Abstract
Past research with the Spontaneously Hypertensive Rat (SHR) model of Attention Deficit/Hyperactivity Disorder showed that adolescent methylphenidate treatment enhanced cocaine abuse risk in SHR during adulthood. Acquisition of cocaine self-administration was faster, and cocaine dose-response functions were shifted upward under fixed-ratio and progressive ratio schedules compared to adult SHR that received adolescent vehicle treatment or to control strains that received adolescent methylphenidate treatment. The current study determined if extending treatment beyond adolescence would ameliorate long-term consequences of adolescent methylphenidate treatment on cocaine abuse risk in adult SHR. Treatments (vehicle or 1.5 mg/kg/day oral methylphenidate) began on postnatal day 28. Groups of male SHR were treated with vehicle during adolescence and adulthood, with methylphenidate during adolescence and vehicle during adulthood, or with methylphenidate during adolescence and adulthood. The group receiving adolescent-only methylphenidate was switched to vehicle on P56. Cocaine self-administration began on postnatal day 77, and groups receiving methylphenidate during adolescence and adulthood were treated either 1-hr before or 1-hr after daily sessions. At baseline under a fixed-ratio 1 schedule, cocaine self-administration (2 hr sessions; 0.3 mg/kg unit dose) did not differ among the four treatment groups. Under a progressive ratio schedule (4.5 hr maximum session length; 0.01 – 1.0 mg/kg unit doses), breakpoints for self-administered cocaine in SHR receiving the adult methylphenidate treatment 1-hr pre-session were not different from the vehicle control group. However, compared to the vehicle control group, breakpoints for self-administered cocaine at the 0.3 and 1.0 mg/kg unit doses were greater in adult SHR that received adolescent-only methylphenidate or received methylphenidate that was continued into adulthood and administered 1-hr post-session. These findings suggest that extending methylphenidate treatment beyond adolescence does not ameliorate explicitly the long-term consequences of adolescent methylphenidate treatment. Pre-session methylphenidate may mask temporarily the detection of an increase in cocaine self-administration following chronic methylphenidate treatment.
Keywords: Adolescence, Attention Deficit/Hyperactivity Disorder, Cocaine, Methylphenidate, Self-administration, Spontaneously Hypertensive Rat
1. Introduction
Methylphenidate is a psychostimulant commonly prescribed for the management of Attention Deficit/Hyperactivity Disorder (ADHD) in children and teenagers. Although an early meta-analysis concluded that stimulant medication initiated in childhood is protective against substance use disorders (SUD) later in life (Wilens et al., 2003), the most recent meta-analysis and Multimodal Treatment Study concluded that stimulant treatment for ADHD initiated in childhood neither protects against nor increases risk of later SUD (Humphreys et al., 2013; Molina et al., 2013). Some evidence that ADHD medication initiation (methylphenidate in particular) during adolescence may have different long-term consequences for adult SUD than initiation in childhood is derived from research specifically analyzing age of treatment onset. One study (Mannuzza et al., 2008) excluded participants with conduct disorder and stratified children into age groups (6–7 vs. 8–12 years) for methylphenidate treatment initiation (lasting 2–4 years). Participants developing adult SUD initiated treatment at a later age than those who never developed SUD, though antisocial personality disorder may have influenced this relationship (Mannuzza et al., 2008). In another study, SUD risk in adulthood increased by a factor of 1.5 for every year older that childhood stimulant treatment began (Dalsgaard et al., 2014). A critical gap in the literature exists, however, regarding SUD in adults who began ADHD treatment as teenagers. Currently, ~20% of teens with ADHD in the United States receive a first diagnosis between ages 11–17, representing an estimated 700,000 people (National Survey of Children’s Health Database, 2011/12). Studying the long-term consequences of adolescent-onset methylphenidate treatment is important because stimulants can change the trajectory of neuronal development during adolescence (Andersen, 2005; Casey and Jones, 2010).
Research using an animal model of ADHD would contribute to understanding the effects of methylphenidate treatment in newly diagnosed teenagers. The spontaneously hypertensive rat (SHR) is the most widely studied and validated animal model of ADHD (Russell, 2011). SHR exhibit frontostriatal neurocognitive deficits during adolescence and adulthood (Gauthier et al., 2014; Harvey et al., 2013; Kantak et al., 2008; Wells et al., 2010) and self-administer greater amounts of cocaine and other drugs of abuse compared to control strains (Chen et al., 2012; dela Pena et al., 2011; Jordan et al., 2014; Marusich et al., 2011; Somkuwar et al., 2013a). These later findings are consistent with epidemiological studies showing that having ADHD carries a 2–3 times greater risk of tobacco, cocaine and marijuana abuse by young adulthood (Lee et al., 2011). Additional preclinical studies demonstrated that adult SHR that had received adolescent methylphenidate treatment acquired cocaine self-administration more rapidly and exhibited upward shifts in cocaine dose-response functions under fixed-ratio (FR) and progressive ratio (PR) schedules compared to adult SHR that had received adolescent vehicle treatment and to control strains that had received adolescent methylphenidate treatment (Harvey et al., 2011). These findings suggest that adolescent methylphenidate treatment further enhanced cocaine abuse risk in adult SHR. In these past studies, methylphenidate treatment was discontinued at the end of adolescence, which was 3 weeks prior to the initiation of cocaine self-administration during adulthood. The current study tested the hypothesis that extending treatment beyond adolescence would ameliorate the long-term consequences of adolescent methylphenidate on cocaine abuse risk in adult SHR. This hypothesis is based on observations that methylphenidate treatment 2 hr before sessions does not increase cocaine choice behavior in adult ADHD patients compared to non-ADHD controls (Collins et al., 2006). However, methylphenidate pretreatment reduces cocaine binding at the dopamine transporter (DAT) (Berglund et al., 2013; Volkow et al., 1995), and this may have masked the effects of chronic methylphenidate treatment on cocaine self-administration in the adult ADHD patients. To test this hypothesis, rats receiving chronic methylphenidate from adolescence into adulthood were treated with methylphenidate either 1 hr before or 1 hr after daily cocaine self-administration sessions and the number of cocaine infusions were determined.
2. Materials and methods
Male SHR/NCrl rats (25 days old on arrival) were housed individually (08:00 h lights on, 20:00 h lights off) and maintained in accordance with the NIH Guide for Care and Use of Laboratory Animals and the Boston University Institutional Animal Care and Use Committee. Figure 1 provides a graphical depiction of the experimental timeline and summary of procedures. Rats began treatment with 1.5 mg/kg (±)-methylphenidate hydrochloride (Sigma-Aldrich, St. Louis, MO) or water vehicle on postnatal day 28 (P28), the start of adolescence in rats (Spear, 2000). Treatments were administered via oyster crackers on Monday-Friday to mimic the clinical practices of oral dosing and medication-free holidays on weekends for young patients with ADHD (Martins et al., 2004). A 1.5 mg/kg/day dose of oral methylphenidate produces peak plasma concentrations between 9–36 ng/ml in rats (Kuczenski and Segal, 2002), which is within peak plasma concentrations (8–40 ng/ml) achieved in pediatric patients (Swanson et al., 1999). This dose of oral methylphenidate also is clinically relevant because it lacks locomotor activating effects (Gerasimov et al., 2000), preferentially increases dopamine and norepinephrine signaling in prefrontal cortex (Berridge et al., 2006), and has procognitive effects in SHR (Harvey et al., 2013; Kantak et al., 2008). The amount of time to consume daily oyster crackers containing methylphenidate or vehicle averaged < 3 min. Groups of SHR were treated with vehicle during adolescence and adulthood (VEH/VEH; n=10), with methylphenidate during adolescence and vehicle during adulthood (MPH/VEH; n=8; 20 ± 0 total days of methylphenidate treatment), or with methylphenidate during adolescence and adulthood (MPH/MPH; n=15). The group receiving adolescent-only methylphenidate was switched to vehicle treatment on P56. Rats receiving adolescent and adult methylphenidate were subdivided into two groups and treated with methylphenidate either 1-hr before (MPH/MPH 1-hr Pre; n=8; 61 ± 3 total days of methylphenidate treatment) or 1-hr after (MPH/MPH 1-hr Post; n=7; 67 ± 2 total days of methylphenidate treatment) daily self-administration sessions that began on P77 (see below). Rats given vehicle during adulthood received treatment 1-hr after daily self-administration sessions.
Figure 1.
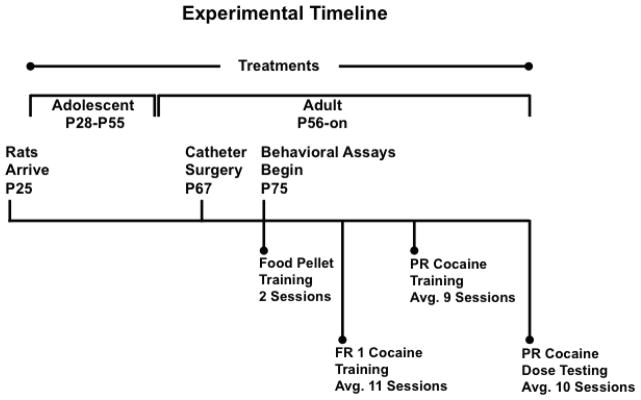
Graphical depiction of the experimental timeline and summary of procedures Abbreviations: postnatal day (P), fixed ratio schedule of reinforcement (FR), progressive ratio schedule of reinforcement (PR).
On P67, catheters were surgically implanted into the right femoral vein under intraperitoneal ketamine (90 mg/kg) and xylazine (10 mg/kg) anesthesia. Surgery, post-surgical care and catheter maintenance were as previously described (Somkuwar et al., 2013a). Methylphenidate and vehicle treatments were suspended the day of and for three days following surgery to prevent interactions with drugs used during surgery and during post-surgical care. Beginning on P75, rats were trained to lever press for 50 food pellets for two days. The average session lengths for the second food pellet session ranged from 29 ± 5 to 36 ± 10 min, with response rates ranging from 1.43 ± 0.24 to 2.38 ± 0.56 responses/min, with no significant group differences. Self-administration training then began in daily (Monday–Friday) 2 hr sessions under an FR1 20-sec timeout schedule of cocaine delivery using a 0.3 mg/kg unit dose (expressed as the hydrochloride salt; National Institute on Drug Abuse Drug Supply Program, Bethesda, MD). The cocaine solution, chamber configuration and session contingencies were as described previously (Somkuwar et al., 2013a). Rats continued self-administration sessions under the FR 1 schedule for a minimum of 10 sessions and until responses stabilized (<10% variation for 5 consecutive sessions and a ratio of 2:1 active to inactive lever responses). Under the FR 1 schedule, cocaine was limited to a maximum of 90 injections per 2 hr session to prevent accidental overdose (8 of 33 rats, equally represented in each treatment group, reached this threshold on 1–4 occasions early in training). Next, rats began training under a PR schedule (Loh and Roberts, 1990) using the 0.3 mg/kg cocaine unit dose for a minimum of 5 sessions and until breakpoints stabilized. This schedule involved a geometric increment in the number of lever presses required for each successive drug infusion (e.g., 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, etc.). PR breakpoint was defined as the last FR completed when the session ended. Once PR breakpoints were stable, a range of cocaine unit doses (0.01, 0.1, 0.3 and 1.0 mg/kg) that included the training dose was substituted in descending order, with 2–3 sessions per dose. Sessions terminated when a rat failed to complete the current FR requirement within 60 min of the last cocaine infusion or 4.5 hr had elapsed, whichever occurred first (3 of 33 rats reached the 4.5 hr threshold on 1–2 occasions during PR training, and 5 of 33 rats reached this threshold on 1 occasion during PR testing with the 1.0 mg/kg unit cocaine dose). The number of active and inactive lever responses, cocaine infusions and PR breakpoints were recorded and analyzed. As all rats were initially trained to lever press for food pellets, sessions to acquire cocaine self-administration were not analyzed. Valid assessment of acquisition of self-administration, as previously reported in SHR and control strains after adolescent methylphenidate treatment (Harvey et al., 2011), requires that rats are given no external inducements to lever press (e.g., prior lever press training for food pellets, food restriction or lever baiting). In the present study, response rates on the first and last FR 1 cocaine self-administration training sessions that followed food pellet training were similar across groups, with overall averages of 0.87 ± 0.14 and 0.66 ± 0.04 responses/min, respectively. As anticipated of rats with prior lever press training, response rates under the FR 1 schedule of cocaine self-administration were not significantly different on the first and last sessions of training, and were significantly lower (p<0.001) than the response rate generated by food reinforcement under the FR 1 schedule (overall average of 1.81 ± 0.20 responses/min).
Baseline performance was defined as the last 5 training sessions under the FR 1 and PR schedules. Values were averaged for individual rats prior to statistical analysis. Also, values from the last 2 dose-testing sessions under the PR schedule were averaged for individual rats prior to statistical analysis. All dependent measures were analyzed by one-factor (treatment group) or two-factor (treatment group X dose) analysis of variance with repeated measures for dose, and by Dunnett’s t-tests that control for type-1 error and permit post-hoc comparisons of methylphenidate groups to the vehicle control group regardless of the outcome of the analysis of variance (Winer et al., 1991).
3. Results
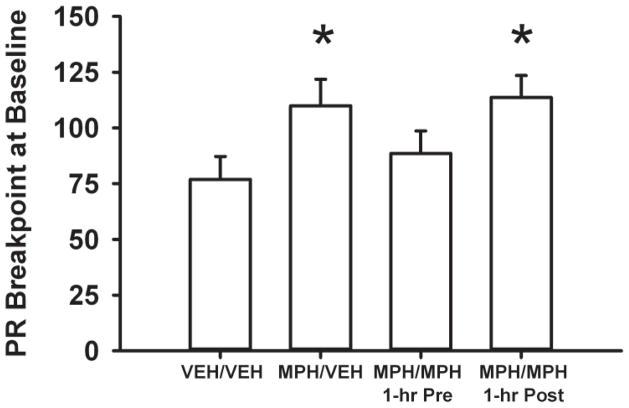
At the FR1 baseline, active and inactive lever responses and cocaine infusions were not significantly different across treatment groups (Figure 2a and 2b). In contrast, under the PR schedule (Figure 3), baseline breakpoints differed among the four treatment groups (F[3, 29] = 2.8, p<0.05). Adult SHR treated with methylphenidate during adolescence and then receiving either vehicle during adulthood (MPH/VEH) or post-session methylphenidate during adulthood (MPH/MPH 1-hr Post) had higher breakpoints at baseline compared to the VEH/VEH control (p ≤ 0.04 and 0.03, respectively). Baseline breakpoints in the adult SHR treated with methylphenidate during adolescence and then receiving pre-session methylphenidate during adulthood (MPH/MPH 1-hr Pre) did not differ from the VEH/VEH control.
Figure 2.
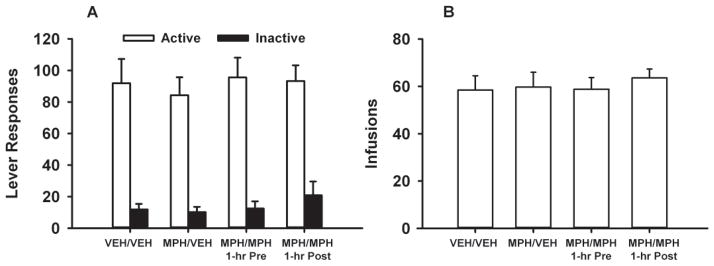
Baseline responding for self-administered cocaine under an FR1 schedule in adult SHR receiving vehicle during adolescence and adulthood (VEH/VEH), methylphenidate during adolescence and vehicle during adulthood (MPH/VEH), methylphenidate during adolescence and adulthood, with adult treatment occurring 1-hr before self-administration sessions (MPH/MPH 1-hr Pre), or methylphenidate during adolescence and adulthood, with adult treatment occurring 1-hr after self-administration sessions (MPH/MPH 1-hr Post). Values are the mean ± S.E.M. active and inactive lever responses (a) and infusions earned (b).
Figure 3.
Baseline breakpoints for self-administered cocaine under the PR schedule in adult SHR receiving vehicle during adolescence and adulthood (VEH/VEH), methylphenidate during adolescence and vehicle during adulthood (MPH/VEH), methylphenidate during adolescence and adulthood, with adult treatment occurring 1-hr before self-administration sessions (MPH/MPH 1-hr Pre), or methylphenidate during adolescence and adulthood, with adult treatment occurring 1-hr after self-administration sessions (MPH/MPH 1-hr Post). Values are the mean ± S.E.M. last FR completed. * p ≤ 0.04 compared to the VEH/VEH control.
During PR testing with a range of cocaine unit doses, analyses of breakpoints revealed dose-related (F[3,29] = 3.4, p ≤ 0.03) and treatment-related (F[3,87] = 97.9, p ≤ 0.001) differences as well as a trend for a treatment group X dose interaction (F[9,87] = 1.8, p ≤ 0.07). Overall, adult SHR receiving MPH/VEH or MPH/MPH 1-hr Post had higher breakpoints than the VEH/VEH control (p ≤ 0.02). The MPH/MPH 1-hr Pre and VEH/VEH groups did not differ. Dunnett’s analysis of treatment groups at each dose revealed that adult SHR with adolescent only methylphenidate (MPH/VEH) had higher breakpoints at the 0.3 (p ≤ 0.04) and 1.0 (p ≤ 0.001) mg/kg cocaine doses compared to the VEH/VEH control (Figure 4a). Methylphenidate treatment continued into adulthood and administered post-session (MPH/MPH 1-hr Post) maintained these elevated breakpoints (p ≤ 0.05 and p ≤ 0.001, respectively, compared to the VEH/VEH control). When methylphenidate treatment was continued and administered pre-session (MPH/MPH 1-hr Pre), breakpoints were not different from the VEH/VEH control at any dose. Analysis of active lever responses during cocaine dose-response testing also revealed dose-related (F[3,87] = 98.7, p ≤ 0.001) and treatment-related (F[3,29] = 4,0, p ≤ 0.02) differences as well as a treatment group X dose interaction (F[9,87] = 2.2 p ≤ 0.03). Overall, adult SHR receiving MPH/VEH or MPH/MPH 1-hr Post emitted more active lever responses than the VEH/VEH control (p ≤ 0.01). Dunnett’s analysis of treatment groups at each dose revealed these differences were significant at the 0.3 and 1.0 mg/kg cocaine doses (p ≤ 0.04 and 0.001, respectively; Figure 4b). Adult SHR receiving MPH/MPH 1-hr Pre did not differ from the VEH/VEH control at any dose for active lever responses. Analysis of inactive lever responses revealed only dose-related differences (F[3,87] = 4.9, p ≤ 0.003), with the highest cocaine dose associated with more inactive lever responses than the lowest cocaine dose, overall (p ≤ 0.001). Inactive lever responses did not differ across treatment groups at any dose (Figure 4b). Lastly, analysis of infusions earned during cocaine dose-response testing revealed dose-related (F[3,87] = 197.8, p ≤ 0.001) and a trend for treatment-related (F[3,29] = 2.6, p ≤ 0.07) differences. Dunnett’s analysis of treatment groups at each dose revealed that adult SHR in the MPH/VEH or MPH/MPH 1-hr Post groups earned more infusions at the 0.3 and 1.0 mg/kg cocaine doses compared to the VEH/VEH control (p ≤ 0.04 and 0.02, respectively; Figure 4c). The MPH/MPH 1-hr Pre group did not differ from the VEH/VEH control at any dose.
Figure 4.
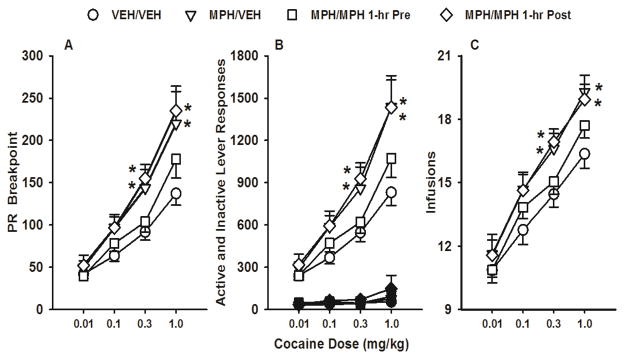
Cocaine dose-response functions under the PR schedule in adult SHR receiving vehicle during adolescence and adulthood (VEH/VEH), methylphenidate during adolescence and vehicle during adulthood (MPH/VEH), methylphenidate during adolescence and adulthood, with adult treatment occurring 1-hr before self-administration sessions (MPH/MPH 1-hr Pre), or methylphenidate during adolescence and adulthood, with adult treatment occurring 1-hr after self-administration sessions (MPH/MPH 1-hr Post). Values are mean ± S.E.M. breakpoints (a), active (white symbols) and inactive (black symbols) lever responses (b), and infusions earned (c). * p ≤ 0.05 compared to the VEH/VEH control.
4. Discussion
Previous research has demonstrated that 1.5 mg/kg/day oral methylphenidate, administered during adolescence and then discontinued, increases the motivation to self-administer cocaine in adult SHR (Harvey et al., 2011). PR breakpoints (last FR completed) were consistently higher across cocaine unit doses (0.01 – 1.0 mg/kg) in methylphenidate-treated SHR (maximum breakpoint ~200) compared to vehicle-treated SHR (maximum breakpoint ~100) or methylphenidate-treated Wistar-Kyoto and Wistar controls (maximum breakpoint ~40). The current study replicated the dose-related performance of SHR, with PR breakpoints reaching a maximum of ~225 after adolescent methylphenidate treatment and a maximum of ~125 after vehicle treatment. Historically, descending- (Harvey et al., 2011) and random-order (Brebner et al., 1999; McGregor et al., 1996) dose tests under a PR schedule in untreated Wistar rats produce overlapping breakpoints (last FR completed) across a range of cocaine doses, suggesting that the in the present study, dose order was not a variable influencing the results. As these findings suggest an unfavorable consequence of adolescent methylphenidate treatment on later cocaine abuse risk in rats with an ADHD phenotype, we explored whether extending methylphenidate treatment beyond adolescence would be protective in SHR. This is important because as many as 65% of adolescents with ADHD continue to meet criteria for the disorder in adulthood, and more than half stop taking ADHD medications by age 18 (McCarthy et al., 2012). Notably, past studies have shown that methylphenidate treatment either reduced or did not modify cocaine use in adult ADHD patients (Collins et al., 2006; Levin et al., 2007). In rhesus monkeys and outbred rats, pretreatment with oral methylphenidate at doses in the therapeutic range either reduced or did not modify cocaine self-administration as well (Czoty et al., 2013; Hiranita et al., 2009; Thanos et al., 2007). In the present study, methylphenidate treatment begun in adolescence and given 1-hr prior to daily sessions in adult SHR did not modify PR breakpoints for cocaine. In contrast, methylphenidate treatment begun in adolescence and given 1-hr after daily sessions in adult SHR increased PR breakpoints to the same degree as adolescent-only methylphenidate treatment. Thus, the timing of daily methylphenidate treatment relative to cocaine access in adulthood is critical for detecting an increase in cocaine abuse risk in SHR. Importantly, group differences under the PR schedule were not dependent on FR 1 baseline performance, as cocaine self-administration behavior was similar across groups under the less demanding FR1 schedule.
The different effects of pre- and post-session methylphenidate may relate to interactions of methylphenidate and cocaine at the dopamine transporter (DAT), which is a critically important target for the reinforcing effects of cocaine (Ramamoorthy et al., 2010) and for ADHD symptom relief (Krause et al., 2005). Among their multiple mechanisms, methylphenidate and cocaine each bind to DAT and inhibit the reuptake of dopamine (Zahniser and Sorkin, 2004). Our past research has shown that 1.5 mg/kg/day oral methylphenidate, administered during adolescence and then discontinued, increased Vmax for dopamine uptake at DAT (DAT function) in medial prefrontal cortex (mPFC) of adult SHR, but not in Wistar-Kyoto and Wistar controls (Somkuwar et al., 2013b). Increased DAT function in SHR would lead to lower basal dopamine tone, a condition that results in abnormally high phasic dopamine responses (Grace, 2001). As cocaine is directly self-administered into the mPFC (Goeders and Smith, 1983; 1986), such a mechanism involving the mPFC may contribute to the increased efficacy and motivating influence of self-administered cocaine in adult SHR treated with methylphenidate during adolescence (Harvey et al., 2011). As a dopamine reuptake inhibitor, methylphenidate administered pre-session may temporarily interfere with increased mPFC DAT function that is normally observed when methylphenidate is not onboard, and in this way, mask detection of an increase in the reinforcing effects of cocaine in adult SHR. DAT inhibition by methylphenidate pretreatment also reduces cocaine binding at DAT (Berglund et al., 2013; Volkow et al., 1995), which could lessen the impact of self-administered cocaine. Post-session methylphenidate, like discontinued methylphenidate, may expose the increase in mPFC DAT function and maintain the enhanced motivation to self-administer cocaine in adult SHR.
In rats, therapeutic doses of orally administered methylphenidate produce peak plasma concentrations ~15 min after administration, with a half-life of ~1 hr (Aoyama et al., 1990; Kuczenski & Segal 2002). Within cortical sites, peak changes in BOLD magnetic resonance signals in rats are attained at 45–90 min following methylphenidate treatment (Easton et al., 2009). Although in the present study the once daily dosing with methylphenidate does not result in steady state plasma levels in rats, the above pharmacokinetic and imaging results suggest that significant amounts of methylphenidate were still present and neurally active at the start of cocaine self-administration sessions in SHR treated 1 hr before, but not 1 hr after the sessions. Methylphenidate pharmacokinetics in pediatric patients, who typically receive either an immediate release (2–3 times daily) or an extended release (once daily) formulation, are different than in rats receiving a once daily dose. In pediatric patients, plasma concentrations peak later (~2 hr) and the half-life and duration of action are longer (~6–12 hr) than in rats (Coghill et al., 2013; Shaywitz et al., 1982; Swanson and Volkow, 2003). Thus, if steady state plasma levels of methylphenidate had been achieved in the SHR in the current study, as is standard for ADHD patients, then increases in DAT function and cocaine abuse risk may not have occurred. However, results show that in rats with steady state plasma levels (achieved via 40 intravenous injections of 0.56 mg/kg methylphenidate over a 6 hr period for 5 days), an increase in striatal DAT function was found 24 hr later (when methylphenidate was not onboard) compared to untreated controls (Calipari et al., 2014). These findings suggest that the long-term consequences of adolescent methylphenidate treatment on DAT function do not depend on achieving steady state plasma levels. Initiating methylphenidate treatment in SHR during adolescence and determining behavior in adulthood when methylphenidate was not onboard may be critical factors for observing increases in DAT function and cocaine abuse risk. Consistent with this view are clinical findings showing the later that twice-daily methylphenidate treatment begins in pediatric ADHD patients, the greater the risk of developing SUD during adulthood (Mannuza et al., 2008).
5. Conclusions
Every animal model of neuropsychiatric disease has limitations (Nestler and Hyman, 2010). Nonetheless, SHR exhibit behavioral and cognitive deficits (Kantak et al., 2008; Russell, 2011; Sagvolden et al., 2005) as well as neurochemical and genetic differences (e.g., Mill et al., 2005 and Roessner et al., 2010) similar to those observed in ADHD. The SHR model of ADHD can help address clinically relevant questions concerning ADHD and SUD more appropriately than research limited to animal models that do not exhibit an ADHD phenotype (e.g., Adriani et al., 2006; Andersen et al., 2002; Brandon et al., 2001; Carlezon et al., 2003; Ferguson and Boctor, 2010; Gill et al., 2012; Thanos et al., 2007). Thus, the SHR model may provide novel insights concerning cocaine abuse risk subsequent to methylphenidate treatment for ADHD initiated during adolescence and extending into adulthood.
Specifically, discontinuation of adolescent methylphenidate treatment for the management of ADHD may place teens at greater risk of abusing cocaine in adulthood, i.e., at a risk beyond that associated with ADHD alone. The idea of extending methylphenidate treatment beyond adolescence to avoid a greater risk of cocaine abuse might only be valid if patients strictly adhere to their medication regimen. However, adherence is irregular among adults with ADHD who oftentimes miss or delay taking their medication (Caisley and Muller, 2012). If adult patients are non-compliant with their medication and cocaine is sampled when methylphenidate is not onboard, then cocaine may have greater reinforcing effects and higher potential for abuse. Our results in SHR advocate for the design of informative clinical studies that specifically probe the long-term consequences of adolescent-onset methylphenidate treatment on adult SUD. Participants should be stratified into groups according to the developmental stage at which treatment began, the specific medication prescribed, and the length of treatment. Continued research with the SHR animal model of ADHD contributes to this understudied public health concern in adolescent patients by elucidating the behavioral and neural mechanisms that account for greater cocaine abuse risk following adolescent-initiated methylphenidate treatment.
Highlights.
Adolescent methylphenidate treatment increases cocaine abuse risk in adult SHR
Extending methylphenidate treatment beyond adolescence is not protective
Treatment prior to cocaine access may mask the effect of chronic methylphenidate
Acknowledgments
This study was supported by NIH grant R01 DA011716. The authors thank Audrey-Jo Santos and Chloe Jordan for technical assistance.
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Britahny M. Baskin, Email: britahny.baskin@gmail.com.
Linda P. Dwoskin, Email: ldwoskin@email.uky.edu.
Kathleen M. Kantak, Email: kkantak@bu.edu.
References
- Adriani W, Leo D, Greco D, Rea M, di Porzio U, Laviola G, Perrone-Capano C. Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2006;31:1946–1956. doi: 10.1038/sj.npp.1300962. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Kotaki H, Iga T. Dose-dependent kinetics of methylphenidate enantiomers after oral administration of racemic methylphenidate to rats. J Pharmacobiodyn. 1990;13:647–652. doi: 10.1248/bpb1978.13.647. [DOI] [PubMed] [Google Scholar]
- Berglund EC, Makos MA, Keighron JD, Phan N, Heien ML, Ewing AG. Oral administration of methylphenidate blocks the effect of cocaine on uptake at the Drosophila dopamine transporter. ACS Chem Neurosci. 2013;4:566–574. doi: 10.1021/cn3002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brebner K, Froestl W, Andrews M, Phelan R, Roberts DC. The GABA(B) agonist CGP 44532 decreases cocaine self-administration in rats: demonstration using a progressive ratio and a discrete trials procedure. Neuropharmacology. 1999;38:1797–1804. doi: 10.1016/s0028-3908(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Caisley H, Muller U. Adherence to medication in adults with attention deficit hyperactivity disorder and pro re nata dosing of psychostimulants: a systematic review. Eur Psychiatry. 2012;27:343–349. doi: 10.1016/j.eurpsy.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC, Jones SR. Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict Biol. 2014;19:145–155. doi: 10.1111/j.1369-1600.2012.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hiler KA, Tolley EA, Matta SG, Sharp BM. Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PLoS One. 2012;7:e44234. doi: 10.1371/journal.pone.0044234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill D, Banaschewski T, Zuddas A, Pelaz A, Gagliano A, Doepfner M. Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies. BMC Psychiatry. 2013;13:237. doi: 10.1186/1471-244X-13-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Levin FR, Foltin RW, Kleber HD, Evans SM. Response to cocaine, alone and in combination with methylphenidate, in cocaine abusers with ADHD. Drug Alcohol Depend. 2006;82:158–167. doi: 10.1016/j.drugalcdep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Martelle SE, Gould RW, Nader MA. Effects of chronic methylphenidate on cocaine self-administration under a progressive-ratio schedule of reinforcement in rhesus monkeys. J Pharmacol Exp Ther. 2013;345:374–382. doi: 10.1124/jpet.113.204321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood - a naturalistic long-term follow-up study. Addict Behav. 2014;39:325–328. doi: 10.1016/j.addbeh.2013.09.002. [DOI] [PubMed] [Google Scholar]
- dela Pena IC, Ahn HS, Choi JY, Shin CY, Ryu JH, Cheong JH. Methylphenidate self-administration and conditioned place preference in an animal model of attention-deficit hyperactivity disorder: the spontaneously hypertensive rat. Behav Pharmacol. 2011;22:31–39. doi: 10.1097/FBP.0b013e328342503a. [DOI] [PubMed] [Google Scholar]
- Easton N, Marshall FH, Marsden CA, Fone KC. Mapping the central effects of methylphenidate in the rat using pharmacological MRI BOLD contrast. Neuropharmacology. 2009;57:653–664. doi: 10.1016/j.neuropharm.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Boctor SY. Cocaine responsiveness or anhedonia in rats treated with methylphenidate during adolescence. Neurotoxicol Teratol. 2010;32:432–442. doi: 10.1016/j.ntt.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Gauthier JM, Tassin DH, Dwoskin LP, Kantak KM. Effects of dopamine D1 receptor blockade in the prelimbic prefrontal cortex or lateral dorsal striatum on frontostriatal function in Wistar and Spontaneously Hypertensive Rats. Behav Brain Res. 2014;268:229–238. doi: 10.1016/j.bbr.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295:51–57. [PubMed] [Google Scholar]
- Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, Swanson JM, Nader MA, Porrino LJ. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacology. 2012;37:2555–2565. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Reinforcing properties of cocaine in the medical prefrontal cortex: primary action on presynaptic dopaminergic terminals. Pharmacol Biochem Behav. 1986;25:191–199. doi: 10.1016/0091-3057(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Grace AA. Psychostimulant actions on dopamine and limbic system function: relevance to the pathophysiology and treatment of ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. pp. 134–157. [Google Scholar]
- Harvey RC, Jordan CJ, Tassin DH, Moody KR, Dwoskin LP, Kantak KM. Performance on a strategy set shifting task during adolescence in a genetic model of attention deficit/hyperactivity disorder: methylphenidate vs. atomoxetine treatments. Behav Brain Res. 2013;244:38–47. doi: 10.1016/j.bbr.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Sen S, Deaciuc A, Dwoskin LP, Kantak KM. Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology. 2011;36:837–847. doi: 10.1038/npp.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther. 2009;329:677–686. doi: 10.1124/jpet.108.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes: a meta-analysis. JAMA Psychiatry. 2013;70:740–749. doi: 10.1001/jamapsychiatry.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Harvey RC, Baskin BB, Dwoskin LP, Kantak KM. Cocaine-seeking behavior in a genetic model of attention-deficit/hyperactivity disorder following adolescent methylphenidate or atomoxetine treatments. Drug Alcohol Depend. 2014;140:25–32. doi: 10.1016/j.drugalcdep.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, Deschepper CF, Dwoskin LP. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behav Neurosci. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- Krause J, la Fougere C, Krause KH, Ackenheil M, Dresel SH. Influence of striatal dopamine transporter availability on the response to methylphenidate in adult patients with ADHD. Eur Arch Psychiatry Clin Neurosci. 2005;255:428–431. doi: 10.1007/s00406-005-0602-x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87:20–29. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Loh EA, Roberts DC. Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology (Berl) 1990;101:262–266. doi: 10.1007/BF02244137. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, 3rd, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S, Tramontina S, Polanczyk G, Eizirik M, Swanson JM, Rohde LA. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14:195–206. doi: 10.1089/1044546041649066. [DOI] [PubMed] [Google Scholar]
- Marusich JA, McCuddy WT, Beckmann JS, Gipson CD, Bardo MT. Strain differences in self-administration of methylphenidate and sucrose pellets in a rat model of attention-deficit hyperactivity disorder. Behav Pharmacol. 2011;22:794–804. doi: 10.1097/FBP.0b013e32834d623e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S, Wilton L, Murray ML, Hodgkins P, Asherson P, Wong IC. Persistence of pharmacological treatment into adulthood, in UK primary care, for ADHD patients who started treatment in childhood or adolescence. BMC Psychiatry. 2012;12:219. doi: 10.1186/1471-244X-12-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Baker G, Roberts DC. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 1996;53:5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Mill J, Sagvolden T, Asherson P. Sequence analysis of Drd2, Drd4, and Dat1 in SHR and WKY rat strains. Behav Brain Funct. 2005;1:24. doi: 10.1186/1744-9081-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, Hoza B, Epstein JN, Wigal T, Abikoff HB, Greenhill LL, Jensen PS, Wells KC, Vitiello B, Gibbons RD, Howard A, Houck PR, Hur K, Lu B, Marcus S. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52:250–263. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Survey of Children’s Health. [last accessed November 7, 2014];Data query from the Child and Adolescent Health Measurement Initiative, Data Resource Center for Child and Adolescent Health website. 2011 Dec; http://www.nschdata.org/browse/survey/results?q=2390&r=1&g=451.
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Balasubramaniam A, See RE, Jayanthi LD. Altered dopamine transporter function and phosphorylation following chronic cocaine self-administration and extinction in rats. Biochem Biophys Res Commun. 2010;391:1517–1521. doi: 10.1016/j.bbrc.2009.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner V, Sagvolden T, Dasbanerjee T, Middleton FA, Faraone SV, Walaas SI, Becker A, Rothenberger A, Bock N. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 2010;167:1183–1191. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Russell VA. Overview of animal models of attention deficit hyperactivity disorder (ADHD) Curr Protoc Neurosci. 2011;Chapter 9(Unit 9.35) doi: 10.1002/0471142301.ns0935s54. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Metzger MA, Schiorbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Hunt RD, Jatlow P, Cohen DJ, Young JG, Pierce RN, Anderson GM, Shaywitz BA. Psychopharmacology of attention deficit disorder: pharmacokinetic, neuroendocrine, and behavioral measures following acute and chronic treatment with methylphenidate. Pediatrics. 1982;69:688–694. [PubMed] [Google Scholar]
- Somkuwar SS, Darna M, Kantak KM, Dwoskin LP. Adolescence methylphenidate treatment in a rodent model of attention deficit/hyperactivity disorder: dopamine transporter function and cellular distribution in adulthood. Biochem Pharmacol. 2013b;86:309–316. doi: 10.1016/j.bcp.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Jordan CJ, Kantak KM, Dwoskin LP. Adolescent atomoxetine treatment in a rodent model of ADHD: effects on cocaine self-administration and dopamine transporters in frontostriatal regions. Neuropsychopharmacology. 2013a;38:2588–2597. doi: 10.1038/npp.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, Williams L, Shoulson I, Wigal S. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 2003;27:615–621. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Wells AM, Janes AC, Liu X, Deschepper CF, Kaufman MJ, Kantak KM. Medial temporal lobe functioning and structure in the spontaneously hypertensive rat: comparison with Wistar-Kyoto normotensive and Wistar-Kyoto hypertensive strains. Hippocampus. 2010;20:787–797. doi: 10.1002/hipo.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown BD, Michels KM. Statistical Principles in Experimental Design. 3. New York: McGraw-Hill; 1991. [Google Scholar]
- Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47 (Suppl 1):80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]