All hypertensive target organ damage essentially develops as a direct and/or indirect consequence of the vascular pathology that results from an exposure to increased intravascular pressures. Such indirect consequences include ischemia and dysfunction of the distal microvasculature without it being directly exposed to elevated pressures. In any event, there are significant differences in the pathogenesis of such injury between target organs which result in different degrees of macro and microvascular disease and are also reflected in the specific clinical phenotypes. Such differences also probably account for the differences in the quantitative relationships between BP increases and the increase in the relative and/or absolute risk for a given target organ damage. Therefore, from the pathogenesis perspective, the spectrum of hypertensive target organ pathology can be broadly separated into (a) macrovascular pathology; (b) cardiac pathology; and (c) microvascular pathology.
At the outset, it also needs to be acknowledged that the following discussion addresses the consequences of hypertension in the context of generic elevation of vascular pressure without trying to separate the pathogenic contribution of individual components of the pressure wave (systolic, diastolic, mean, pulse). One of the important paradigm shifts in hypertension has been the recognition of the importance of systolic rather than mean or diastolic BP as a predictor of adverse outcomes, particularly in older individuals. Similarly, the important issues of BP lability and the relative merits of the various BP measurement approaches (clinic, home, ambulatory) are also not addressed but some relevant references are cited.
(a) Macrovascular Pathology
If the increase in target organ intravascular pressures is severe enough to exceed a critical threshold, it can result in acute barotrauma to the vascular wall with severe disruptive vascular and microvascular injury as is characteristically observed in malignant/accelerated hypertension. Lesions of fibrinoid necrosis and/or thrombosis are frequently observed and the vascular injury is usually wide spread and involves multiple organs (brain, eyes, heart, kidney). However, in the vast majority of patients, BP increases are less severe and target organ damage develops after years/decades of chronic hypertension. The pathogenesis of hypertensive vascular disease in such individuals is more complex and varied. In addition to the direct deleterious impact of elevated pressures on the vessel wall, activation of other hypertension modulated pathogenic pathways contribute to the vascular pathology. These not only include endothelial dysfunction and oxidative stress, but also the myriad changes in vascular structure and function that occur with normal aging even in the absence of overt hypertension. Indeed, the major vascular changes observed with aging, such as increases in arterial stiffness, pulse wave velocity and pulse pressure due to the deterioration of the vascular wall elastin network, are also observed in an exaggerated form with hypertension in younger individuals. In fact, the similarities between aging and hypertension are so considerable that hypertension can be considered as “accelerated cardiovascular aging”. Similarly, the increasing atherosclerosis with aging is further enhanced by hypertension and the associated endothelial dysfunction, and additionally contributes to target organ dysfunction and adverse cardiovascular outcomes. Cerebrovascular disease with ischemic, or more uncommonly hemorrhagic stroke, provides the most dramatic and consequential illustration of hypertensive macrovascular pathology.
(b) Cardiac Pathology
The increased aortic pulsatile load associated with hypertensive large vessel disease leads to increased left ventricular stress and hypertrophy (LVH) with increased myocardial oxygen consumption and decreased contractile and coronary flow reserve. Over time, the left ventricle becomes stiffer and diastolic filling becomes impaired with resulting diastolic dysfunction. Moreover, because the heart is mostly perfused during the diastole unlike other organs, the central arterial stiffening in elder hypertensive individuals with the attendant fall in diastolic pressure, constitutes an additional risk factor for ischemic cardiac events. The pathogenesis of systolic dysfunction on the other hand is probably more complex and likely requires additional mechanisms such as through hypertension promoted atherosclerotic coronary artery disease with its ischemic and fibrotic sequelae.
(c) Microvascular Pathology
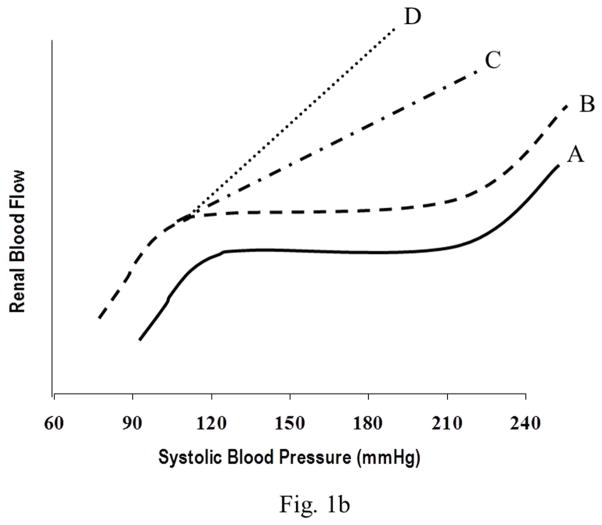
This is the least frequent form of hypertensive target organ damage and differs very substantially in its pathophyisology from that of macrovascular disease as has been clearly demonstrated in experimental animal models using BP radiotelemetry. Unlike the larger vessels which are directly exposed to the elevated BP, the microvasculature (capillaries) is protected by the myogenic autoregulatory responses of the precapillary resistance vessels. Through proportionate arteriolar vasoconstriction, the pressure in these capillary beds is maintained relatively constant and normal. Therefore, as long as the BP increases, episodic or sustained, are within the autoregulatory range, the target organ capillaries are not exposed to the hypertension. This is particularly relevant for hypertensive kidney damage. Most end stage renal disease (ESRD) is due to a severe loss of glomerular capillaries and filtration capacity rather than macrovascular disease. Because the autoregulatory mechanisms are intact in the vast majority of patients with essential hypertension, the glomeruli and GFR are largely spared and only a very slowly progressive form of vascular pathology termed benign nephrosclerosis is seen. Downstream ischemic glomerular loss does occur but is relatively modest and therefore, does not result in ESRD except in some genetically susceptible individuals/groups (African-Americans) who exhibit a more accelerated course. Therefore, although essential hypertension is second only to diabetes as a population risk factor for ESRD because of its huge prevalence, the individual risk for developing hypertensive ESRD is exceedingly low (~0.5%). By contrast, if renal disease is present and a significant loss of functional renal mass has occurred irrespective of etiology, renal autoregulation is impaired which results in an increased transmission of even modest systemic hypertension to glomerular capillaries. This enhanced susceptibility to hypertensive glomerular injury is manifested as a greatly reduced BP threshold at which progressive glomerular injury develops. These differing patterns of quantitative relationships between BP and renal damage and their relationship to renal autoregulatory capacity are illustrated in Fig. 1(a & b). Consistent with these interpretations, it has been shown that antihypertensive agents such as dihydropyridine calcium channel blockers that can adversely alter autoregulatory capacity may also adversely impact the slope of the relationship between BP and microvascular renal damage. Conversely, very modest differences if any, are observed between antihypertensive classes for macrovascular disease or cardiovascular outcomes.
Fig. 1.
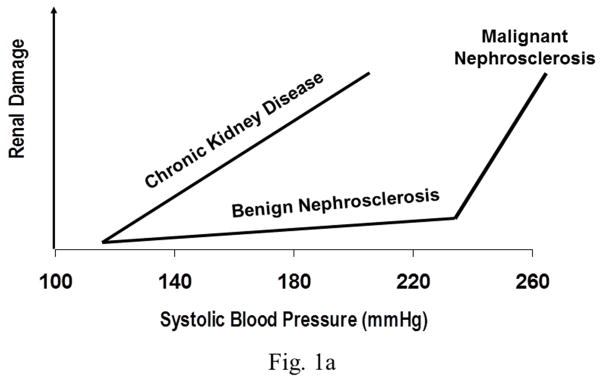
Fig. 1a. The differing BP thresholds and slopes of the relationship between BP and renal damage in patients with uncomplicated hypertension (benign and malignant nephrosclerosis) and those with diabetic and non-diabetic CKD.
Fig. 1b. Illustration of the spectrum of pressure/flow relationships in the renal vascular bed in hypertension. Pattern A represents the normal renal autoregulatory responses observed in uncomplicated hypertension and shows the constancy of renal blood flow (RBF) despite BP changes within the autoregulatory range. Pattern B indicates the ambient renal vasodilation but preserved autoregulation after uninephrectomy. Pattern C illustrates the impaired RBF autoregulatory responses observed in the 5/6 renal ablation model. Pattern D shows the complete loss of renal autoregulation in 5/6 renal ablated rats treated with dihydropyridine CCBs. Although RBF is depicted as the dependent variable, the same relationships are expected to obtain for glomerular pressures, given that the autoregulatory resistance changes are confined to the preglomerular vasculature.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anil K. Bidani, Email: abidani@lumc.edu, Professor of Medicine, Director, Division of Nephrology and Hypertension, Loyola University Chicago, 2160 South First Avenue, Maywood, IL 60153, (708) 202-4120, FAX 708-202-7978
Karen A. Griffin, Professor of Medicine, Fellowship Program Director, Division of Renal Disease and Hypertension, Loyola University Medical Center, Renal Section Chief, Edward Hines, Jr. V.A., Phone 708-202-4120, Fax 708-202-7978
SUGGESTED READINGS
- Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertens. 2009;54:393–398. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertens. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, Levy D. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7(6):476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, Najjar SS. Aging, hypertension and the heart. In: Izzo JL Jr, Sica DA, Black HR, editors. Hypertension Primer. The essentials of high blood pressure. 4. Lippincott Williams & Wilkins; 2008. pp. 184–188. [Google Scholar]
- Messeril FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertens. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- Stergiou GS, Parati G. How to best assess blood pressure? The ongoing debate on the clinical value of blood pressure average and variability. Hypertens. 2011;57:1041–1042. doi: 10.1161/HYPERTENSIONAHA.111.172924. [DOI] [PubMed] [Google Scholar]