conditions. * p<0.05 for main effect in two-way repeated measures ANOVA. + p<0.05 post hoc Holm-Sidak test after two-way ANOVA.
4. Discussion
Previous studies have demonstrated robust effects of repeated stress on the morphology and physiology of BLA neurons. Moreover, repeated stress during adulthood most commonly causes dendritic growth and spinogenesis of neurons in the LAT and BA subregions (Adamec et al., 2012; Hill et al., 2011; Hill et al., 2012; Mitra et al., 2005; Vyas et al., 2002; Vyas et al., 2006), but with different patterns of morphological changes across nuclei (Nietzer et al., 2011; Padival et al., 2013b). However, little is known about the effects of stress on morphology of BLA neurons in adolescents. The current study demonstrates that repeated stress has very different effects on the morphology of adolescent BA and LAT neurons. In adult rats, repeated stress had little impact on LAT neuron total dendritic length, but increased spine number of LAT neurons. Conversely, repeated stress increased dendritic length and attenuated spine density in LAT neurons in adolescent rats. Repeated stress was also found to augment both dendritic length and spine number in the BA of adult rats, but had no significant effect on BA neuronal total dendritic length or spine density in adolescent rats.
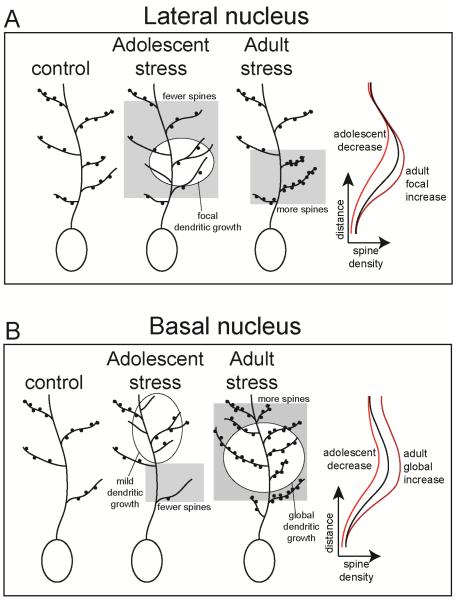
On a finer level of analysis, the effects of stress on the anatomical distribution of spines across the dendritic tree and the site of dendritic growth were also different across nuclei and age. In BA and LAT neurons, the highest proportion of spines tend to be in 3rd order branches, and the 2nd – 3rd order branches have the greatest proportion of dendritic length. This anatomical distribution was altered by stress. Specifically, in adult rats, repeated stress subtly increased spine number and dendritic length of LAT neurons across a range of the dendritic tree, particularly at middle distances, leading to a similar anatomical pattern of length and distribution of spines in control and stress groups. In contrast, in adolescent rats, repeated stress selectively decreased LAT neuron spines on several proximal branch orders, along with a complementary focal increase of dendritic length at 3rd order branches. The overall impact is a small increase of LAT neuron spine density at proximal dendrites in adult rats, but a decrease of spine density over a wide range of the dendrite in adolescent rats (Fig 10).
Figure 10. Schematic representation of age-dependent effects of stress.
A) In LAT neurons of adult rats, repeated stress caused a focal increase of dendritic spines, leading to a focal increase of spine density. In contrast, in adolescent rats, repeated stress caused focal dendritic growth coupled with more widespread decrease of spine number. This led to a global decrease of spine density in adolescent rats. B) In BA neurons of adult rats, repeated stress caused global increases of spine number and dendritic length. The balance was a global increase of spine density. In contrast, in adolescent rat repeated stress decreased spine number and increased dendritic length in complementary dendritic branches, leading to global decrease of spine density.
Repeated stress increased BA neuronal spines at middle and distal distances in adult rats, leading to a shift in the relative distribution of spines towards more distal sites, in parallel with increased dendritic length. In contrast, repeated stress did not shift the relative distribution of spines across the dendritic tree of BA neurons in adolescent rats, but caused a small decrease of proximal spine number along with a small increase of distal dendritic length. The overall effect was an increased spine density in BA neurons of adult rats, but a global decrease in adolescent rats (Fig 10). The effects of stress are similar when analyzed by Sholl's method or by branch order, with few small differences (e.g. focal decrease of BA dendritic spine number in Sholl analysis but not branch order analysis). This small difference is likely due to a dilution of some focal differences when analyzed across branch order.
Observed differences in dendritic growth and spine distribution are expected to produce significant differences in the effects of stress on synaptic physiology. A change in spine number likely indicates a change in excitatory synaptic input. Excitatory inputs to cortical-like neurons are filtered by dendritic properties, leading to different kinetics of somatic excitatory postsynaptic potentials (EPSPs) that originate at different distances along the dendrite (Andreasen and Lambert, 1998; Magee, 2000; Turner, 1988). Therefore, a shift in the relative anatomical distribution of excitatory inputs can lead to a shift in the overall shape and amplitude of somatic EPSPs (e.g. Rall et al., 1967; Rall, 1967). A shift in these kinetic properties of EPSPs will have a large impact on synaptic integration, and ultimately neuron plasticity and action potential firing (Larkum et al., 2004; Oviedo and Reyes, 2005; Schaefer et al., 2003; Williams, 2005). Furthermore, in many cortical-like regions, there is a specific proximal-distal topography of synaptic inputs from different sources across the dendritic tree (Amaral and Witter, 1989; Bollmann and Engert, 2009; French and Totterdell, 2002; Ishizuka et al., 1990; Jia et al., 2010; Markram et al., 1997; Petreanu et al., 2009; Richardson et al., 2009; Steward, 1976). While the topography of inputs to BLA neurons is still not clear, these shifts in spine distribution after stress may point to a change in the ability of a specific set of inputs to drive BLA neuronal activity, and guide BLA-dependent behaviors.
Previous studies indicate differences in the effects of stress on LAT and BA neuron morphology in adult rats (Nietzer et al., 2011; Padival et al., 2013b). Other studies hint towards different effects of stress on BLA neuronal morphology in adults and adolescents. Five weeks of chronic social instability stress through adolescence and early adulthood reduced dendritic length and spine density of secondary dendrites in BA neurons when measured as adults, whereas the same stress applied in adults leads to opposite changes (Tsai et al., 2014). Repeated restraint stress from weaning to adolescence can increase dendritic length of BA neurons (Eiland et al., 2012). Post-weaning social isolation increased segmenting of BA neurons measured from adult rats, but had no significant effect on total dendritic length or spine density (Wang et al., 2012). While those studies did not compare adult and adolescent morphology, they point to different and sometimes opposite effects of stress on BA neurons in adolescence, as previous studies found that stressful experiences during adulthood causes BA neuronal spinogenesis and dendritic growth. However, based on the current literature it is difficult to make inferences about age differences in the effects of stress on LAT neuronal morphology. The results from the current study bridge this gap in the literature and contribute to our understanding of age differences by contrasting LAT and BA nuclei, focusing on the effects of stress during adolescence on adolescent amygdala neuron morphology, and directly comparing the same exact stress experience between adolescents and adults.
There are a number of factors that may contribute to differences in the effects of stress on BLA neuronal morphology across age. One possibility is that repeated stress during adolescence stunts normal development of the BLA. Between adolescence and middle adulthood (~P90), there is an increase in dendritic branching of BA neurons (Koss et al., 2014), with no significant difference of dendritic length or spine density. However, there may be some dendritic retraction of BA neurons between early adulthood and middle adulthood (Bergstrom et al., 2010). This implies that between adolescence and middle adulthood there is a process of dendritic growth and increased branching of BA neurons, followed by dendritic retraction. Our current results support an interpretation of growth followed by later retraction. A stunting of this process by repeated stress may explain the reduced dendritic complexity and the subtle shift of dendritic length in adolescents. Other causes may include age differences in the responsiveness of the hypothalamic-pituitary-adrenal axis (Romeo et al., 2006; Sapolsky and Meaney, 1986; Walker et al., 1991), or responsiveness of neurons to stress factors and neuromodulators (Braga et al., 2004; Jiang et al., 2009; Sandi et al., 2008).
The current studies point to distinct effects of repeated stress in adult and adolescent rats on the morphology of BA and LAT neurons. Increased excitatory drive of BLA neurons is expected to lead to increased anxiety and fear behavior. This expectation holds well in adult rats, where increased excitatory drive is observed in parallel with increased BLA-related anxiety and fear behavior. While repeated stress during adolescence often does increase fear and anxiety-like behavior, the current studies indicate that these behavioral changes occur in the absence of increased number of excitatory synaptic inputs. Our results suggest that other factors may be driving increased anxiety and fear behavior after repeated stress in adolescent rats. For instance, reduced GABAergic function and increased membrane excitability could increase BLA neuronal responsivity, and both have been previously observed in adolescent rats after repeated stress (Braga et al., 2004; Hetzel and Rosenkranz, 2014; Jacobson-Pick and Richter-Levin, 2012; Jiang et al., 2009; Liu et al., 2014; Tzanoulinou et al., 2014; Maslova et al., 2002; McCormick et al., 2008; Toth et al., 2008). However, the loss of spines, perhaps reflective of loss of specific glutamatergic inputs, layered on top of those changes in adolescent rats may lead to a loss of specificity of responses. Loss of specificity of amygdala neuronal responses can lead to fear generalization (Ghosh and Chattarji, 2014). Increased BLA neuronal excitability, impaired GABAergic regulation, and increased plasticity of intrinsic connectivity contribute to fear generalization (Shaban et al, 2006; Bergado-Acosta et al, 2008; Ball et al, 2012; Ghosh and Chattarji, 2014). However, impaired function of the prefrontal cortex and hippocampus (Zelinski et al, 2010; Xu and Sudhof, 2013), and their input to the BLA, also contributes to BLA-mediated fear generalization (Bergado-Acosta et al, 2008; Sangha et al, 2009; Lihktik et al, 2014). Our current results indicate that repeated stress leads to increased fear generalization. This is not likely due to non-associative sensitization, as the footshock intensity utilized is lower than reported to be required for non-specific behavioral sensitization (Laxmi et al, 2003; Baldi et al, 2004; Siegmund and Wotjak 2007), and exploration of the open field after stress was suppressed more by the generalized tone than in the non-tone condition. If behavioral sensitization were the root cause of generalization, reduced exploration would be expected even in the non-tone condition. However, contribution of sensitization cannot be entirely ruled out in these conditions. Using different approaches, previous studies also demonstrate increased fear generalization after prepubertal stress (Morrissey et al, 2010; Sampath et al, 2014) or adult stress (Hoffman et al, 2014).
The BA and LAT play different roles in acquisition and extinction of cued and contextual fear, and perhaps different roles in phasic and prolonged anxiety (Calandreau et al., 2005; Davis et al., 2010; Onishi and Xavier, 2010; Orsini et al., 2011; Vlachos et al., 2011). Through differences in anatomical projections to nucleus accumbens and prefrontal cortex, these nuclei are also expected to play different roles in depressive behaviors. An understanding of the differences in the effects of stress on these two nuclei across age may lead to better predictability of the effects of stress on these different forms of affective behavior. This can lead to insight into age differences in anxiety and depressive symptoms after repeated exposure to stress, and treatments to target these differences.
Highlights.
Repeated stress decreases spine density in adolescent lateral amygdala neurons
Repeated stress decreases spine density in adolescent basal amygdala neurons
These effects of stress differ from adults in the direction of changes and in the location of changes across the dendrite
In parallel, repeated stress increases fear generalization in adolescent rats
Acknowledgements
Supported by NIH (MH084970). The funding sources had no role in study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication. The authors have no conflicts of interest to declare.
The authors wish to thank Mitch Beales for upkeep of microscope and software used for neuronal reconstructions, and Dr. Gloria Meredith for providing training in neuronal reconstructions. Funding for this project was provided by National Institutes of Health (MH084970) to JAR. They had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Abbreviations
- ANOVA
Analysis of variance
- BA
Basal nucleus of the amygdala
- BLA
Basolateral area of the amygdala
- EPSP
Excitatory postsynaptic potential
- Fig
Figure
- i.p.
Intraperitoneal
- LAT
Lateral nucleus of the amygdala
- P
Postnatal day
- SD
Standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec R, Blundell J, Burton P. Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiol Behav. 2005;86:75–91. doi: 10.1016/j.physbeh.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Adamec R, Hebert M, Blundell J, Mervis RF. Dendritic morphology of amygdala and hippocampal neurons in more and less predator stress responsive rats and more and less spontaneously anxious handled controls. Behav Brain Res. 2012;226:133–146. doi: 10.1016/j.bbr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Lambert JD. Factors determining the efficacy of distal excitatory synapses in rat hippocampal CA1 pyramidal neurones. J Physiol. 1998;507:441–462. doi: 10.1111/j.1469-7793.1998.441bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Antunes R, Moita MA. Discriminative auditory fear learning requires both tuned and nontuned auditory pathways to the amygdala. J Neurosci. 2010;30(29):9782–7. doi: 10.1523/JNEUROSCI.1037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E, Lorenzini CA, Bucherelli C. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem. 2004;81(3):162–6. doi: 10.1016/j.nlm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ball JM, Hummos AM, Nair SS. Role of sensory input distribution and intrinsic connectivity in lateral amygdala during auditory fear conditioning: a computational study. Neuroscience. 2012;224:249–67. doi: 10.1016/j.neuroscience.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learn Mem. 2008;15(3):163–71. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, Smith RF, Mollinedo NS, McDonald CG. Chronic nicotine exposure produces lateralized, age-dependent dendritic remodeling in the rodent basolateral amygdala. Synapse. 2010;64:754–764. doi: 10.1002/syn.20783. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am J Psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Engert F. Subcellular topography of visually driven dendritic activity in the vertebrate visual system. Neuron. 2009;61:895–905. doi: 10.1016/j.neuron.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004;29:45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD, Davis TL, Jiang A, Cohen MS, Stern CE, Belliveau JW, Baer L, O'Sullivan RL, Savage CR, Jenike MA, Rosen BR. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Brinley-Reed M, Mascagni F, McDonald AJ. Synaptology of prefrontal cortical projections to the basolateral amygdala: an electron microscopic study in the rat. Neurosci Lett. 1995;202:45–48. doi: 10.1016/0304-3940(95)12212-5. [DOI] [PubMed] [Google Scholar]
- Calandreau L, Desmedt A, Decorte L, Jaffard R. A different recruitment of the lateral and basolateral amygdala promotes contextual or elemental conditioned association in Pavlovian fear conditioning. Learn Mem. 2005;12:383–388. doi: 10.1101/lm.92305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb C, Aoki C, Milner T, Kaneko T, LeDoux J. Glutamate immunoreactive terminals in the lateral amygdaloid nucleus: a possible substrate for emotional memory. Brain Res. 1992;593:145–158. doi: 10.1016/0006-8993(92)91303-v. [DOI] [PubMed] [Google Scholar]
- Farb CR, Ledoux JE. Afferents from rat temporal cortex synapse on lateral amygdala neurons that express NMDA and AMPA receptors. Synapse. 1999;33:218–229. doi: 10.1002/(SICI)1098-2396(19990901)33:3<218::AID-SYN6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol. 2002;446:151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- Ganzel B, Casey BJ, Glover G, Voss HU, Temple E. The aftermath of 9/11: effect of intensity and recency of trauma on outcome. Emotion. 2007;7:227–238. doi: 10.1037/1528-3542.7.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci. 2014 doi: 10.1038/nn.3888. doi: 10.1038/nn.3888. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hetzel A, Rosenkranz JA. Distinct effects of repeated restraint stress on basolateral amygdala neuronal membrane properties in resilient adolescent and adult rats. Neuropsychopharmacology. 2014;39:2114–2130. doi: 10.1038/npp.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, McEwen BS. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex. 2011;21:2056–2064. doi: 10.1093/cercor/bhq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, Cravatt BF, Hillard CJ, Chattarji S, McEwen BS. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry. 2012;18(10):1125–35. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AN, Lorson NG, Sanabria F, Foster Olive M, Conrad CD. Chronic stress disrupts fear extinction and enhances amygdala and hippocampal Fos expression in an animal model of post-traumatic stress disorder. Neurobiol Learn Mem. 2014;112:139–47. doi: 10.1016/j.nlm.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Li C, Rainnie DG, Muly EC. Effects of stress on AMPA receptor distribution and function in the basolateral amygdala. Brain Struct Funct. 2014;219:1169–1179. doi: 10.1007/s00429-013-0557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Richter-Levin G. Short- and long-term effects of juvenile stressor exposure on the expression of GABAA receptor subunits in rats. Stress. 2012;15:416–424. doi: 10.3109/10253890.2011.634036. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Carnevale NT. Passive normalization of synaptic integration influenced by dendritic architecture. J Neurophysiol. 1999;82:3268–3285. doi: 10.1152/jn.1999.82.6.3268. [DOI] [PubMed] [Google Scholar]
- Jia H, Rochefort NL, Chen X, Konnerth A. Dendritic organization of sensory input to cortical neurons in vivo. Nature. 2010;464:1307–1312. doi: 10.1038/nature08947. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology. 2009;34:410–423. doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- Korte SM, De Boer SF, Bohus B. Fear-potentiation in the elevated plus-maze test depends on stressor controllability and fear conditioning. Stress. 1999;3(1):27–40. doi: 10.3109/10253899909001110. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Senn W, Luscher HR. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb Cortex. 2004;14:1059–1070. doi: 10.1093/cercor/bhh065. [DOI] [PubMed] [Google Scholar]
- Laxmi TR, Stork O, Pape HC. Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res. 2003;145(1-2):89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17(1):106–13. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZP, Song C, Wang M, He Y, Xu XB, Pan HQ, Chen WB, Peng WJ, Pan BX. Chronic stress impairs GABAergic control of amygdala through suppressing the tonic GABAA receptor currents. Mol Brain. 2014;7:32. doi: 10.1186/1756-6606-7-32. doi: 10.1186/1756-6606-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslova LN, Bulygina VV, Markel AL. Chronic stress during prepubertal development: immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology. 2002;27:549–561. doi: 10.1016/s0306-4530(01)00092-0. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey MD, Mathews IZ, McCormick CM. Enduring deficits in contextual and auditory fear conditioning after adolescent, not adult, social instability stress in male rats. Neurobiol Learn Mem. 2010;95(1):46–56. doi: 10.1016/j.nlm.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietzer SL, Bonn M, Jansen F, Heiming RS, Lewejohann L, Sachser N, Asan ES, Lesch KP, Schmitt AG. Serotonin transporter knockout and repeated social defeat stress: impact on neuronal morphology and plasticity in limbic brain areas. Behav Brain Res. 2011;220:42–54. doi: 10.1016/j.bbr.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Onishi BK, Xavier GF. Contextual, but not auditory, fear conditioning is disrupted by neurotoxic selective lesion of the basal nucleus of amygdala in rats. Neurobiol Learn Mem. 2010;93:165–174. doi: 10.1016/j.nlm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo H, Reyes AD. Variation of input-output properties along the somatodendritic axis of pyramidal neurons. J Neurosci. 2005;25:4985–4995. doi: 10.1523/JNEUROSCI.0562-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival M, Quinette D, Rosenkranz JA. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology. 2013a;38:1748–1762. doi: 10.1038/npp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival MA, Blume SR, Rosenkranz JA. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013b;246:230–242. doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. Third The Rat Nervous System; Paxinos: 2004. p. 1328. [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Rosenkranz JA, Morshedi MM, Sullivan EM, Meredith GE. Amphetamine-associated contextual learning is accompanied by structural and functional plasticity in the basolateral amygdala. J Neurosci. 2010;30:4676–4686. doi: 10.1523/JNEUROSCI.6165-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Farb CR, He Y, Janssen WG, Rodrigues SM, Johnson LR, Hof PR, LeDoux JE, Morrison JH. Distribution of NMDA and AMPA receptor subunits at thalamo-amygdaloid dendritic spines. Brain Res. 2007;1134:87–94. doi: 10.1016/j.brainres.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967;30:1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Smith TG, Nelson PG, Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967;30:1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Blundon JA, Bayazitov IT, Zakharenko SS. Connectivity patterns revealed by mapping of active inputs on dendrites of thalamorecipient neurons in the auditory cortex. J Neurosci. 2009;29:6406–6417. doi: 10.1523/JNEUROSCI.0258-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath D, Sabitha KR, Hegde P, Jayakrishnan HR, Kutty BM, Chattarji S, Rangarajan G, Laxmi TR. A study on fear memory retrieval and REM sleep in maternal separation and isolation stressed rats. Behav Brain Res. 2014;273:144–54. doi: 10.1016/j.bbr.2014.07.034. [DOI] [PubMed] [Google Scholar]
- Sangha S, Narayanan RT, Bergado-Acosta JR, Stork O, Seidenbecher T, Pape HC. Deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory. J Neurosci. 2009;29(50):15713–20. doi: 10.1523/JNEUROSCI.2620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Cordero MI, Ugolini A, Varea E, Caberlotto L, Large CH. Chronic stress-induced alterations in amygdala responsiveness and behavior--modulation by trait anxiety and corticotropin-releasing factor systems. Eur J Neurosci. 2008;28:1836–1848. doi: 10.1111/j.1460-9568.2008.06451.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Larkum ME, Sakmann B, Roth A. Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J Neurophysiol. 2003;89:3143–3154. doi: 10.1152/jn.00046.2003. [DOI] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Lüthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9(8):1028–35. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007;41(10):848–60. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J Comp Neurol. 1994;342:232–248. doi: 10.1002/cne.903420207. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Bennur S, Ghosh S, Tomar A, Anilkumar S, Chattarji S. Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130151. doi: 10.1098/rstb.2013.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007;2007:71203. doi: 10.1155/2007/71203. doi:10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tsai SF, Huang TY, Chang CY, Hsu YC, Chen SJ, Yu L, Kuo YM, Jen CJ. Social instability stress differentially affects amygdalar neuron adaptations and memory performance in adolescent and adult rats. Front Behav Neurosci. 2014;8:27. doi: 10.3389/fnbeh.2014.00027. doi: 10.3389/fnbeh.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DA. Waveform and amplitude characteristics of evoked responses to dendritic stimulation of CA1 guinea-pig pyramidal cells. J Physiol. 1988;395:419–439. doi: 10.1113/jphysiol.1988.sp016927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S, Garcia-Mompo C, Castillo-Gomez E, Veenit V, Nacher J, Sandi C. Long-term behavioral programming induced by peripuberty stress in rats is accompanied by GABAergic-related alterations in the Amygdala. PLoS One. 2014;9:e94666. doi: 10.1371/journal.pone.0094666. doi: 10.1371/journal.pone.0094666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Roth A, Hausser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–937. doi: 10.1152/jn.2001.85.2.926. [DOI] [PubMed] [Google Scholar]
- Vlachos I, Herry C, Luthi A, Aertsen A, Kumar A. Context-dependent encoding of fear and extinction memories in a large-scale network model of the basal amygdala. PLoS Comput Biol. 2011;7:e1001104. doi: 10.1371/journal.pcbi.1001104. doi:10.1371/journal.pcbi.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct. 2012;217:337–351. doi: 10.1007/s00429-011-0355-4. [DOI] [PubMed] [Google Scholar]
- Williams SR. Encoding and decoding of dendritic excitation during active states in pyramidal neurons. J Neurosci. 2005;25:5894–5902. doi: 10.1523/JNEUROSCI.0502-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339(6125):1290–5. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EL, Hong NS, Tyndall AV, Halsall B, McDonald RJ. Prefrontal cortical contributions during discriminative fear conditioning, extinction, and spontaneous recovery in rats. Exp Brain Res. 2010;203(2):285–97. doi: 10.1007/s00221-010-2228-0. [DOI] [PubMed] [Google Scholar]
- Zhang W, Hetzel A, Shah B, Atchley D, Blume SR, Padival MA, Rosenkranz JA. Greater physiological and behavioral effects of interrupted stress pattern compared to daily restraint stress in rats. PLoS One. 2014;9:e102247. doi: 10.1371/journal.pone.0102247. doi: 10.1371/journal.pone.0102247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–474. doi: 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA. Repeated restraint stress enhances cue-elicited conditioned freezing and impairs acquisition of extinction in an age-dependent manner. Behav Brain Res. 2013;248:12–24. doi: 10.1016/j.bbr.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]