Abstract
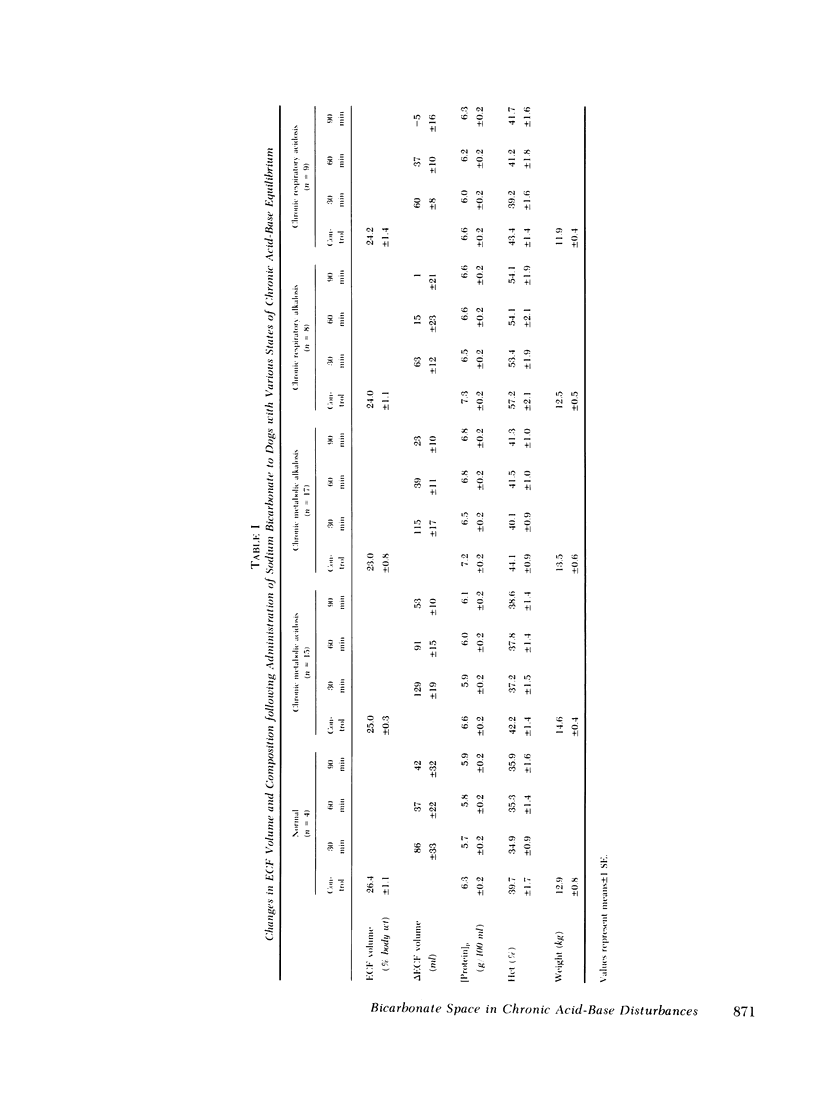
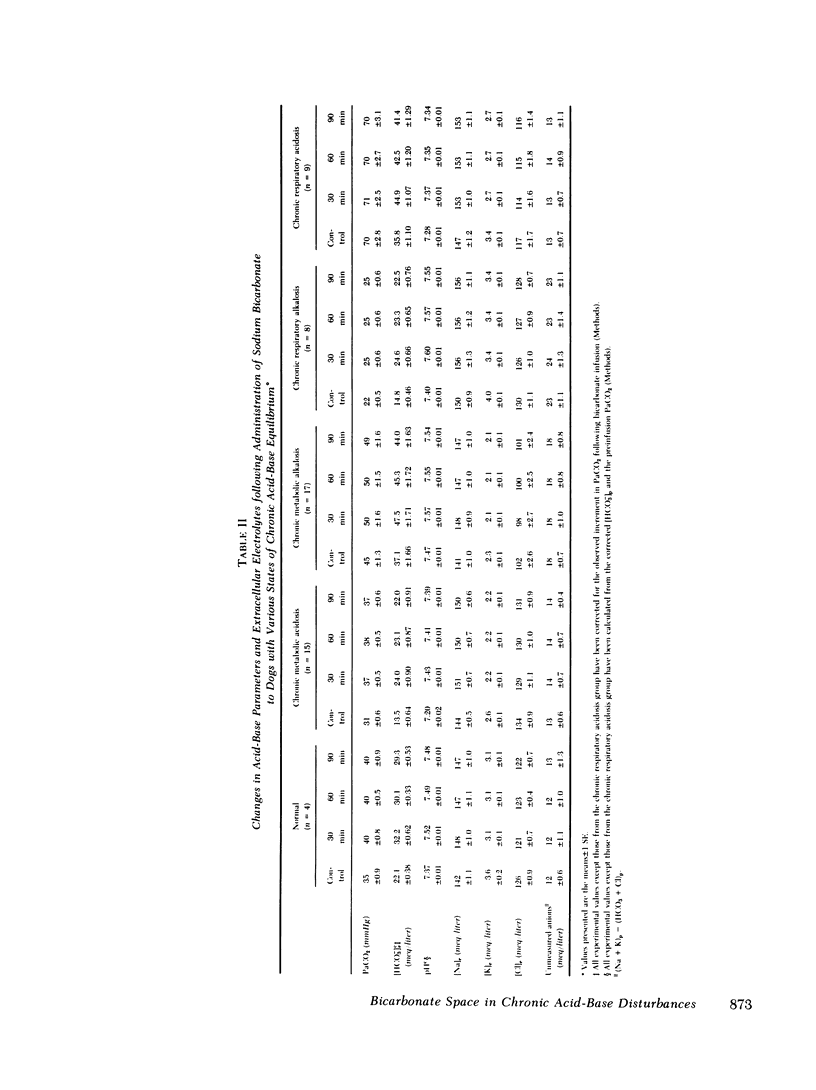
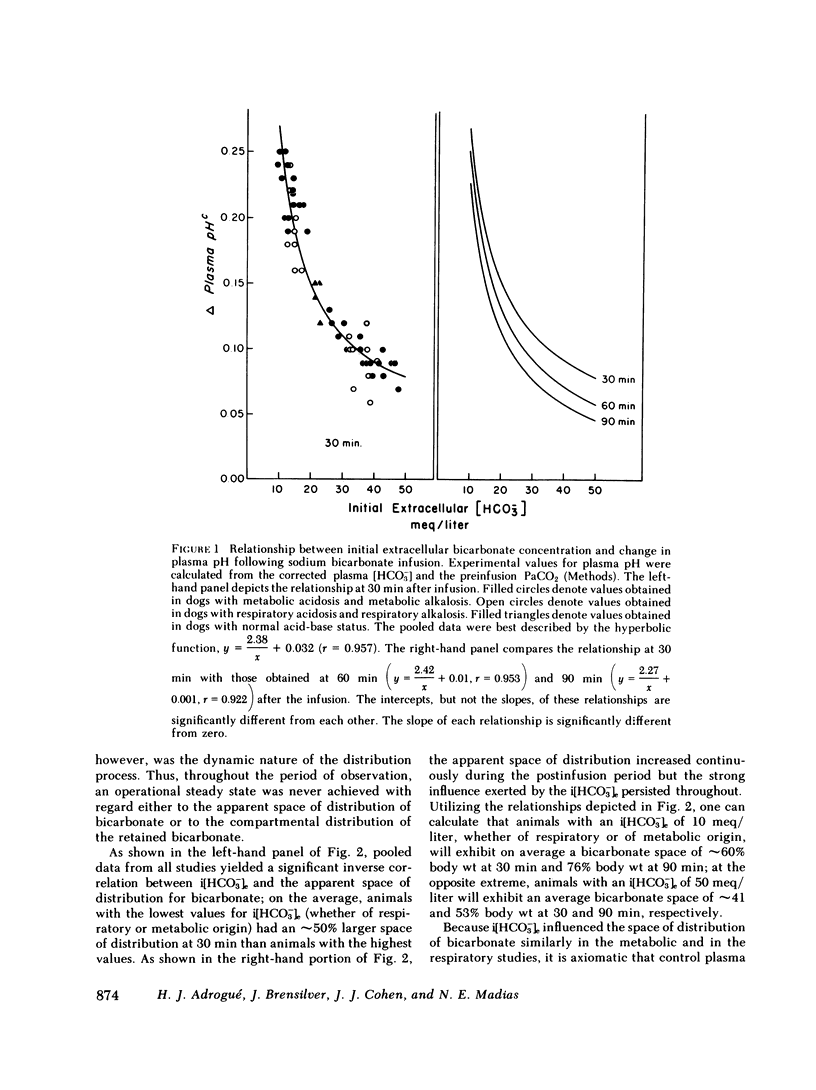
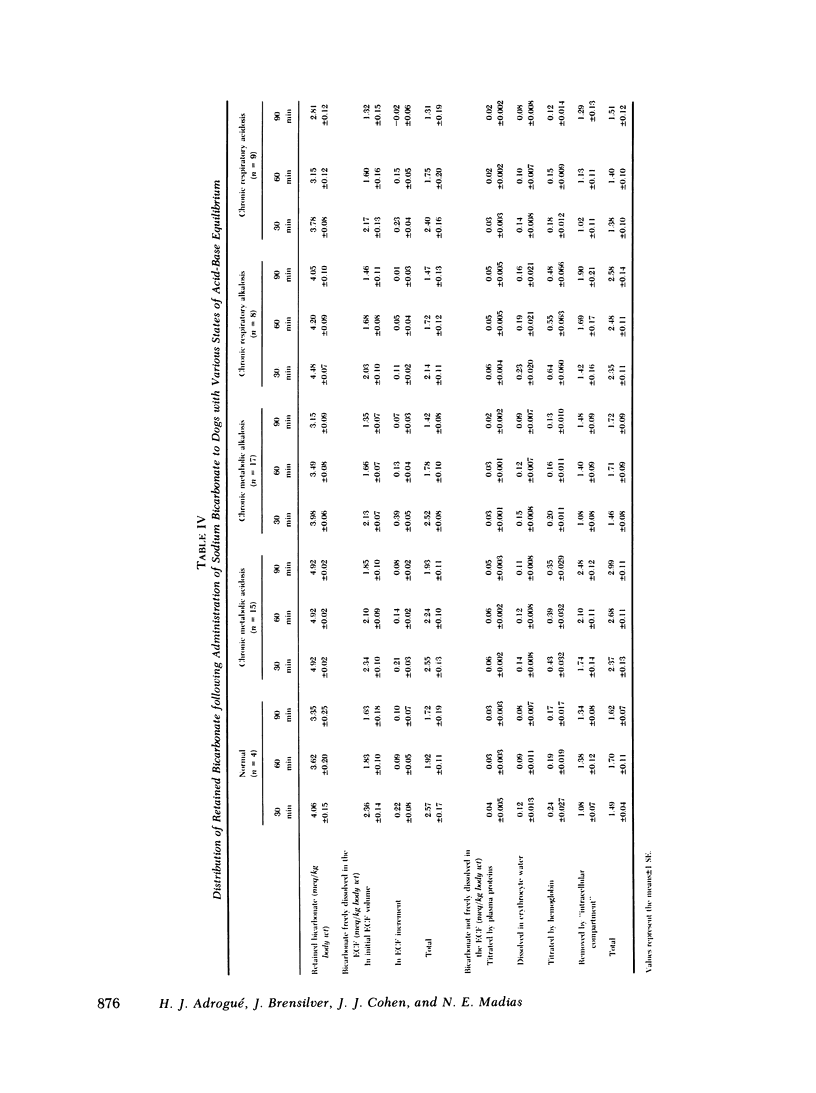
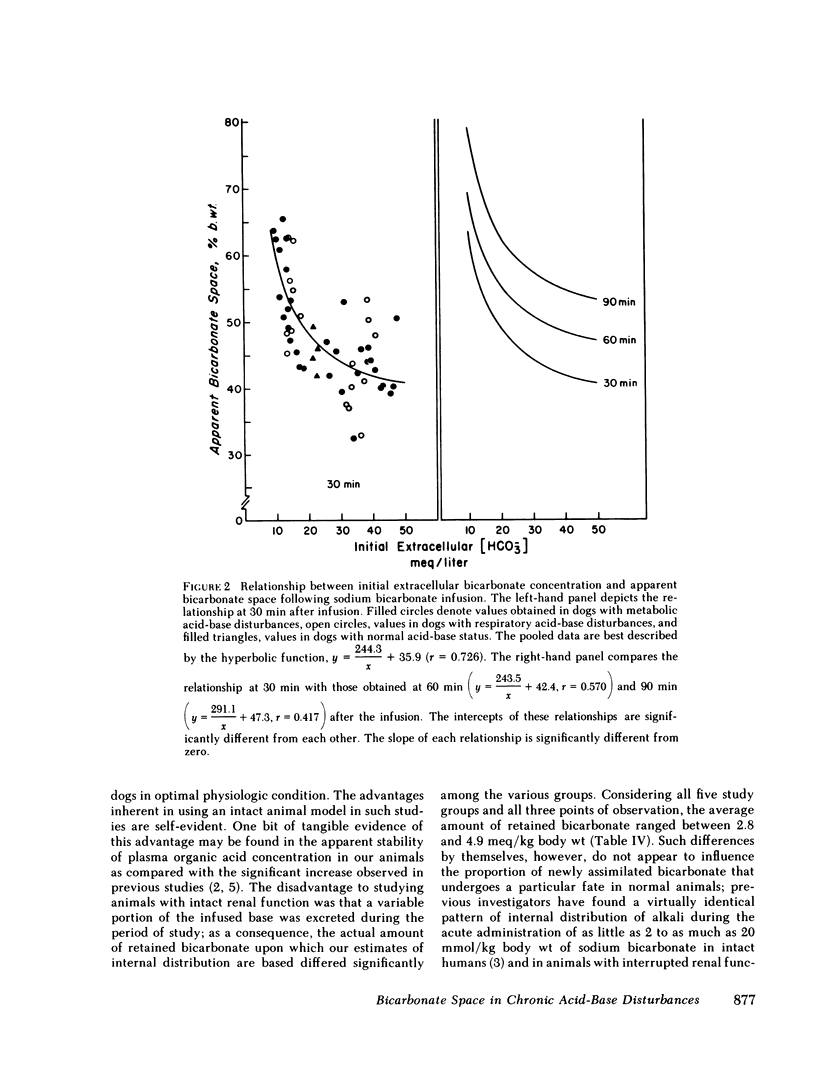
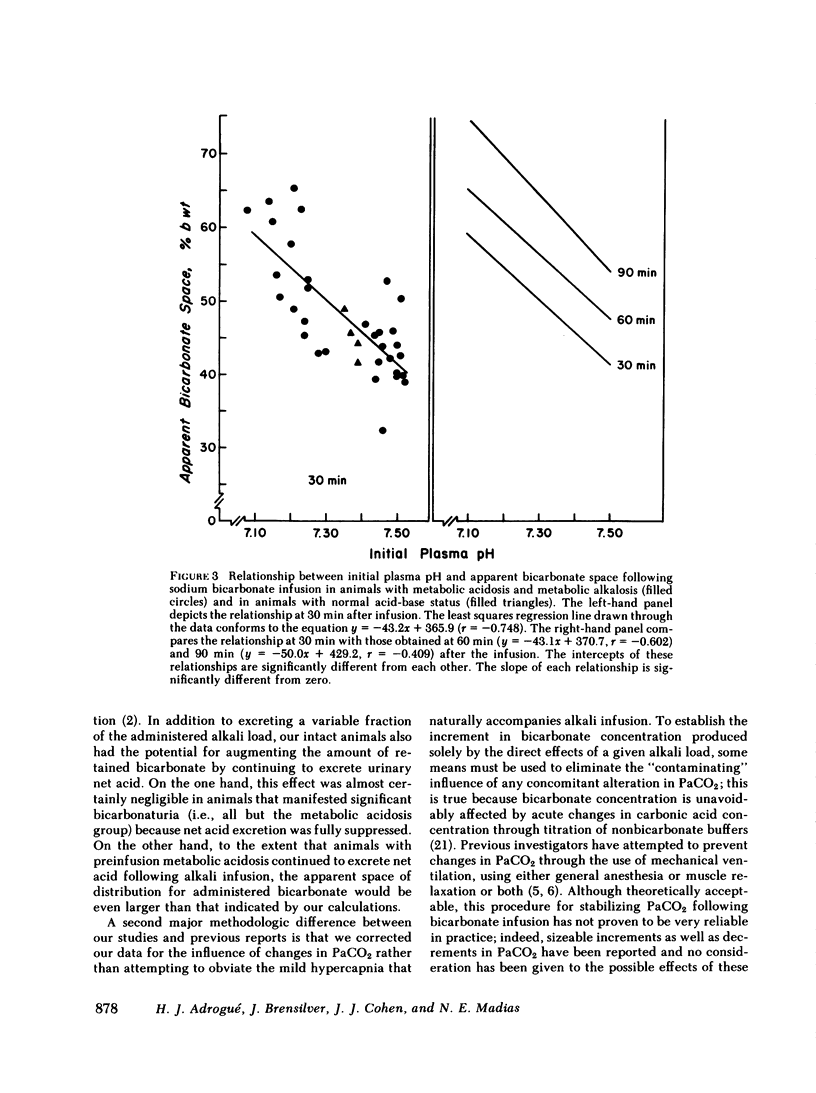
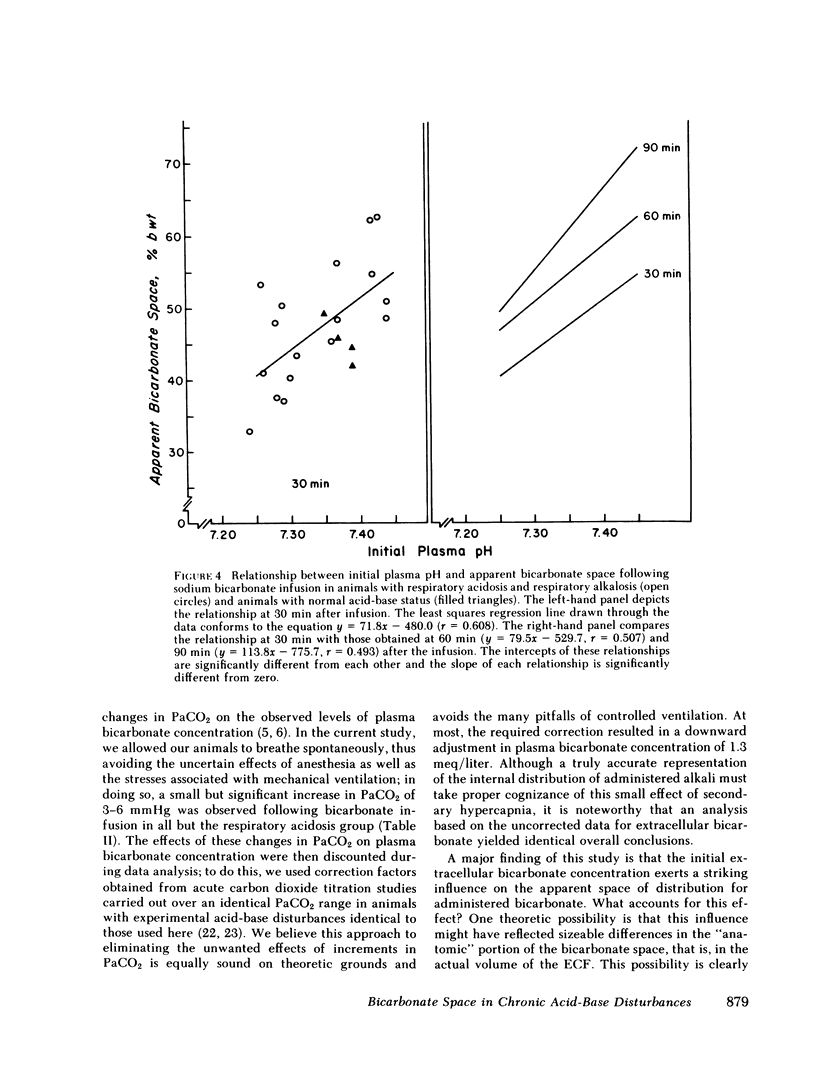
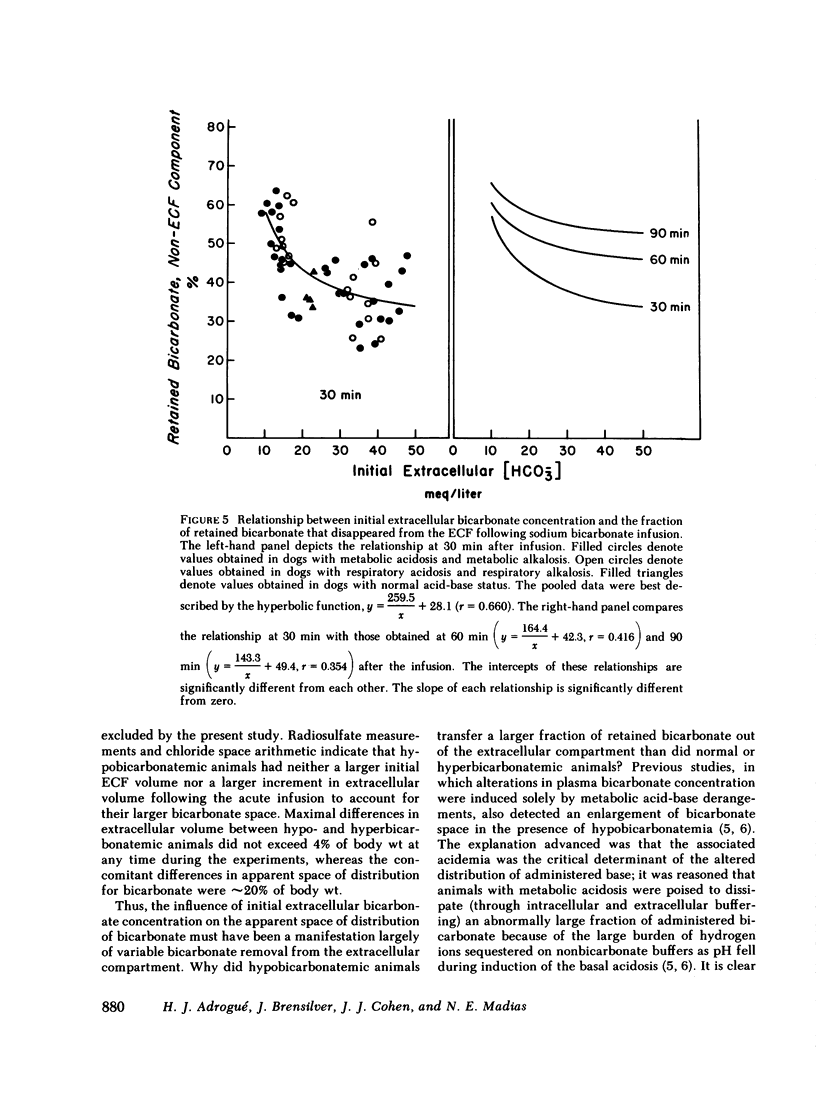
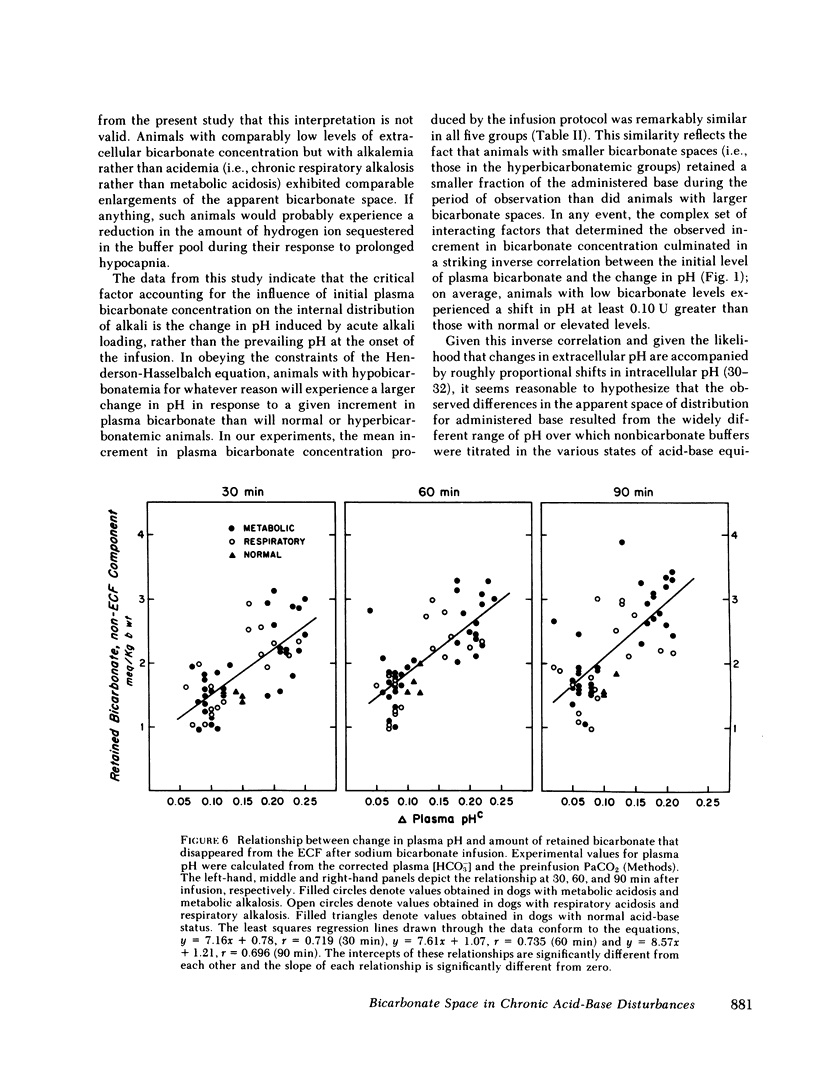
Previous workers have shown that metabolic acidosis increases the apparent space through which administered bicarbonate is distributed. This finding has been ascribed to the accompanying acidemia and to the consequent availability of a large quantity of hydrogen ion that accumulates on nonbicarbonate tissue buffers during the development of acidosis. To test this hypothesis, bicarbonate space was measured in dogs with a broad range of steady-state plasma [HCO-3] in association with alkalemia as well as with acidemia. Appropriate combinations of pH and plasma [HCO-3] were achieved by pretreating the animals to produce graded degrees of each of the four cardinal, chronic acid-base disorders. Metabolic acidosis (n = 15) was produced by prolonged HCl-feeding; metabolic alkalosis (n = 17) by diuretics and a chloride-free diet; and respiratory acidosis (n = 9) and alkalosis (n = 8) by means of an environmental chamber. Animals with normal acid-base status (n = 4) were also studied. Sodium bicarbonate (5 mmol/kg) was infused over 10 min to the unanesthetized animals; observations were carried out over 90 min. The results obtained from animals with metabolic acid-base disturbances demonstrated an inverse relationship between bicarbonate space and initial plasma pH, confirming the previous findings of others. By contrast, the results obtained in animals with respiratory acid-base disturbances demonstrated a direct relationship between bicarbonate space and initial plasma pH. The pooled data revealed that bicarbonate space is, in fact, quite independent of the initial pH but is highly correlated with the initial level of extracellular [HCO-3]; dogs with low extracellular [HCO-3] (congruent to 10 meq/liter) whether acidemic or alkalemic, have a bicarbonate space that is 25% larger than normal and some 50% larger than in dogs with high extracellular [HCO-3] (congruent to 50 meq/liter). We conclude from these results that the increased bicarbonate space in metabolic acidosis (and respiratory alkalosis) does not reflect the availability of more hydrogen ions for release during bicarbonate administration, but merely evidences the wider range of titration (delta pH) of nonbicarbonate buffers that occurs during alkali loading whenever plasma [HCO-3] is low.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. I. THE RESPONSE OF MUSCLE CELLS TO CHANGES IN CO2 TENSION OR EXTRACELLULAR BICARBONATE CONCENTRATION. J Clin Invest. 1965 Jan;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrogué H. J., Brensilver J., Madias N. E. Changes in the plasma anion gap during chronic metabolic acid-base disturbances. Am J Physiol. 1978 Oct;235(4):F291–F297. doi: 10.1152/ajprenal.1978.235.4.F291. [DOI] [PubMed] [Google Scholar]
- BRADLEY A. F., SEVERINGHAUS J. W., STUPFEL M. Accuracy of blood pH and PCO2 determinations. J Appl Physiol. 1956 Sep;9(2):189–196. doi: 10.1152/jappl.1956.9.2.189. [DOI] [PubMed] [Google Scholar]
- BRADLEY A. F., SEVERINGHAUS J. W., STUPFEL M. Variations of serum carbonic acid pK with pH and temperature. J Appl Physiol. 1956 Sep;9(2):197–200. doi: 10.1152/jappl.1956.9.2.197. [DOI] [PubMed] [Google Scholar]
- Burnell J. M. In vivo response of muscle to changes in CO2 tension or extracellular bicarbonate. Am J Physiol. 1968 Dec;215(6):1376–1383. doi: 10.1152/ajplegacy.1968.215.6.1376. [DOI] [PubMed] [Google Scholar]
- De Sousa R. C., Harrington J. T., Ricanati E. S., Shelkrot J. W., Schwartz W. B. Renal regulation of acid-base equilibrium during chronic administration of mineral acid. J Clin Invest. 1974 Feb;53(2):465–476. doi: 10.1172/JCI107580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEBISCH G., BERGER L., PITTS R. F. The extrarenal response to acute acid-base disturbances of respiratory origin. J Clin Invest. 1955 Feb;34(2):231–245. doi: 10.1172/JCI103076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garella S., Dana C. L., Chazan J. A. Severity of metabolic acidosis as a determinant of bicarbonate requirements. N Engl J Med. 1973 Jul 19;289(3):121–126. doi: 10.1056/NEJM197307192890303. [DOI] [PubMed] [Google Scholar]
- Gennari F. J., Goldstein M. B., Schwartz W. B. The nature of the renal adaptation to chronic hypocapnia. J Clin Invest. 1972 Jul;51(7):1722–1730. doi: 10.1172/JCI106973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. B., Gennari F. J., Schwartz W. B. The influence of graded degrees of chronic hypercapnia on the acute carbon dioxide titration curve. J Clin Invest. 1971 Jan;50(1):208–216. doi: 10.1172/JCI106475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuri R. N., Agulian S. K., Bogharian K. K. Intracellular bicarbonate of skeletal muscle under different metabolic states. Am J Physiol. 1976 Jan;230(1):228–232. doi: 10.1152/ajplegacy.1976.230.1.228. [DOI] [PubMed] [Google Scholar]
- Madias N. E., Ayus J. C., Adrogué H. J. Increased anion gap in metabolic alkalosis: the role of plasma-protein equivalency. N Engl J Med. 1979 Jun 21;300(25):1421–1423. doi: 10.1056/NEJM197906213002507. [DOI] [PubMed] [Google Scholar]
- POLAK A., HAYNIE G. D., HAYS R. M., SCHWARTZ W. B. Effects of chronic hypercapnia on electrolyte and acid-base equilibrium. I. Adaptation. J Clin Invest. 1961 Jul;40:1223–1237. doi: 10.1172/JCI104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINI M. E., WOLF A. V. Refractometric determination of total solids and water of serum and urine. J Biol Chem. 1957 Apr;225(2):869–876. [PubMed] [Google Scholar]
- Relman A. S. Metabolic consequences of acid-base disorders. Kidney Int. 1972 May;1(5):347–359. doi: 10.1038/ki.1972.46. [DOI] [PubMed] [Google Scholar]
- Rettori V., Gral T., Massry S. G., Villamil M. F. Exchangeable potassium content and distribution in normal subjects and uraemic patients on chronic haemodialysis. Clin Sci. 1972 Jun;42(6):673–684. doi: 10.1042/cs0420673. [DOI] [PubMed] [Google Scholar]
- Russell C. D., Illickal M. M., Maloney J. V., Jr, Roeher H. D., DeLand E. C. Acute response to acid-base stress in the dog. Am J Physiol. 1972 Sep;223(3):689–694. doi: 10.1152/ajplegacy.1972.223.3.689. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ W. B., ORNING K. J., PORTER R. The internal distribution of hydrogen ions with varying degrees of metabolic acidosis. J Clin Invest. 1957 Mar;36(3):373–382. doi: 10.1172/JCI103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER R. B., CLARK J. K., BARKER E. S., CROSLEY A. P., Jr, ELKINTON J. R. The acute effects in man of rapid intravenous infusion of hypertonic sodium bicarbonate solution. I. Changes in acid-base balance and distribution of the excess buffer base. Medicine (Baltimore) 1955 Feb;34(1):51–95. doi: 10.1097/00005792-195502000-00003. [DOI] [PubMed] [Google Scholar]
- SWAN R. C., AXELROD D. R., SEIP M., PITTS R. F. Distribution of sodium bicarbonate infused into nephrectomized dogs. J Clin Invest. 1955 Dec;34(12):1795–1801. doi: 10.1172/JCI103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWAN R. C., PITTS R. F. Neutralization of infused acid by nephrectomized dogs. J Clin Invest. 1955 Feb;34(2):205–212. doi: 10.1172/JCI103073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz W. B., Silverman L. A large environmental chamber for the study of hypercapnia and hypoxia. J Appl Physiol. 1965 Jul;20(4):767–774. doi: 10.1152/jappl.1965.20.4.767. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Bleich H. L., Schwartz W. B. The renal response to acid loads in metabolic alkalosis; an assessment of the mechanisms regulating acid excretion. J Clin Invest. 1966 Apr;45(4):562–572. doi: 10.1172/JCI105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M., SELDIN D. W., GROLLMAN A. An evaluation of radiosulfate for the determination of the volume of extracellular fluid in man and dogs. J Clin Invest. 1953 Apr;32(4):299–311. doi: 10.1172/JCI102739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]



