Abstract
Coronary artery disease is a leading cause of death and disability worldwide with contemporary treatment strategies employing both optimal medical therapy and catheter based percutaneous coronary intervention (PCI) with drug eluting stents (DES). While DES have dramatically reduced restenosis rates, their use has been associated with an increased risk of late stent thrombosis and accelerated neointimal atherosclerosis (i.e. “neoatherosclerosis”) both major contributors to late stent failure. The underlying substrate of late DES failure is likely related to vascular endothelial dysfunction such as poor endothelial regrowth and barrier function (i.e. “endothelial healing”). Initial concerns with 1st generation DES have lead to improvements in mechanical and biologic properties of current 2nd generation DES, which inhibit endothelial regrowth to a lesser extent, lessening late stent failure and resulting in an overall improved safety profile. Current guidelines recommend duration of at least one year of dual anti-platelet therapy with aspirin and a thienopyridine agent such as clopidogrel or prasugrel as sufficient to prevent late thrombotic complications. Recent studies, however, suggest a shorter duration of dual anti-platelet therapy may be equally as safe and efficacious in preventing stent thrombosis with newer generation DES. However, higher risk populations such as patients receiving 1st generation DES or those with increased risk for future ischemic events may benefit from a longer duration (i.e. 30 months) of DAPT to prevent major cardiovascular events with the caveat that such an approach may be associated with an increased risk for bleeding. This review examines the vascular responses to 1st and second generation DES and recent clinical trials examining DAPT duration.
Introduction
Coronary artery disease is a leading cause of death and disability[1]. Treatment strategies aimed at reducing events in patients with coronary artery disease (CAD) have employed both optimal medical therapy and catheter based percutaneous coronary intervention (PCI) with drug eluting stents (DES). While DES have dramatically reduced restenosis rates compared with bare metal stents (BMS), initial concerns with their use surrounded an increased risk of late (i.e. greater than 30 days after implant) stent thrombosis (LST), mainly observed with 1st generation DES. The primary substrate underlying LST is poor endothelialization and the recommendations for extended (one-year) dual anti-platelet therapy with aspirin and clopidogrel were implemented with the belief this might reduce this risk. More recently, newer generation DES utilizing thinner stent struts, improved polymer biocompatibility and lower drug concentration have demonstrated superior endothelialization in animal models and intravascular imaging studies. However both 1st and current generation DES tend to develop accelerated collections of foamy macrophages within the neointima (termed “neoatherosclerosis”) which may contribute to late thrombotic events when compared to bare metal stent. In this review, we will discuss the pre-clinical and clinical data supporting the use of specific durations of DAPT in patients receiving DES.
Pathophysiology of Late Stent Thrombosis after DES Implantation
The approval of 1st generation sirolimus eluting (SES) and paclitaxel eluting stents (PES) by the United States Food and Drug Administration was based upon randomized clinical trial data of short-term (< one year) duration [2, 3]. The major endpoints of these trials were based on measures of stent restenosis and both DES demonstrated major benefits without other serious adverse events. However, these trials were never powered to examine safety endpoint such as stent thrombosis.
A number of case reports and observational studies describing late stent thrombosis in patients more than one year after DES implantation raised initial concerns[4, 5]. Coincident with these studies, we also described the vascular responses in human pathologic samples taken from patients receiving these devices[6]. By comparing 23 autopsies of human DES implants of more than 30 days duration to 25 bare metal stent (BMS) implants matched for age, sex, stented artery and duration of implant, we demonstrated delayed arterial healing as defined by persistent fibrin, minimal neointimal formation and incomplete endothelialization in DES compared to BMS cases. Endothelialization was complete in most BMS sections consistent with earlier pathologic studies which suggested near compete healing by 3 to 4 months. In DES, some samples remained unhealed as far as 40 months after implant. Late stent thrombosis (LST), defined as any platelet rich thrombus occupying 25% of lumen 30 days after DES implantation, was observed in 14 of 23 patients receiving DES. The major pathologic finding distinguishing late thrombosed from patent DES was evidence of a significantly greater delay in arterial healing characterized by lack of endothelialization and persistent fibrin deposition at a mean of approximately 6 months after DES implantation[7]. These data suggested that lack of complete arterial healing after DES was the common factor underlying all cases of DES late stent thrombosis.
Our findings were complimented by angioscopic studies in patients receiving BMS and DES which found incomplete neointimal coverage in most sirolimus eluting stent (SES) implants[8]. Furthermore clinical data continued to demonstrate increased thrombotic events in patients receiving 1st generation DES and indicated the most important risk factor for such events was withdrawal of dual anti-platelet therapy[5]. Although the American Heart Association and the American College of Cardiology recommended 12 months of dual antiplatelet therapy with aspirin and the P2Y12 inhibitor clopidogrel, data from the SIRTAX and Post-SIRTAX registries in Bern and the RESEARCH and T-SEARCH registries in Rotterdam indicated that stent thromboses continued to occur steadily, at a constant rate of 0.6% per year at least out to 4 years after stent implantation and perhaps beyond[9]. Thus it seems clear that in some patients receiving 1st generation DES, arterial healing continues to be delayed for many years.
2nd generation DES such as everolimus eluting stents (EES), Endeavor-zotarolimus eluting stents (E-ZES), and Resolute Integrity zotarolimus eluting stents (R-ZES) were designed with thinner strut backbone stents, less polymer and drug loading, and eluted analogues of sirolimus such as everolimus and zotarolimus, which in some cases have improved lipophilicity potentially increasing tissue retention and cellular targeting. In preclinical-models of arterial stenting, EES, E-ZES, and R-ZES demonstrate superior endothelialization to 1st generation DES at similar timepoints suggesting the duration of healing might also be superior in humans[10]. Indeed, human autopsy samples of 1st generation DES compared to 2nd generation DES (i.e. EES) have recently shown that EES demonstrates superior strut coverage at similar timepoints[11]. Although head-to-head trials of 1st generation DES (i.e. SES) versus EES have not conclusively shown a reduced incidence of stent thrombosis, all have been probably been underpowered to detect such a difference. However, in aggregrate they appear to demonstrate superiority of EES [12]. OCT studies in patients receiving these stents have also been conducted and suggest superior strut coverage in EES[13]. The implications of these findings for the duration of DAPT after DES will be discussed below.
Mechanisms of Delayed Healing by mTOR inhibitors
The majority of DES used in clinical practice are designed to elute pharmacologic agents such as sirolimus that inhibit the mammalian target of rapamycin (mTOR), a member of the phosphatidylinositol kinase-related family of serine/threonine kinase. Although animal studies suggest that inhibitors of mTOR delay endothelial cell growth and recovery, the precise cellular mechanisms are still being elucidated.
mTOR interacts with several proteins to form two distinct complexes named mTOR complex 1 (mTORC1) and 2 (mTORC2) each of which each has distinct sensitivities to rapamycin. Each mTOR complex integrates information from upstream signaling and activates downstream effectors to control distinct cellular mechanisms needed for arterial repair[14]. mTORC1 is the better characterized of the mTOR complexes and integrates signaling from multiple signals including growth factors released upon arterial injury to affect process critical for endothelial coverage after injury such as migration and proliferation. SRL inhibits mTORC1 but not mTORC2 through specific binding of the FKBP12, a ubiquitous, cytosolic 12-KD FK506–binding protein and key stabilizing component of ryanodine (RyR2) intracellular calcium release channels in various cell types[15]. SRL has subnanomolar affinity to FKBP12 with 50% inhibitory concentration for the mTORC1 signaling pathway at this subnanomolar dose range[16]. mTORC1 directly phosphorylates translational regulators eukaryotic initial factor 4E-binding protein 1 (4EBP-1) and S6 kinase (S6K1). The regulation of proteins critical for cell proliferation and migration might in fact be the most important mechanism by which mTORC1 regulates endothelialization. We recently showed that inhibition of S6K1 in human endothelial cells was far more effective at inhibiting cell proliferation versus 4EBP-1[17]. Moreover, sirolimus’s effect on inhibiting endothelial proliferation could be rescued by overexpressing S6K.
The relationship of sirolimus to mTORC2 is more complex. Because short-term treatment with sirolimus does not inhibit mTORC2 signaling, this complex was originally thought to be sirolimus insensitive. However, the situation was made more complex by the observation that long-term treatment with sirolimus inhibits mTORC2 signaling in some cell types including endothelial cells[18]. Less is understood about the mTORC2 complex including its upstream effectors. It does respond to growth factors including insulin through poorly understood mechanisms. mTORC2 controls several kinases including Akt and serum- and glucocorticoid-induced protein kinase 1 (SGK1). Inhibition of mTORC2 also likely affects endothelial recovery after DES placement by impacting endothelial survival through the downstream affects on Akt. Akt is involved in promoting the expression of cell barrier proteins, such as vascular endothelial (VE) cadherins, important in endothelial survival[19]. Many other mechanisms by which inhibition of mTORC2 affect endothelial recovery have yet to be discovered but undoubtedly exist. Other aspects also affect endothelial recovery. Local drug concentrations are increased by overlapping DES, encountered in approximately one-quarter of interventional procedures, which may lead to increased percentage of uncovered stent struts[20]. Interestingly, systemic drugs may also adversely affect the mTOR signaling pathway. Metformin, a biguanide and commonly used diabetic drug, also inhibits mTORC1 through both AMP kinase (AMPK) dependent and independent pathways (Figure 1). In a pre-clinical model, Metformin was shown to inhibit mTOR signaling pathway via the AMPK dependent mechanism highlighting the susceptibility of the mTOR signaling pathway to alteration cellular energy homeostasis[17].
Figure 1.
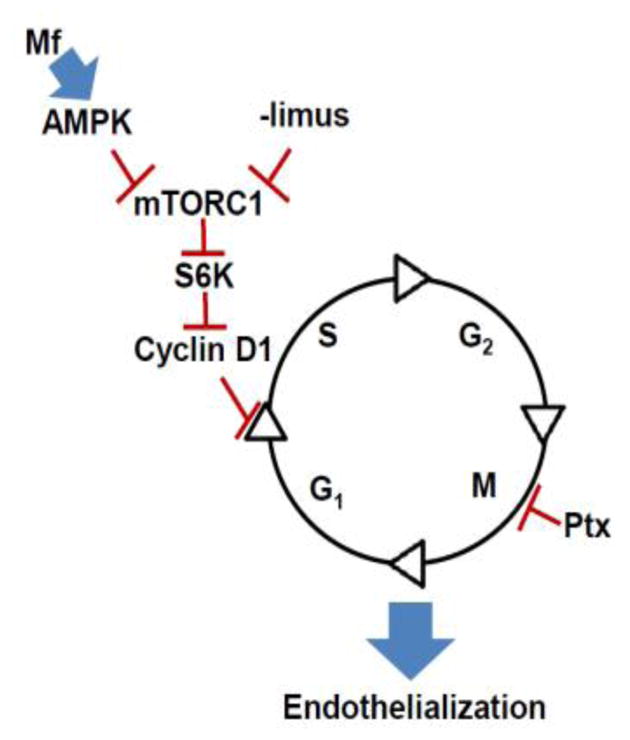
Role of mTOR in Vascular Endothelial Growth. Effect of –limus based agents on the endothelial cell cycle in conjunction with Metformin (Mf), a biguanide and common anti-diabetic drug (G1 = gap phase 1, S = Synthesis, G2 = Gap 2, M = Mitosis). Metformin activates AMP kinase (AMPK) and inhibits mTOR complex 1 (mTORC1) downstream effectors (S6K and Cyclin D1) to prevent S/G1 transition similar to -limus based agents.
Additionally outside of pharmacologic factors related to eluted drugs, various mechanical factors are related to poor endothelial healing and late stent failure. These factors include polymer hypersensitivity leading to eosinophilic infiltration and persistently poor endothelial healing as well as stent malapposition[21, 22]. Some of these factors have been overcome by advances in stent technology in newer generation DES with more biocompatible polymers, newer alloys and stent designs. The use of newer, lipophilic – limus based mTOR inhibitors (i.e. everolimus, zotarolimus) have also allowed lower drug concentrations lessening drug toxicity when compared to the prototype, sirolimus. Furthermore everolimus has been shown to have a more favorable vascular response in a pre-clinical diabetic animal model after DES implantation suggesting it may have a role in promoting endothelial integrity (Table 1)[23]. While improvements in design (i.e. mechanical and biologic) factors may have largely addressed etiologies of poor endothelial coverage after 1st generation DES placement; an intact endothelium may display poor endothelial barrier function that may act as a substrate for neointimal atherosclerosis known as “neoatherosclerosis” (Figure 2). Neoatherosclerosis is the development of foamy macrophages within the neointima which overlies the deployed stent and is accelerated in DES compared with BMS[24]. The use of –limus based DES may contribute to poor endothelial barrier function, leading to neoatherosclerosis which is increasing seen as a common substrate that underlie late stent failure leading to instent restenosis and thrombosis.
Table 1.
In Vitro and In Vivo Assessment of Vascular Response of Comparator Limus-Based Drug-Eluting and Bare Metal Stents (BMS) Diabetic Animal Model
| Stent | Drug (μg/mm2) | Polymer | Depth (mm) | Release Kinetics | NIH | ENDO | In vitro migration | In vitro apoptosis | In vitro proliferation | EBF |
|---|---|---|---|---|---|---|---|---|---|---|
| BMS | None | None | 81 | NA | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| SES | SRL (1.4) | PEVA+PBMA | 152.6 | 80% | ⇊ | ⇊ | ⇊ | ↓ | ⇊ | ⇊ |
| EES | EVL (1.0) | FP | 88.6 | 80% | ⇊ | ↔ | ↓ | ⇊ | ↓ | ↔ |
SRL = sirolimus, EVL = everolimus, PEVA = polyethylene-co-vinyl acetate, PBMA = poly n-butyl methacrylate, FP = fluropolymer, NIH = neointimal hyperplasia, ENDO = endothelialization, EBF = endothelial barrier function
Figure 2.
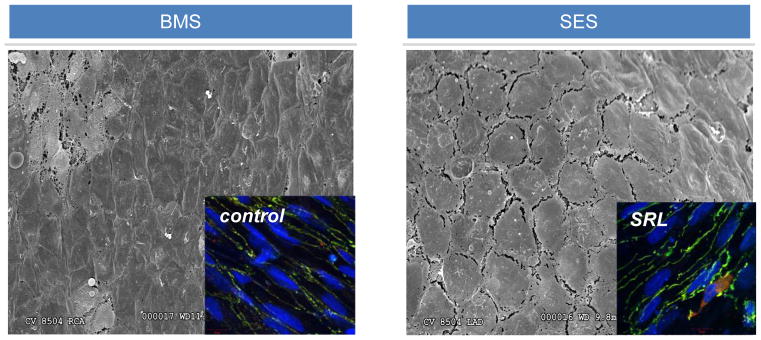
Poorly Formed Endothelial Cell Junctions Following Stent Placement. When compared with bare metal stents (BMS), scanning electron microscopy of rabbit iliac s treated with sirolimus eluting stents (SES, Cypher, Johnson and Johnson) have poorly formed endothelial junctions compared with bare metal stent (BMS) treated arteries and subsequent endothelial barrier dysfunction. Insets show immunohistochemistry of key endothelial barrier proteins, VE cadherin and p120, with higher colocalization in control treated human endothelial cells compared with those treated with sirolimus (SRL).
Neoatherosclerosis
Previously post mortem studies of patients with late stent failure/stent related deaths have demonstrated both 1) poor endothelial coverage and 2) neointimal atherosclerosis (“neoatherosclerosis”) as a common substrate of late stent failure. Key features of neoatherosclerosis include foamy macrophages, thin cap fibroatheroma and lipid infiltration or plaque rupture. Accelerated neoatherosclerosis is seen with 1st generation DES placement (mean ~ 420 days) compared with BMS (mean ~ 2160 days) and may play a role in the greater observed incidence of late stent and very late stent thrombosis(25). We previously reported in an autopsy series of 1st and 2nd generation DES that the incidence of neoatherosclerosis was approximately 30% in both 1st and second generation (i.e. EES) DES[11].
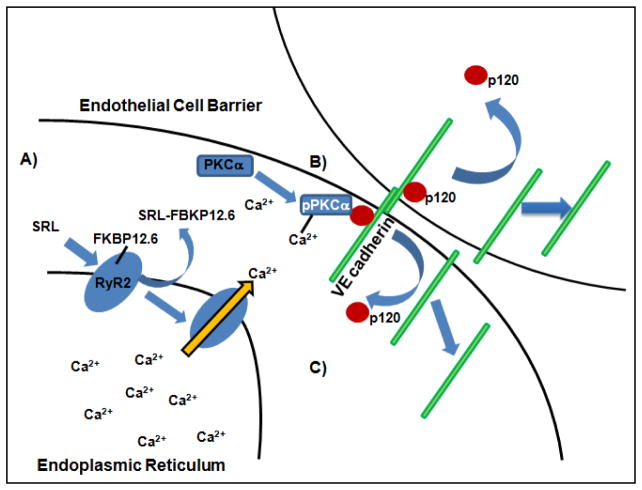
We recently demonstrated a mechanism by which mTOR inhibition inhibits endothelial barrier formation. We showed that sirolimus-FKBP12 interaction impairs barrier formation by increasing intracellular calcium via destabilization of ryanodine intracellular calcium release channels and subsequent activation of calcium sensitive protein kinase C alpha (PKCα), a serine/threonine kinase important in vascular endothelial (VE) cadherin barrier function through its interaction with p120-catenin (p120) (Figure 3)[26]. This study demonstrated that the impairment in barrier formation that occurs after endothelial cells are treated with mTOR inhibitors occurs because of off-target effects of the drug itself rather than as a direct consequence of mTOR inhibition. These differences are likely exacerbated by diabetes where PKC activation is also associated with accelerated atherosclerosis suggesting that neoatherosclerosis is likely a major contributor to in stent restenosis especially in diabetes[27]. These data may also explain why the incidence of neoatherosclerosis is not different between 1st and 2nd generation DES since both employ mTOR inhibitors.
Figure 3.
Role of mTOR in Vascular Endothelial Barrier Function. A) The prototypical –limus agent, Sirolimus (SRL), displaces FKBP12.6 from RyR2 calcium release channel (blue oval) specifically in vascular endothelial cells resulting in increased intracellular free Ca2+. B) PKCα is activated and destabilized the p120-VE cadherin interaction. C) p120 and eventually VE cadherin move from the membrane to the intracellular space leading to impaired endothelial barrier function.
Six versus twelve or twenty-four months of DAPT after DES to Prevent Stent Thrombosis
The delay in arterial repair seen after DES placement and the observation that withdrawal of anti-platelet therapy is an important risk factor for late stent thrombosis suggests prolonged DAPT might be protective against late stent related complications. Indeed some observational studies initially did suggest extending DAPT beyond one year was associated with reduced risk of myocardial infarction after DES implantation while others demonstrated increased risk for bleeding without any reduction in ischemic events[28–32]. Complicating interpretation of these studies it the fact that stent technology as discussed above has improved healing responses so that the duration of DAPT needed to prevent late stent-related events might actually be different depending on stent type.
It is also important to separate the issue of prolonged DAPT for the purposes of reducing stent-related events from protection from non-culprit mediated thrombotic events arising from atherosclerosis progression. Because of data generated in humans and animals demonstrating superior stent strut coverage in 2nd versus 1st generation DES, smaller trials have focused on whether curtailing the duration of DAPT for 6 months or less might be non-inferior to 12 or 24 months duration. In most of these trials (summarized in table 2), 2nd generation DES such as E-ZES and EES were the major stent type used though some of these trials had limited numbers of 1st generation DES. In general, many of these trials were probably not appropriately powered to detect low frequency events such as stent thrombosis. Conversely, it could also be said that lack of any significant difference between treatment regimens might be interpreted to mean that even if significant numbers of patients were included to show superiority of one regimen over the other, small but statistically significant differences might not be clinically meaningful. In aggregate these trials appear to demonstrate that to prevent stent related events, 6 months of DAPT is non-inferior to 12 or 24 months of DAPT though heterogeneity of stent types used is a limiting factor in interpreting these data. These trials appear to confirm that the mandatory period of DAPT for 2nd generation or later DES is 6 months and that prolonged DAPT (i.e. beyond 6 months) does not further reduce the risk of stent-related events and may increase the risk of significant bleeding.
Table 2.
Completed Short-term DAPT Trials
| Trial Name | N | DAPT Duration | DES Type | Conclusion |
|---|---|---|---|---|
| OPTIMIZE (31) | 3,120 | 3 vs. 12 | E-ZES (N = 3,119; 1563 3 months DAPT; 1556 12 months) | 3-mo DAPT was non-inferior to 12-mo for D/MI/stroke/major bleeding, without significantly increasing risk for ST |
| EXCELLENT (35) | 1,443 | 6 vs. 12 | EES and SES (N=1443; 514 6 months DAPT; 672 12 months) | 6-mo DAPT did not increase risk of target vessel failure at 12 mo after implantation of DES compared to 12-mo DAPT; however, the non-inferiority margin was wide and study was underpowered for death or MI |
| PRODIGY (29) | 1,970 | 6 vs 24 | E-ZES (N = 502) PES (N = 505) EES (N = 501) BMS (N = 505) (983 6 months DAPT; 987 24 months) Essentially all stent types evenly randomized to 6 or 24 months. |
24-mo clopidogrel not more effective than 6 mo clopidogrel in reducing composite death, MI, or cerebrovascular accident. |
| RESET (36) | 2,117 | 3 vs. 12 | E-ZES (N=1059 randomized to 3 months DAPT) 12 months of DAPT group included R- ZES (N=559) SES (N=383) EES (N=404) |
E-ZES 3-mo DAPT was non-inferior to standard therapy (12 months clopidogrel and other DES) with respect to the primary endpoint (composite all-cause death, MI, or ST) |
| SECURITY (37) | 1399 | 6 vs. 12 | 2nd Gen DES (E-ZES=934, EES (N=457), Nobori (N=250), Biomatrix N=166) All Randomized to 6 months DAPT (N=682 versus 12 months N=717) |
6 months DAPT non-inferior to 12 months regarding the primary composite end point of cardiac death, MI, stroke, definite or probable ST or BARC type 3 or 5 bleeding at 12 months follow-up |
| ITALIC (38) | 2301 | 6 vs. 24 | EES | Non-inferiority for one year D/MI/Stroke/TVR/ major bleed between DAPT regimens |
| ISAR-SAFE (unpublished) | 4005 6-month event-free | 6 vs. 12 | All DES | Non-inferiority for 9 month D/MI/ST/stroke, major bleeding between DAPT regimens |
Abbreviations: D=death, MI=myocardial infarction, ST=stent thrombosis; BARC=bleeding Academic Research Consortium, TVR=target vessel revascularization
Twelve versus Thirty months of DAPT After Stenting for Prevention of Thrombotic Events
Recently, the 12 or 30 months of Dual Antiplatelet therapy after Drug Eluting Stents Trial was published[33]. This trial, a very large international, multicenter, randomized study, was designed to determine the benefits and risks of continuing DAPT therapy (either clopidogrel or prasugrel) beyond one year in patients who had received coronary stents. Patients were only eligible for the trial if after 12 months of DAPT they had not had a major adverse cardiovascular or cerebrovascular event, repeat revascularization, or moderate to severe bleeding. Longer-term treatment with DAPT reduced the risk of stent thrombosis, and myocardial infarction with an increase in moderate bleeding. A reduction in myocardial infarction not related to stent thrombosis accounted for 55% of the treatment benefit. It must be noted that a fairly large number of patients in the trial (approximately 27%) received 1st generation paclitaxel eluting stents and that this group appeared to demonstrate larger reductions in ischemic events following longer duration DAPT[34]. Prolonged DAPT had the least effect in reducing ischemic events in patients receiving 2nd generation DES (i.e. EES) (p=0.05 for interaction term), suggesting that the benefit of prolonged DAPT may be dependent upon stent type.
One potentially concerning and unexpected finding was that all-cause mortality was higher for the prolonger DAPT group, largely due to an increase in non-cardiovascular deaths. The number of cancer-related deaths was increased in the prolonged DAPT arm and the investigators posited that this might be due to the increased number of patients in this arm with a history of cancer.
In summary, the DAPT trial demonstrates a clear benefit in terms of reduction of ischemic events for patients receiving DES who undergo prolonged (i.e. 30 months) of DAPT. However, the increase in all-cause mortality and bleeding in the prolonged therapy arm suggests that this approach should not be generalized. Clinicians need to take into account individual patient factors such as risk of bleeding, type of stent implanted, and overall risk of future ischemic events to tailor individual DAPT regimens.
Conclusions
DES have revolutionized the percutaneous treatment of coronary artery disease but also raised concerns about the optimal duration of DAPT to mitigate the increased risk of stent thrombosis seen with 1st generation DES due to delayed healing. More recent data has demonstrated that 2nd generation DES appear to re-endothelialize at a more rapid rate than 1st generation DES likely due to improvements in drug itself, drug load, polymer formulation, and backbone stent design. Recent clinical trials appear to demonstrate that in patients receiving 2nd generation or later DES it appears safe to discontinue DAPT at 6 months from a stent-thrombosis perspective. However, to prevent ischemic events not necessarily related to the stent itself 30 months of DAPT appears to be superior to 12 months with the caveat that prolonged therapy increases risk of bleeding. Thus, DAPT duration is likely an individualized decision based on patient clinical and procedural risk factors.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE Investigators S. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. The New England journal of medicine. 2003;349(14):1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME Investigators T-I. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. The New England journal of medicine. 2004;350(3):221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 4.McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, Pichard AD, Satler LF, Waksman R, Serruys PW. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364(9444):1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 5.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Jama. 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 6.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. Journal of the American College of Cardiology. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115(18):2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 8.Kotani J, Awata M, Nanto S, Uematsu M, Oshima F, Minamiguchi H, Mintz GS, Nagata S. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. Journal of the American College of Cardiology. 2006;47(10):2108–2111. doi: 10.1016/j.jacc.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 9.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369(9562):667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 10.Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, Wilson PS, Skorija K, Cheng Q, Xu X, Gold HK, Kolodgie FD, Virmani R. Endothelial cell recovery between comparator polymer-based drug-eluting stents. Journal of the American College of Cardiology. 2008;52(5):333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD, Virmani R. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129(2):211–223. doi: 10.1161/CIRCULATIONAHA.113.001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park KW, Kang SH, Velders MA, Shin DH, Hahn S, Lim WH, Yang HM, Lee HY, Van Boven AJ, Hofma SH, Kang HJ, Koo BK, Oh BH, Park YB, Kandzari DE, Kim HS. Safety and efficacy of everolimus- versus sirolimus-eluting stents: a systematic review and meta-analysis of 11 randomized trials. American heart journal. 2013;165(2):241–250. e244. doi: 10.1016/j.ahj.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Kim BK, Kim JS, Park J, Ko YG, Choi D, Jang Y, Hong MK. Comparison of optical coherence tomographic assessment between first- and second-generation drug-eluting stents. Yonsei medical journal. 2012;53(3):524–529. doi: 10.3349/ymj.2012.53.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77(4):513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 16.Bierer BE, Mattila PS, Standaert RF, Herzenberg LA, Burakoff SJ, Crabtree G, Schreiber SL. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib A, Karmali V, Polavarapu R, Akahori H, Nakano M, Yazdani S, Otsuka F, Pachura K, Davis T, Narula J, Kolodgie FD, Virmani R, Finn AV. Metformin impairs vascular endothelial recovery after stent placement in the setting of locally eluted mammalian target of rapamycin inhibitors via S6 kinase-dependent inhibition of cell proliferation. Journal of the American College of Cardiology. 2013;61(9):971–980. doi: 10.1016/j.jacc.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer cell. 2006;10(2):159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98(2):147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 20.Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112(2):270–278. doi: 10.1161/CIRCULATIONAHA.104.508937. [DOI] [PubMed] [Google Scholar]
- 21.Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109(6):701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 22.Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. Journal of the American College of Cardiology. 2011;57(4):390–398. doi: 10.1016/j.jacc.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 23.Habib A, Karmali V, John MC, Polavarapu R, Nakazawa G, Pachura K, Davis T, Kolodgie FD, Virmani R, Finn AV. Everolimus-eluting stents improve vascular response in a diabetic animal model. Circulation Cardiovascular interventions. 2014;7(4):526–532. doi: 10.1161/CIRCINTERVENTIONS.113.001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R. One step forward and two steps back with drug-eluting-stents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis. JACC Cardiovascular imaging. 2009;2(5):625–628. doi: 10.1016/j.jcmg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. Journal of the American College of Cardiology. 2011;57(11):1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habib A, Karmali V, Polavarapu R, Akahori H, Cheng Q, Pachura K, Kolodgie FD, Finn AV. Sirolimus-FKBP12.6 impairs endothelial barrier function through protein kinase C-alpha activation and disruption of the p120-vascular endothelial cadherin interaction. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(10):2425–2431. doi: 10.1161/ATVBAHA.113.301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circulation research. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, Kramer JM, Harrington RA, Matchar DB, Kandzari DE, Peterson ED, Schulman KA, Califf RM. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. Jama. 2007;297(2):159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 29.Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, Castriota F, Colombo F, Tebaldi M, Fuca G, Kubbajeh M, Cangiano E, Minarelli M, Scalone A, Cavazza C, Frangione A, Borghesi M, Marchesini J, Parrinello G, Ferrari R Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study I. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125(16):2015–2026. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- 30.Park SJ, Park DW, Kim YH, Kang SJ, Lee SW, Lee CW, Han KH, Park SW, Yun SC, Lee SG, Rha SW, Seong IW, Jeong MH, Hur SH, Lee NH, Yoon J, Yang JY, Lee BK, Choi YJ, Chung WS, Lim DS, Cheong SS, Kim KS, Chae JK, Nah DY, Jeon DS, Seung KB, Jang JS, Park HS, Lee K. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. The New England journal of medicine. 2010;362(15):1374–1382. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- 31.Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB, 3rd, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ, Jr, Nicolela EL, Jr, Perin MA, Devito FS, Labrunie A, Salvadori D, Jr, Gusmao M, Staico R, Costa JR, Jr, de Castro JP, Abizaid AS, Bhatt DL Investigators OT. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. Jama. 2013;310(23):2510–2522. doi: 10.1001/jama.2013.282183. [DOI] [PubMed] [Google Scholar]
- 32.Collet JP, Silvain J, Barthelemy O, Range G, Cayla G, Van Belle E, Cuisset T, Elhadad S, Schiele F, Lhoest N, Ohlmann P, Carrie D, Rousseau H, Aubry P, Monsegu J, Sabouret P, O’Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Beygui F, Vicaut E, Montalescot G investigators A. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet. 2014;384(9954):1577–1585. doi: 10.1016/S0140-6736(14)60612-7. [DOI] [PubMed] [Google Scholar]
- 33.Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR, Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Lee PV, Rinaldi MJ, Massaro JM the DSI. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garratt KN, Weaver WD, Jenkins RD, Pow TK, Mauri L, Kereiakes DJ, Winters KJ, Christen T, Allocco DJ, Lee D. Prasugrel Plus Aspirin Beyond 12 Months Is Associated With Improved Outcomes After Taxus Liberte Paclitaxel-Eluting Coronary Stent Placement. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.013570. [DOI] [PubMed] [Google Scholar]
- 35.Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, Han KR, Choi JH, Choi SH, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Jang Y, Kim HS. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125(3):505–513. doi: 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- 36.Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Park BE, Kang WC, Lee SH, Yoon JH, Hong BK, Kwon HM, Jang Y Investigators R. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation) Journal of the American College of Cardiology. 2012;60(15):1340–1348. doi: 10.1016/j.jacc.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 37.Colombo A, Chieffo A, Frasheri A, Garbo R, Masotti-Centol M, Salvatella N, Oteo Dominguez JF, Steffanon L, Tarantini G, Presbitero P, Menozzi A, Pucci E, Mauri J, Cesana BM, Giustino G, Sardella G. Second-Generation Drug-Eluting Stent Implantation Followed by 6- Versus 12-Month Dual Antiplatelet Therapy: The SECURITY Randomized Clinical Trial. Journal of the American College of Cardiology. 2014;64(20):2086–2097. doi: 10.1016/j.jacc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Gilard M, Barragan P, Noryani AA, Noor HA, Majwal T, Hovasse T, Castellant P, Schneeberger M, Maillard L, Bressolette EE, Wojcik J, Delarche N, Blanchard D, Jouve B, Ormezzano O, Paganelli F, Levy G, Sainsous J, Carrie D, Furber A, Berland J, Darremont O, Le Breton H, Lyuycx-Bore A, Gommeaux A, Cassat C, Kermarrec A, Cazaux P, Druelles P, Dauphin R, Armengaud J, Dupouy P, Champagnac D, Ohlmann P, Endresen KK, Benamer H, Kiss RG, Ungi I, Boschat JJ, Morice MC. Six-month versus 24-month dual antiplatelet therapy after implantation of drug eluting stents in patients non-resistant to aspirin: ITALIC, a randomized multicenter trial. Journal of the American College of Cardiology. 2014 doi: 10.1016/j.jacc.2014.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.