Abstract
Recent studies in zebrafish have shown that exposure to ethanol in tank water affects various behaviors, including locomotion, anxiety and aggression, and produces changes in brain neurotransmitters, such as serotonin and dopamine. Building on these investigations, the present study had two goals: first, to develop a method for inducing voluntary ethanol intake in individual zebrafish, which can be used as a model in future studies to examine how this behavior is affected by various manipulations, and second, to characterize the effects of this ethanol intake on different behaviors and the expression of hypothalamic orexigenic peptides, galanin (GAL) and orexin (OX), which are known in rodents to stimulate consumption of ethanol and alter behaviors associated with alcohol abuse. Thus, we first developed a new model of voluntary intake of ethanol in fish by presenting this ethanol mixed with gelatin, which they readily consume. Using this model, we found that individual zebrafish can be trained in a short period of time to consume stable levels of 10% or 20% ethanol (v/v) mixed with gelatin and that their intake of this ethanol-gelatin mixture leads to pharmacologically-relevant blood ethanol concentrations which are strongly, positively correlated with the amount ingested. Intake of this ethanol-gelatin mixture increased locomotion, reduced anxiety, and stimulated aggressive behavior, while increasing expression of GAL and OX in specific hypothalamic areas. These findings, confirming results in rats, provide a method in zebrafish for investigating with forward genetics and pharmacological techniques the role of different brain mechanisms in controlling ethanol intake.
Keywords: zebrafish, ethanol, orexin, galanin
1. Introduction
Alcohol is one of the most abused drugs in the world. Studies of neurobiological mechanisms underlying alcohol abuse have demonstrated the importance of different neurochemical systems that are responsive to ethanol exposure and, in turn, promote intake [1, 2]. While these studies have been performed mostly in humans and rodents [3, 4], zebrafish are being increasingly utilized as an animal model for studying the effects of ethanol on behavior and brain neurotransmitters known to be involved in alcoholism. Zebrafish are highly prolific, resilient, and one of the lower order vertebrate species in which complex brain function and behavior may be studied in the laboratory. A large number of zebrafish mutants have already been described (www.zfin.org), providing a valuable tool for researchers interested in ethanol-gene interactions. Studies in zebrafish show that acute exposure to ethanol in the tank water, at low to moderate concentrations, reduces anxiety and increases locomotion, aggression, conditioned place preference and shoaling [5–8], whereas chronic exposure to ethanol in the water leads to the development of tolerance and withdrawal which then increases anxiety and decreases shoaling [9, 10]. These ethanol-induced behavioral changes in zebrafish, which are shown to enhance consummatory behavior in humans and rodents [11–13], are associated with marked changes in neurotransmitters in the zebrafish brain. Acute ethanol exposure increases whole brain levels of dopamine, serotonin and their metabolites and suppresses levels of glutamate and gamma-aminobutyric acid (GABA) [14–16], while chronic ethanol exposure has little impact on these neurotransmitters [15]. Ethanol consumption in rodents has similar effects on these neurotransmitters, which are sometimes stimulated by acute consumption of low amounts of ethanol while unaffected or suppressed by chronic consumption of high amounts of ethanol [17]. This evidence supports the use of zebrafish in investigating the relationship of ethanol with these neurochemicals.
In addition to these neurotransmitters, there are a number of orexigenic peptides, which in rodents are found to stimulate the consumption of ethanol and to be strongly affected by ethanol [18–21] and in humans are believed to have a role in alcoholism [22, 23]. These include galanin (GAL) and orexin/hypocretin (OX), which show increased expression in rats administered or trained to drink ethanol [19, 20, 24] and higher expression in inbred and outbred rodent strains that spontaneously overconsume ethanol [25, 26]. Whereas central injections of GAL and OX have been shown to stimulate food intake in zebrafish [27, 28], these peptides have yet to be examined in zebrafish exposed to ethanol in the water and in relation to various behaviors known to promote intake. To date, studies of OX in zebrafish have focused on arousal-related behaviors and shown this peptide to regulate the sleep-wake cycle, as in rodents [25, 29]. The evidence demonstrates that OX overexpression increases locomotor activity, decreases rest, and consolidates wakefulness [30, 31], while the ablation of OX neurons increases sleep and alters behavioral responses to external stimuli [32] and OX receptor mutants exhibit short and fragmented sleep in the dark [33]. There are few studies of GAL in zebrafish, with one report showing overexpression of GAL to increase rest and decrease locomotor activity [31] and another study showing the inhibition of GAL translation to have little impact on other orexigenic peptides [34]. With no studies examining the effect of ethanol exposure on orexigenic peptides in zebrafish, the possibility that the peptides are stimulated by ethanol in this species, similar to rodents, would provide support for their role in mediating the ingestion of ethanol and also other behaviors known to predict or be associated with alcohol abuse [3, 11, 35, 36].
Building on the reports in zebrafish examining the effects of ethanol exposure in the tank water, our goals for this investigation were to: 1) establish a model for inducing voluntary ethanol ingestion in zebrafish, which could reliably elevate blood ethanol concentration (BEC) to pharmacological levels; 2) characterize the effect of voluntary ethanol intake on different behaviors that have an established role in promoting ethanol consumption in rodents and humans; and 3) determine whether exposure to ethanol through voluntary ingestion has effects on orexigenic peptides known to stimulate ethanol intake in rodents and believed to have a role in alcoholism in humans. The model presented in this study could be valuable in performing more targeted genetic, epigenetic, molecular and pharmacological studies of mechanisms controlling ethanol consumption and abuse.
2. Methods
2.1. Animals and Housing
Adult zebrafish (Danio rerio) of the AB strain were bred in our facility (Rockefeller University, New York, NY) following standard procedures [37], from the breeding pairs purchased from ZIRC (Eurgene, Oregon). Fish were housed in 3 L tanks with constant water flow (Aquatic Habitats, Apopka, FL), which consisted of reverse osmosis water with salts (Instant Ocean, .25ppt and 500–700uS). Adult male and female fish between 6 and 12 months of age were used in this study, maintained on a 12:12 hr light-dark cycle (9 am lights on and 9 pm lights off) in 24.5–25°C water. All fish were housed in groups of 10 in 3L tanks (Aquatic Habitats). Fish were fed twice daily, at 10 am with gelatin containing shrimp as described below and at 4 pm with Zeigler Adult Diet (Aquatic Habitats). The facility was fully accredited by AAALAC. Protocols were approved by the Rockefeller University Animal Care and Use Committee and followed the NIH Guide for the Care and Use of Laboratory Animals. We used 4 different sets of zebrafish in this study in the following manner: Set 1 (N = 48) to establish the model of voluntary ethanol consumption and measure brain peptides and BEC, Set 2 (N = 48) to measure the effect of ethanol consumption on behavior, Set 3 (N = 42) determine the effect of ethanol consumptions on BEC at different time point, Set 4 (N = 16) to measure the effect of soaking in ethanol on BEC and Set 5 (N = 8) to measure the effect of soaking in ethanol on brain peptides and BEC.
2.2 Experimental design and procedures
2.2.1 Model of voluntary ethanol consumption
With gelatin-based foods used for administering special nutrients and antibiotics to aquarium fish [38, 39], we chose to use this gelatin diet to train the zebrafish to consume ethanol. Numerous studies conducted in our laboratory and others, indicating that rats not specially bred to drink ethanol will readily consume a 10% or 20% ethanol solution that in turn leads to pharmacologically relevant BEC levels when presented in a binge model [18, 40, 41], led us to formulate three types of gelatin that contained either 0%, 10% or 20% ethanol (v/v). We first prepared the gelatin (Knox Gelatin, Kraft Foods, Northfield, IL) by combining one packet (1.8 g) of gelatin with 120 ml of hot water and stirring until dissolved. Then, we poured 2.5 ml of melted gelatin into a condiment cup and combined it with 100 mg (wet weight, with excess water removed) of 2-day-old brine shrimp nauplii (Brine Shrimp Direct, Ogden, UT). We then added into each cup 2.5 ml of liquid consisting of water or 20% or 40% ethanol solution, for a final gelatin mixture containing 0%, 10%, or 20% ethanol (v/v) in 5 ml of gelatin, respectively. The mixture of gelatin with shrimp and different ethanol concentrations was allowed to set for 1 hr in a sealed cup at 4°C and then kept on ice. These ethanol-gelatin meals were made fresh daily and were given 1 hr into the light cycle. For the first 3 days of training, the fish were housed in groups of 4 in 3L tanks and fed with plain gelatin (500 mg/per feeding/tank), with water flow turned off during feeding and resumed after gelatin removal. Starting on the 4th day (first day of ethanol feeding, D1), fish in the 3L tanks were group fed for 5 min with pre-weighed 0%, 10% or 20% ethanol-gelatin mixture, and then the gelatin was removed with a fine brine shrimp net, blotted and re-weighed. After 3 days of group training, the fish on D4 were individually housed in 1.5 L tanks and fed daily for 5 min from D4-D14 with 500 mg of gelatin containing 0%, 10% or 20% ethanol, and then the gelatin was re-weighed to determine the amount consumed. Blood was collected from individual fish in the manner described below, to determine the effect of ethanol-gelatin intake on BEC.
2.2.2 Behavioral testing
In addition to gelatin intake, other behaviors of the fish were also examined in a Novel Test tank on D20 and then in a Mirror Test on D22. To acclimate them to handling, each animal, once daily from D15-D19, was caught in a soft white net (PetSmart, Phoenix, AZ) and held out of their tank for 10 s before being gently released back into the tank. On test days, fish were fed for 5 min in their home cage with gelatin containing 0%, 10% or 20% ethanol, as described above, and their intake was measured. Fifteen min after completion of this meal, the fish were individually netted into the test tank filled with system water at 25.5°C, and their behavior was video recorded twice for 60 s: first, 30 s after having been placed in the tank, indicating their response to novelty (1st min), and second, 10 min later, indicating their response in a habituated state (10th min).
The Novel Test tank was used first to assess the effect of ethanol-gelatin ingestion on locomotor activity and anxiety, using methods established in other laboratories [7, 42]. Briefly, the tank (2 L, 2 L, rectangular, Colombia University) was divided into 4 equal vertical sections and 3 horizontal sections by drawing lines on the outside of the tank, with each resulting grid being 5 × 5 cm, double the average adult body length. The following behaviors were scored during the 1st and 10th min of the test: 1) locomotor activity (calculated as the total number of horizontal + vertical crossings), and 2) exploratory behavior, an indicator of anxiety (calculated as the time spent in the top, middle and bottom sections and latency to reach the top). Behaviors were scored from videotapes, with the camera facing the longer, gridded wall of the tank.
The Mirror test was then used to assess in individual fish the effect of ethanol-gelatin intake on aggressive behavior, using methods established in other laboratories [6, 7, 43, 44] that scored fish responses to their own mirror image. A mirror was cut to fit into the tank and was placed at a 22.5° angle behind a partition, in the back or front of the experimental 1.5 L trapezoidal tank (Aquatic Habitats) on alternate days. The mirror was placed inside rather than outside the tank, in order to focus the fish on their reflection in the mirror instead of on the wall of the tank. The tank was divided into 4 equal sections drawn with a black marker on the bottom of the tank. In addition, a line was drawn 0.5 cm from the mirror, representing the “contact zone”, and another line was drawn 2.5 cm from the first line (based on the average adult body length), representing the “approach zone”. With the camera placed above the tanks, the following behaviors were scored: 1) percent time in S1 (contact zone + approach zone); 2) latency to 1st approach and 1st contact; 3) number of entries into approach zone and contact zone; and 4) percent time in contact zone.
2.2.3 Quantification of behavior
All videos were recorded with a Panasonic Lumix DMC TS25 in 720p HD and viewed in MPEG-4 Movie format. All behaviors were manually scored using a stop watch by a trained observer blind to the treatment of the groups. Locomotion was scored by counting entries into each of the 4 horizontal sections [6, 7] and 3 vertical sections [42, 45]. Time in each layer was recorded and calculated as percent time spend in each section relative to the total time of 60s [6, 7]. Latency to reach the top layer of the tank was scored as the time it took for two-thirds of the body length to cross the line that separated the middle from the top layer of the tank [46]. The Mirror test was scored by measuring the time it took for fish to approach and contract the mirror and the number of entries into the approach zone and contact zone, and then calculating the percent time spent in the S1 zone (“contact zone” plus “approach zone”) and the contact zones [42, 46].
2.3. Digoxigenin-labeled in situ hybridization (ISH) histochemistry to measure peptide neuronal density
In situ hybridization with digoxigenin-labeled probes was used to measure the density of neurons expressing the orexigenic peptide genes. Fish were fed for 5 min with gelatin containing 0%, 10% or 20% ethanol, and at 30 min after completion of the meal, they were anesthetized in an ice water bath for 5–10 min until they lost equilibrium and ocular movement. Immediately after, blood was collected, and fish were euthanized by decapitation. For the brain dissection, the fish head was placed ventral side up on moistened filter paper which was placed on the top of a petri dish filled with ice. As previously described [47], forceps were used to remove the gills, jaw, eyes and surrounding tissue. The skull was cracked open, and the base of the skull was gently removed. The whole brain was pulled out of the skull by the spinal cord. The brain was immediately placed in 4% paraformaldehyde in phosphate buffer solution (0.1 M, pH 7.2), fixed overnight at 4°C, and then cryoprotected in 25% sucrose for 24 to 48 hrs at 4°C. Afterwards, the brain was frozen at −80°C for 1–2 hrs, then cut with a cryostat in 40 μm serial sagittal sections, allowing alternate free-floating sections to be used for measurement of GAL and OX mRNA using ISH. Specifically, ISH was performed by processing the sections consecutively as follows: 10 min each in 0.001% proteinase K, 4% paraformaldehyde, and acetylation solution, between each step, 2 × 5 min wash in PB (0.1 M pH 7.2), followed by hybridization for 18 hrs at 65°C and then a 20-min wash in 5 × SSC and 30-min wash in 50% formamide, with both at 65°C, and 30 min in RNase A (1 μg/ml) at 37°C. After 2–4 hrs in a 1.5% blocking solution, the sections were then incubated in AP-conjugated sheep anti-digoxigenin Fab fragments (1:500, Roche, Indianapolis, IN) for 18 hrs and developed in freshly prepared color developer (50 μl of 4-nitroblue tetrazolium chloride solution, 37.5 μl of 5-bromo-4-chloro-3-indolyl-phosphate solution in 10 ml Tris buffer, pH 9.5; Roche). Finally, the sections were fixed for 10 min in 4% paraformaldehyde, then mounted, air-dried, dehydrated, cleared and coverslipped. The sense probe control was performed in the same tissue, and no signal was detected. All procedures were conducted at room temperature, unless otherwise indicated. Zebrafish GAL and OX cDNA plasmids were generously provided by Dr. Pertti Panula (University of Helsinki, Finland), and the digoxigenin (DIG)-labelled riboprobes (sense and antisense) were made as described [34, 48].
2.4. Semiquantification of digoxigenin-labeled ISH
The GAL- and OX-expressing cells in the hypothalamus were viewed using a Leitz microscope with a 20X illumination objective, and the images were captured with a Nikon DXM 1200 digital camera (Nikon, Tokyo, Japan). The density of the GAL- and OX-expressing neurons was measured using Image-Pro Plus software (Version 4.5, Media Cybernetics Inc., Silver Spring, MD), as described in detail [49, 50], and the measure of cell density is expressed as number of cells/μm2. In all analyses, only those cells containing a nucleus (≥ 20 μm2) on one plane in each section were counted. The average cell density for the 0% (control), 10% and 20% ethanol groups was then compared and analyzed statistically, with the GAL cells evident in the ventral and caudal zones of the periventricular hypothalamus and the OX cells evident in the anterior hypothalamus.
2.5. Ethanol exposure in tank water
In addition to the effect of ethanol consumption on hypothalamic peptides, we also examined peptide responses to ethanol in the tank water by holding the fish in an ethanol-system water solution (0%, 0.25% or 0.5% ethanol) for 1 hr in a 3L home tank with a lid [7, 51]. Blood collection was carried out as described below (Section 2.6) and assayed for levels of BEC. To determine the effect of ethanol treatment on orexigenic peptides, an additional group of fish was treated with 0% or 0.25% ethanol, and as described below, their blood was collected for measurements of BEC and their brain was collected for analysis of the peptides.
2.6. Blood ethanol concentration
As previously described [52, 53], immediately after the fish were anesthetized in ice water bath, each one was wrapped in a paper towel with its tail end exposed to air and was then placed on a second paper towel on top of a Petri dish filled with ice, with its tail hanging off. The tail was cut above the anal fin to sever the dorsal aorta. Holding the fish between the fingers at a 60–90° angle, blood was collected in a heparinized capillary tube by gently squeezing the fish from head to tail. The entire procedure took less than 1.5 min per fish. The capillary tubes were capped with clay on the bottom, covered with parafilm, and centrifuged at 3.1 RPM for 8 min at 4°C, with each fish yielding 5–10 ul of blood and 2–7 ul of serum. Serum was removed from the tube and stored in −80°C until it was assayed for BEC using an Analox GM8 Alcohol Analyzer (Lunenberg, MA). When the serum volume was not sufficient for standard BEC measurements, it was diluted with PBS (pH 7.4), and BEC levels were calculated accordingly.
2.7 Specific Experiments
2.7.1. Experiment 1: Voluntary ingestion of ethanol-gelatin and effect on BEC in individual zebrafish
The goal of this experiment in zebrafish was to establish a model of voluntary ethanol ingestion and to relate this ingestion to BEC. After the initial 3-day training to consume gelatin, the zebrafish (N = 48) on the fourth day of training (D4) were randomly assigned to one of three groups (n = 16/group), and for the next 11 days (D4-D14), were given a daily meal of gelatin containing 0% (control), 10% or 20% ethanol and the amount ingested was measured. To determine the effect of ingesting the ethanol-gelatin mixture on BEC, a different set of fish (N = 42) was similarly trained to consume 0%, 10% or 20% ethanol-gelatin and their individual intake was recorded daily from D4-D14. They were then sacrificed on D14 at 5, 15 or 30 min after the 5-min meal, and their blood was collected for measurements of BEC. With our evidence showing that the zebrafish ingest more of the 10% ethanol-gelatin compared to gelatin alone, we examined an additional set of fish (n = 6/group) to determine if this could be due to a change in appetite induced by the ethanol. We found that fish that had consumed a 10% ethanol-gelatin meal tended to consume less of a subsequent meal of 0% ethanol-gelatin provided 3 hrs later (49 ± 6 mg/g body weight) than did fish that consumed 0% ethanol-gelatin at both meals (85 ± 10 mg/g bodyweight, ns), suggesting that the increased intake of the ethanol-gelatin mixture was not due specifically to ethanol-induced changes in appetite.
2.7.2. Experiment 2: Voluntary ingestion of ethanol-gelatin and effects on behavior
This experiment examined the effects of voluntary intake of ethanol-gelatin vs. gelatin alone on different behaviors, using the same set of zebrafish examined in Experiment 1 after completing the intake measurements from D4-D14. After these fish were habituated to handling from D15-D19, their behaviors were first examined on D20 in a Novel Test tank and then on D22 in a Mirror Test, 15 min after completion of the 0%, 10% or 20% ethanol-gelatin meal. To determine the effect of ethanol-gelatin intake on locomotion and anxiety, the Novel Test tank was used to measure locomotor activity, latency to reach the top zone of the tank, and location of swimming during the 1st and 10th min of the test. To determine the impact of ethanol-gelatin intake on aggressive behavior, the Mirror test was used to examine their responses to their own mirror image, by measuring the percent time spent in the S1 zone, including both the approach and contact zones of the mirror.
2.7.3. Experiment 3: Voluntary intake of ethanol-gelatin and effects on expression of orexigenic peptides GAL and OX
This experiment examined the effect of voluntary ethanol-gelatin ingestion on mRNA expression of the orexigenic peptides, GAL and OX, in the hypothalamus. Zebrafish (N = 18) were trained to consume 0%, 10% or 20% ethanol-gelatin as described above (n = 8/group), and then on the last day of training (D14), they were sacrificed 30 min after meal completion and their blood was collected for measurements of BEC and their brains were dissected for peptide analyses using in situ hybridization. To compare these results to those produced by ethanol in the tank water, we measured in an additional set of zebrafish (N = 8) gene expression of the two peptides along with BEC after 1 hr of soaking in 0.25% ethanol (v/v) compared to water (n = 4/group).
2.8. Statistical Analysis
Correlations between gelatin intake at each day, ethanol intake with BEC, and BEC with peptide expression, were analyzed using the Pearson product-moment correlation coefficient. Differences between the effects of ethanol on behaviors or gene expression at single time-points were tested with a one-way ANOVA, followed up by Tukey’s post-hoc test or by unpaired, two-tailed t-tests as appropriate. Differences in the effects of multiple doses of ethanol at multiple time-points were tested with a repeated-measures ANOVA (when time was a within-subject factor) followed up by pairwise comparisons using Tukey’s HSD, or with a one-way ANOVA followed up by Tukey’s post-hoc test as appropriate. Differences in the effects of multiple doses of ethanol in multiple brain regions (in the same fish) were tested with a repeated-measures ANOVA (with brain area as the within-subject factor), with a significant interaction effect followed up by a one-way ANOVA and then pairwise comparisons using Tukey’s HSD. Data were determined to be distributed normally using the Shapiro-Wilk test. Significance was determined at p < 0.05. Data are reported as mean ± standard error of the mean (S.E.M.).
3. Results
3.1. Experiment 1: Voluntary ingestion of ethanol-gelatin and effects on BEC in individual zebrafish
The goal of this experiment was to establish a model of voluntary ethanol ingestion and to characterize the ingestion pattern of gelatin with or without ethanol (0%, 10%, 20%, n = 16/group) and determine the relationship between the amount of ethanol-gelatin consumed and measurements of BEC. As shown in Fig. 1A, daily ingestion (mg/g body weight) started to stabilize around D9, with day-to-day intake from D11 through D14 significantly, positively correlated (r = +0.69, p < 0.01). Gelatin intake from D9 to D14 averaged 43 ± 4.7 mg with 0% ethanol (control), 73 ± 7 mg with 10% ethanol, and 75 ± 4 mg with 20% ethanol, yielding an average ethanol intake by each fish of 7.3 ± 0.7 mg at 10% ethanol and 15.0 ± 0.7 mg at 20% ethanol. These data from D4 to D14 yielded a significant main effect of ethanol-gelatin intake [F(2,27) = 4.42, p < 0.01] and time [F(10,270) = 4.70, p < 0.01] but no ethanol-gelatin x time interaction [F(20, 270) = 1.30, ns], with post-hoc comparisons showing the main effect on intake to be due to a significant increase in intake of 10% ethanol-gelatin (+50%, p < 0.01) with no change in intake of 20% ethanol-gelatin (ns) compared to 0% ethanol-gelatin control. To measure the impact of ethanol-gelatin ingestion on BEC over time, a separate set of zebrafish (N = 42) was trained to consume 0%, 10% or 20% ethanol-gelatin (n = 10–12/group/time point) and sacrificed on D14 at either 5, 15 or 30 min after 5 min of consumption, at which time their trunk blood was collected. Analysis with a one-way ANOVA revealed a significant main effect of ethanol-gelatin intake on BEC at 5 min [F(2,31) = 21.14, p < 0.01], 15 min [F(2,29) = 40.01, p < 0.01] and 30 min [F(2,29) = 28.34, p < 0.01] (Fig. 1B), with post-hoc comparisons showing this effect to be due to a significant increase in BEC from 10% and 20% ethanol-gelatin at all three time points compared to 0% ethanol-gelatin control (p < 0.01) and higher after intake of 20% ethanol-gelatin compared to 10% ethanol-gelatin at all time points (p < 0.01). Further, the amount of ethanol-gelatin consumed (mg gelatin/g body weight) was strongly, positively correlated with BEC when measured at 5 min (r = +0.65, p < 0.05), 15 min (r = +0.77, p < 0.01) and 30 min (r = +0.73, p < 0.05) after intake of 10% ethanol-gelatin and also at 5 min (r = +0.61, p < 0.05), 15 min (r = +0.88, p < 0.01) and 30 min (r = +0.92, p < 0.01) after intake of 20% ethanol-gelatin. To compare these BEC values to those measured in fish that were soaked in ethanol, we examined an additional set of fish (n = 8/group) and found that soaking for 1 hr in 0.25% or 0.5% ethanol, a paradigm commonly used [6, 7, 43, 54], has a significant effect on BEC [F(2,23) = 141.56, p < 0.01], with a significant increase at both the 0.25% (129 ± 9 mg/dl, p < 0.05) and 0.5% (222 ± 15 mg/dl, p < 0.05) ethanol concentrations. Together, these results indicate that zebrafish voluntarily ingest stable amounts of ethanol in gelatin and that this consumption increases BEC to levels that are pharmacologically relevant.
Fig. 1.
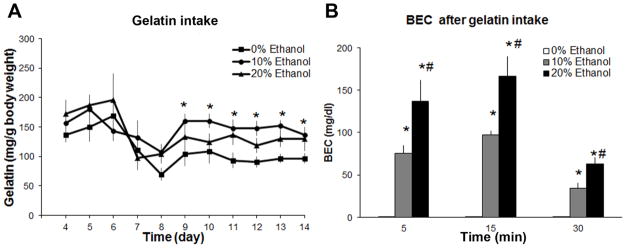
Daily feeding with gelatin containing 0%, 10% or 20% ethanol produced stable intake in zebrafish. (A) Intake of gelatin containing 10% ethanol resulted in greater consumption than that of gelatin with 0% ethanol (starting on D9) (n = 16/group). (B) Intake of 10% or 20% ethanol-gelatin led to greater blood ethanol concentration (BEC) levels than consumption of 0% ethanol-gelatin, while consumption of 20% ethanol-gelain led to higher BEC levels than consumption of 10% ethanol-gelatin (n = 10–12/group). *p < 0.05 vs. 0% ethanol-gelatin, #p < 0.05 vs. 10% ethanol-gelatin.
3.2. Experiment 2: Voluntary ingestion of ethanol-gelatin and effects on different behaviors in zebrafish
This experiment examined the effects of voluntary ingestion of ethanol-gelatin on different behaviors in zebrafish, first in a Novel Tank test and then in a Mirror test. Table 1 displays the results for all the ANOVAs used to analyze the behavioral tests.
Table 1.
Statistical results for the effect of ethanol-gelatin ingestion on behavior in the Novel Tank and Mirror Tests. Groups were 0%, 10%, and 20% ethanol-gelatin. Time-points were 1st and 10th minute.
| df | F | p | |
|---|---|---|---|
| Novel Tank Test | |||
| Locomotor activity | |||
| Ethanol-gelatin | 1,42 | 69.61 | 0.01 |
| Time | 1,42 | 22.10 | 0.01 |
| Ethanol-gelatin x Time | 2,42 | 2.16 | ns |
| Latency to reach top | |||
| Ethanol-gelatin | 2,45 | 4.37 | 0.01 |
| Location of swimming | |||
| % time on top | |||
| Ethanol-gelatin | 1,44 | 3.28 | 0.05 |
| Time | 1,44 | 19.10 | 0.02 |
| Ethanol-gelatin x time | 2,44 | 5.55 | 0.05 |
| % time in the middle | |||
| Ethanol-gelatin | 1,44 | 4.53 | 0.01 |
| Time | 1,44 | 13.2 | 0.01 |
| Ethanol-gelatin x time | 3,44 | 2.83 | ns |
| % time in the bottom | |||
| Ethanol-gelatin | 1,44 | 33.01 | 0.01 |
| Time | 2,44 | 5.43 | 0.01 |
| Ethanol-gelatin x time | 2,44 | 5.55 | 0.01 |
| Mirror Test | |||
| % time in S1 | |||
| Ethanol-gelatin | 2,46 | 14.01 | 0.01 |
| Time | 1,46 | 1.31 | ns |
| Ethanol-gelatin x time | 2,46 | 1.81 | ns |
| % time in contact zone | |||
| Ethanol-gelatin | 2,46 | 6.12 | 0.05 |
| Time | 1,46 | 10.39 | 0.01 |
| Ethanol-gelatin x time | 2,46 | 5.01 | 0.05 |
| Latency to approach | |||
| Ethanol-gelatin | 2,44 | 7.92 | 0.05 |
| Latency to contact | |||
| Ethanol-gelatin | 2,44 | 1.25 | ns |
| Entries into approach zone | |||
| Ethanol-gelatin | 2,46 | 2.21 | ns |
| Time | 1,46 | 6.20 | 0.05 |
| Ethanol-gelatin x time | 2,46 | 0.11 | ns |
| Entries into contact zone | |||
| Ethanol-gelatin | 2,46 | 1.25 | ns |
| Time | 1,46 | 10.01 | 0.05 |
| Ethanol-gelatin x time | 2,46 | 0.76 | ns |
Novel Tank Test
To determine the impact of ethanol-gelatin intake on locomotor behavior and anxiety in a novel tank, we recorded different behavioral measures in zebrafish (N = 47) during the 1st and 10th min of the test after the 0%, 10% and 20% ethanol-gelatin meal. We observed a main effect of ethanol-gelatin intake on locomotor activity, calculated as the total number of horizontal and vertical crossings, with a significant change in activity over time (Fig. 2A) but no ethanol x time interaction, with a significant effect of 20% (p < 0.001) but not 10% (ns) ethanol-gelatin intake compared to 0% ethanol-gelatin control. We also observed a significant main effect of ethanol-gelatin intake on the latency to reach the top zone, with post-hoc tests showing 20% ethanol-gelatin compared to control to significantly decrease the time taken for zebrafish to reach the top zone (p < 0.01) and 10% ethanol-gelatin to produce a small, statistically insignificant decrease in time (ns) (Fig. 2B).
Fig. 2.
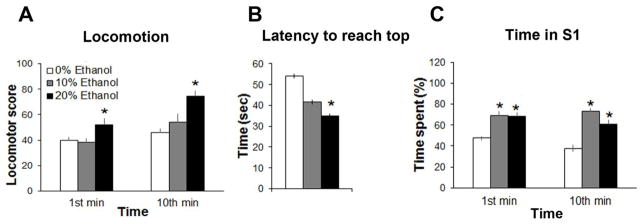
Intake of ethanol-gelatin significantly altered zebrafish behavior. (A) Zebrafish in the novel tank test became hyperactive following intake of 20% ethanol-gelatin compared to 10% or 0% ethanol-gelatin. (B) Latency to reach the top of the tank was decreased after intake of 20% but not 10% ethanol-gelatin compared to 0% ethanol-gelatin in the novel tank test. (C) Intake of 10% or 20% ethanol-gelatin increased the time spent in the S1 zone, which represents “contact zone” plus “approach zone” in the mirror stimulation test. *p < 0.05 vs. 0% or 10% ethanol-gelatin, n = 16/group.
Examination of the location of swimming during the 1st and 10th min of the test revealed changes in the measures of percent time spent in the top, middle and bottom sections of the tank (Table 2). Analysis of time spent in the top section revealed a significant main effect of ethanol-gelatin intake and time with an interaction between ethanol-gelatin and time, and post-hoc tests showing a significant increase in percent time spent on top after consumption of 20% (p < 0.01) but not 10% (ns) ethanol-gelatin compared to control and no difference between the two ethanol groups (ns). Pairwise comparisons showed the percent time spent in the top section to increase with time (p < 0.01). Analysis of the time spent in the middle section also revealed a significant main effect of ethanol-gelatin and time but no interaction between ethanol-gelatin and time, with post-hoc tests showing a significant increase after consumption of 20% (p < 0.05) but not 10% (ns) ethanol-gelatin and no difference between the two ethanol groups (ns) and with pairwise comparisons showing the percent time spent in the middle section overall to increase over time (p < 0.05). The time spent in the bottom section was also significantly affected by ethanol-gelatin and time as was the interaction between ethanol-gelatin and time, with post-hoc tests showing a significant decrease after consumption of both 20% (p < 0.05) and 10% (p < 0.05) ethanol-gelatin and with pairwise comparisons showing the percent time spent at the bottom of the tank to decrease after consumption of 10% (p < 0.01) but not 20% (ns) ethanol-gelatin compared to control. Together, these data demonstrate that voluntary consumption of ethanol-gelatin significantly increases anxiety in zebrafish.
Table 2.
Consumption of ethanol increases: 1) exploration in a novel environment as demonstrated by a decrease in the time that fish spent in the bottom of the tank and an increase in the time spent in the top of the tank; and 2) aggressive behavior in a mirror test as demonstrated by a decrease in the latency to approach and contact the mirror and an increase in the time spent in close proximity to the mirror. p < 0.05 vs. 0% ethanol, n = 16/group.
| 1st min | 10th min | |||||
|---|---|---|---|---|---|---|
| 0% | 10% | 20% | 0% | 10% | 20% | |
| Novel Tank Test | ||||||
| % Time on top | 2 ± 0.9 | 5 ± 2.7 | 14 ± 5.1 | 16 ± 4.4 | 32 ± 5.9* | 29 ± 2.1* |
| % Time in middle | 8 ± 2.9 | 14 ± 1.1 | 32 ± 2.9* | 27 ± 4.6 | 36 ± 4.1 | 33 ± 3.9* |
| % Time on bottom | 91 ± 3.5 | 81 ± 7.1 | 55 ± 9.2* | 57 ± 8.0 | 33 ± 5.4* | 48 ± 5.6 |
| Mirror Test | ||||||
| Latency to approach | 12.2 ± 3.2 | 2.8 ± 0.6* | 4.1 ± 1.5* | n/a | n/a | n/a |
| Latency to contact | 12.8 ± 3.3 | 3.7 ± 0.9* | 4.4 ± 1.5* | n/a | n/a | n/a |
| Entries into contact zone | 5.8 ± 0.6 | 4.6 ± 0.5 | 5.9 ± 1.0 | 6.8 ± 0.8 | 7.4 ± 0.9 | 8.3 ± 0.8 |
| Entries into approach zone | 6.1 ± 0.9 | 5.1 ± 0.8 | 5.9 ± 1.6 | 7.0 ± 0.7 | 7.6 ± 1.0 | 9.3 ± 0.7 |
| % Time in contact zone | 34.4 ± 2.6 | 57.6 ± 6.9* | 65.4 ± 4.9* | 32.0 ± 5.1 | 61.6 ± 5.5* | 52.3 ± 7.4* |
| % Time in S1 | 48.0 ± 3.0 | 65.8 ± 4.7* | 68.4 ± 5.1* | 37.7 ± 5.6 | 73.0 ± 6.3* | 61.4 ± 6.6* |
Mirror Test
To determine the impact of voluntary ethanol-gelatin consumption on aggressive behavior in zebrafish (N = 48), individual fish were tested for their responses to their own mirror image. Analysis of the percent time spent in the S1 zone, including both the approach and contact zones of the mirror, revealed a significant main effect of ethanol-gelatin intake, with no main effect of time and no interaction between time and treatment (Fig. 2C) and post-hoc tests showed this main effect to reflect a significant increase in time spent in the S1 zone after ingestion of both the 10% and 20% ethanol-gelatin compared to control (p < 0.05) and no difference between the two ethanol-gelatin groups (ns). Analysis of the percent time spent specifically in the contact zone of the mirror revealed a significant main effect of ethanol-gelatin as well as time and a significant interaction between ethanol-gelatin and time, with post-hoc analysis showing this effect to be due to intake of both the 10% (+80%, p < 0.01) and 20% (+77%, p < 0.01) ethanol-gelatin with no difference between the two ethanol groups (ns). A significant main effect of ethanol-gelatin was also obtained for the latency to approach and contact the mirror, with a significant decrease in both measures observed after consumption of 10% (p < 0.05) and 20% (p < 0.05) ethanol-gelatin but no difference between the two ethanol groups (ns) (Table 2). While there was no main effect of ethanol-gelatin intake on the number of entries into the approach or contact zones, there was a significant main effect of time on these measures, with pairwise comparisons showing that entries into these zones increased with time (p < 0.05). There were no time x treatment interaction effects for the number of entries into approach and contact zones. Together, these behavioral tests demonstrate that voluntary ethanol consumption has significant, stimulatory effects on both exploration and aggressive behavior.
3.3. Experiment 3: Voluntary intake of ethanol-gelatin and effects on expression of orexigenic peptides, GAL and OX
With no studies to date examining the impact of ethanol on orexigenic peptides in the brain, this experiment examined the effect of voluntary ethanol-gelatin intake (0%, 10% or 20% ethanol-gelatin) on mRNA expression of the orexigenic peptides, GAL and OX, in the hypothalamus (n = 8/group). As in Experiment 1, measurements of BEC revealed a significant main effect of ethanol-gelatin intake [F(2,21) = 28.0, p < 0.01], with both the 10% and 20% ethanol-gelatin producing a significant increase in BEC (p < 0.01), with the 20% ethanol-gelatin producing higher BEC (63 ± 8 mg/dl) than 10% ethanol-gelatin (31 ± 5 mg/dl, p < 0.001), and with BEC being strongly, positively correlated with the amount of 10% (r = +0.82, p < 0.01) and 20% (r = +0.91, p < 0.01) ethanol-gelatin consumed. Analysis of the peptides revealed a main effect of ethanol-gelatin on mRNA expression of GAL [F(2, 21) = 140.19, p < 0.01] and OX [F(2, 23) = 11.06, p < 0.01] in the hypothalamus (Fig. 3). The effect on GAL was anatomically localized [F(2,21) = 162.17, p < 0.01], occurring specifically in the ventral (Hv) [F(2,21) = 23.98, p < 0.01] and caudal (Hc) [F(2,21) = 149.35, p < 0.01] zones of the periventricular hypothalamus, with no significant interaction between ethanol-gelatin intake and brain area [F(2,21) = 60.65, p < 0.01] (Fig 4). In both groups (10% and 20%), pairwise comparisons showed ethanol-gelatin consumption to significantly increase the expression of GAL mRNA in both the Hv (+22%, p < 0.01) and Hc (+239%, p < 0.01), with a significantly larger effect observed in the Hc zone (ηp2 = 0.93 vs. ηp2 = 0.69) (Fig 4). Ethanol-gelatin intake also increased OX mRNA expression in the anterior hypothalamic region (+60%, p < 0.01) (Fig 4), again with no difference observed between the two ethanol groups (ns). Exposure to ethanol by soaking in 0.25% ethanol (v/v) compared to water (n = 4/group), which increased BEC to 114 ± 9 mg/dl, also significantly increased the density of GAL mRNA in both the Hv (5.0 vs 3.8 objects/μm2 x10−5, p < 0.05) and Hc (5.2 vs 1.3 objects/μm2 x10−5, p < 0.05) zones of the periventricular hypothalamus and of OX mRNA in the anterior region of the hypothalamus (2.4 vs 1.4 objects/μm2 x10−5, p < 0.05). Together, these two experiments demonstrate that voluntary ethanol-gelatin intake has a stimulatory effect on hypothalamic expression of both GAL and OX and that these effects are similar to those observed with exposure to ethanol in tank water.
Fig. 3.
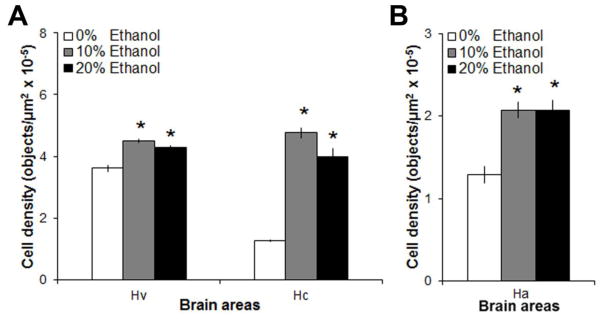
Intake of 10% or 20% ethanol-gelatin increased the density of (A) galanin mRNA-expressing neurons in the ventral (Hv) and caudal (Hc) region of the periventricular nucleus and of (B) orexin mRNA-expressing neurons in the anterior hypothalamus (Ha), as measured by digoxigenin-labeled in situ hybridization. *p < 0.05 vs. 0% ethanol-gelatin, n = 8/group.
Fig. 4.
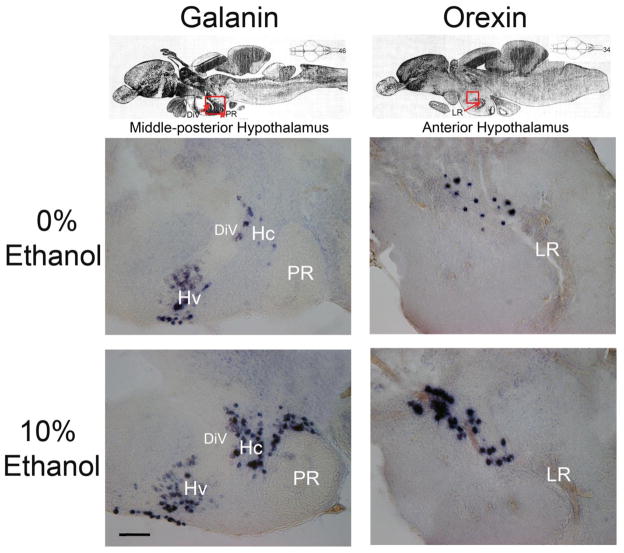
Photomicrographs illustrate changes in the density of galanin (column A) and orexin (column B) mRNA-expressing neurons in the hypothalamus in response to consumption of 20% ethanol-gelatin compared to 0% ethanol-gelatin, as revealed in sagittal sections with digoxigenin-labeled in situ hybridization. (A) Representative images from sagittal sections at the 46th section plane of the zebrafish brain, demonstrating a stimulatory effect of ethanol on galanin expression in the mid-posterior hypothalamus, outlined in red in the top panel. (B) Representative images from sagittal sections at the 34th section plane of the zebrafish brain, demonstrating a stimulatory effect of ethanol on orexin expression in the anterior hypothalamus, outlined in red in the top panel. DiV, diencephalic ventricle; Hc, caudal zone of periventricular hypothalamus; Hv, ventral zone of periventricular hypothalamus; LR, lateral recess of diencephalic ventricle; PR, posterior recess of diencephalic ventricle. Scale bar = 100μm. Top panels adapted from Wullimann, Rupp, and Reichert, Neuroanatomy of the Zebrafish Brain (1996), with permission from Birkhäuser Verlag [64].
4. Discussion
This study describes a method for inducing fish to voluntary ingest ethanol by mixing it with gelatin, which allows one to measure ethanol-gelatin intake in individual fish that can be related to their BEC. This novel model produces pharmacologically relevant BEC, which leads to profound changes in behavior that in rodent and human studies are associated with alcohol abuse. These behavioral changes induced by ethanol-gelatin ingestion are accompanied by a strong stimulatory effect on orexigenic peptides known in other species to have a role in mediating these behaviors.
4.1. Model of voluntary intake of ethanol-gelatin in zebrafish
In this study, we established a model that is easy to set up and allows one to reliably measure voluntary ingestion of ethanol mixed with gelatin and the resulting BEC in individual zebrafish. There is only one publication that has measured BEC in zebrafish, and this study pooled samples from 12 fish and showed that soaking for 10 min in 0.125–1% ethanol solution increased BEC to 50–170 mg/dl [55]. Our present results demonstrate that ingestion of the 10% or 20% ethanol-gelatin mixture causes an increase in BEC, to 86 mg/dl and 152 mg/dl, respectively, during the first 15 min after completion of this 5-min meal. These levels are comparable to those produced by soaking zebrafish for 1 hr in 0.25% ethanol (129 mg/dl), allowing us to compare behavioral changes induced by our model of ethanol ingestion to those induced by the commonly-used procedure of ethanol soak (0.025–1%) [7]. These BEC levels produced by ethanol-gelatin intake were also comparable to those observed in rats (up to 134 mg/dl) consuming a pharmacologically relevant amount of ethanol [18] and higher than the minimum value in humans (80 mg/dl) that qualifies as binge drinking [56]. Measurements of ethanol concentration in the zebrafish brain have shown that soaking in 1% ethanol in the water increases brain levels to 4.2 ug/mg in 20 min and 6.3 ug/mg in 1 hr [45], with 6 hr required for equilibration of the brain concentration with tank concentration [9]. Of particular note is our additional finding that the amount ethanol-gelatin consumed by each zebrafish is strongly, positively correlated with BEC (r = +0.76). It is interesting that the fish consumed significantly more of the gelatin when it contained 10% ethanol vs. 0% ethanol. As fish that consumed this 10% ethanol-gelatin did not consume more of a subsequent 0% ethanol-gelatin meal than did fish that consumed 0% ethanol-gelatin at both meals, the increased intake of ethanol-gelatin may not due to an ethanol-induced change in appetite. Instead, zebrafish may have a preference for the pharmacological effects or the taste of ethanol-gelatin, at least at the 10% concentration, which in humans and rodents is perceived as containing both sweet and bitter taste components [57, 58]. This preference is similar to what we and others have observed in outbred rodents when presented with 10% or 20% ethanol using an intermittent-access model [18, 40, 41]. These findings suggest that our new model of voluntary ingestion of ethanol-gelatin, which yields stable measures of intake within just 14 days and increases BEC in individual fish to pharmacologically-relevant levels, will provide a valuable tool for investigating the effects of ethanol intake on behavior and brain neurochemical systems, as well as the effect of genetic and pharmacological manipulations on ethanol consumption.
4.2. Effect of voluntary intake of ethanol-gelatin on behavior in zebrafish
The results indicate that our model of voluntary ethanol-gelatin intake produces pharmacologically relevant BEC levels that significantly affect behavior in zebrafish. Overall, the behavioral changes induced by ingestion are similar to those produced by soaking in ethanol, validating this new model of voluntary consumption. The ethanol-gelatin caused a significant increase in locomotion and a decrease in anxiety, as indicated by increased exploration in zebrafish that ingested the 20% ethanol-gelatin. Measurements of locomotor activity (number of vertical and horizontal transitions) in a novel environment revealed a significant increase after ingestion of the 20% ethanol-gelatin, during the 1st min as well as the 10th min of the test after habituation. The long-lasting nature of this hyperactivity suggests that that the ethanol-exposed fish failed to habituate to this novel environment [7], although it cannot be ruled out that it does not reflect increased sensitivity over the course of the test to the pharmacokinetic-induced changes in ethanol levels. This increased activity is similar to that observed in wildtype zebrafish exposed for 1 hr to a low or intermediate concentration (0.25% and 0.5%) of ethanol in the water, in contrast to the reduced locomotor activity induced by a higher concentration (1%) of ethanol [7]. We additionally found the latency to reach the top of the tank to be significantly reduced by ingestion of the 20% ethanol-gelatin, indicating increased exploratory behavior that is associated with reduced anxiety [51]. This behavioral change caused by ethanol-gelatin intake in AB zebrafish is similar to that observed in wildtype zebrafish exposed for 5 min to 0.3% ethanol in the water [5]. The location of swimming was also affected by 20% ethanol-gelatin, with an increase in the percent time spent in the top of the tank during both the 1st and 10th min of the test again reflecting an increase in exploratory activity and possibly reduced anxiety with time. These results are consistent with findings in wildtype zebrafish exposed repeatedly for 1 hr to 0.25% and 0.5% ethanol in the water [7, 51] and also in AB zebrafish exposed for 20 min to 1% and 1.5% ethanol [10, 43]. Further measurements revealed significant effects of ethanol-gelatin intake on aggressive behavior, with the both 10% and 20% ethanol-gelatin significantly increasing the time spent in close proximity to the mirror and decreasing the latency to approach and contact the mirror, suggesting an increase in aggression. Again, these effects induced by ethanol-gelatin intake are similar to those observed in wildtype zebrafish with 0.25% and 0.50% ethanol in the water [6, 7, 43], although they are opposite to findings in AB zebrafish [10, 43]. In addition to these similarities between the effects of ethanol-gelatin intake and ethanol in the tank water, it is interesting that these behavioral effects in zebrafish are similar to those observed in rodents and humans, which in response to ethanol at low-to-moderate doses exhibit an increase in locomotor activity and aggressive behavior along with a decrease in anxiety [12, 18, 22, 36, 59]. The finding that 10% ethanol-gelatin significantly increases aggressive behavior while having no impact on exploratory behavior suggests that these specific behaviors are differentially sensitive to BEC [36, 60]. Together, these findings validate our new model of voluntary ingestion of ethanol-gelatin in zebrafish, which increases BEC to pharmacological levels that are strongly, positively correlated with the amount consumed and produces behavioral changes similar to those induced by ethanol in the water, as well as by ethanol consumption in rodents.
4.3. Effect of voluntary ingestion of ethanol-gelatin intake on hypothalamic orexigenic peptides in zebrafish
Our results additionally demonstrate that voluntary intake of ethanol-gelatin stimulates the expression of the orexigenic peptides, GAL and OX, in the hypothalamus of zebrafish. This effect was found to be anatomically specific, occurring for GAL in the ventral (+22%) and caudal (+239%) zones of the periventricular hypothalamus, with a significantly greater effect in the caudal compared to ventral zone, and for OX (+60%) in the anterior periventricular hypothalamus. These findings with ethanol-gelatin intake in zebrafish are similar to evidence obtained in rats, showing ethanol consumption [18–20] as well as ethanol administration [20, 21] to increase mRNA levels of these two peptides. The lack of a clear dose response relationship for the two ethanol concentrations, as also shown in rats [20, 21], suggests that mRNA expression may not be sensitive to differential BEC levels within the first 30 min following ethanol ingestion. Although we cannot rule out the possibility that intake of gelatin itself affected the orexigenic peptides, the findings that 20% ethanol-gelatin stimulated the peptides without gelatin intake beyond that of 10%, together with our result that ethanol soak also stimulated GAL and OX mRNA, supports the idea that ethanol intake under our new model of voluntary ethanol-gelatin intake is sufficient to significantly alter neurochemical processes in the brain.
Whereas these peptides have yet to be examined in zebrafish in relation to their effects on ethanol intake, there is evidence that both GAL and OX mRNA expression in the hypothalamus of fish is stimulated by fasting [61–63] and that intracerebroventricular injection of GAL or OX increases food intake in fish between 1 hr and 8 hrs post-injection [27–29, 61, 64], pointing to their involvement in feeding regulation in fish. While yet to be tested, the possibility that they are involved in promoting ethanol ingestion in fish is supported by abundant evidence in the rodent literature, showing central injection of GAL or OX to stimulate ethanol drinking as well as food intake and genetic manipulation of these peptides to affect behaviors known to predict or be associated with ethanol overconsumption, including novelty seeking, locomotor activity, anxiety and aggression [3, 21, 26, 36, 65]. With this study focusing on the measures of exploration in a novel environment, which is an indicator of anxiety, and aggressive behavior, there is some evidence in the fish literature pointing to the possibility that orexigenic peptides mediate the effects of ethanol on behavior. While little is known about the relationship of these peptides to aggressive behavior and of GAL to ethanol intake, intracerebroventricular injection of OX in goldfish does stimulate locomotor activity [61, 64], a behavior closely associated with the overconsumption of ethanol in the rodent [11, 35], and studies in rats have linked endogenous GAL expression to increased locomotor activity in a novel environment [11] and show central injection of GAL to reduce anxiety [66, 67]. Collectively, our findings demonstrate for the first time a direct relationship between ethanol and the orexigenic peptides in zebrafish, demonstrating that voluntary ingestion of ethanol dramatically changes peptide expression. Future studies are needed to determine whether these peptides, in turn, promote novelty seeking, anxiety and aggression, behaviors which increase the probability of ethanol drinking.
5. Conclusion
This novel model of voluntary ethanol consumption in zebrafish, closely resembling the pattern of ethanol consumption observed in humans and rodents, results in pharmacologically relevant BEC that leads to significant changes in behavior and orexigenic peptides which parallel changes previously observed in other species. These results support the ability of this model to be used for forward genetic studies that may allow one to characterize the effect of a single gene mutation on ethanol consumption and vulnerability to ethanol abuse in zebrafish.
Highlights.
Zebrafish consume stable and pharmacologically-relevant levels of ethanol-gelatin
Ethanol-gelatin intake correlates with blood ethanol concentration
Ethanol-gelatin intake leads to similar blood ethanol levels to ethanol soak
Ethanol-gelatin intake significantly changes behavior and orexigenic peptides
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R01AA12882. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would like to thank Dr. Jessica Barson (The Rockefeller University, New York, NY) for helping with statistical analysis and manuscript preparation, Dr Pertti Panula (University of Helsinki, Finland) for generously donating zebrafish GAL and OX cDNA plasmids, Nathan McKenney and Adedeji Afolalu (The Rockefeller University) for helping to set up our zebrafish facility, Kevin Ying for assisting with fish husbandry, and Dr. Akira Akabayashi for establishing the BEC analysis protocol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barson JR, Morganstern I, Leibowitz SF. Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiol Behav. 2011;104:128–37. doi: 10.1016/j.physbeh.2011.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barson JR, Morganstern I, Leibowitz SF. Neurobiology of consummatory behavior: mechanisms underlying overeating and drug use. ILAR J. 2012;53:35–58. doi: 10.1093/ilar.53.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thanos PK, Wang GJ, Volkow ND. Positron emission tomography as a tool for studying alcohol abuse. Alcohol Res Health. 2008;31:233–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlai R. Zebra fish: an uncharted behavior genetic model. Behav Genet. 2003;33:461–8. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- 7.Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–82. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 8.Mathur P, Berberoglu MA, Guo S. Preference for ethanol in zebrafish following a single exposure. Behav Brain Res. 2011;217:128–33. doi: 10.1016/j.bbr.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dlugos CA, Brown SJ, Rabin RA. Gender differences in ethanol-induced behavioral sensitivity in zebrafish. Alcohol. 2011;45:11–8. doi: 10.1016/j.alcohol.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Mathur P, Guo S. Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behav Brain Res. 2011;219:234–9. doi: 10.1016/j.bbr.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barson JR, Morganstern I, Leibowitz SF. Complementary roles of orexin and melanin-concentrating hormone in feeding behavior. Int J Endocrinol. 2013;2013:983964. doi: 10.1155/2013/983964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubow EF, Boxer P, Huesmann LR. Childhood and adolescent predictors of early and middle adulthood alcohol use and problem drinking: the Columbia County Longitudinal Study. Addiction. 2008;103 (Suppl 1):36–47. doi: 10.1111/j.1360-0443.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 13.Radwanska K, Kaczmarek L. Characterization of an alcohol addiction-prone phenotype in mice. Addict Biol. 2012;17:601–12. doi: 10.1111/j.1369-1600.2011.00394.x. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee D, Gerlai R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav Brain Res. 2009;200:208–13. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatterjee D, Shams S, Gerlai R. Chronic and acute alcohol administration induced neurochemical changes in the brain: comparison of distinct zebrafish populations. Amino Acids. 2014;46:921–30. doi: 10.1007/s00726-013-1658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puttonen HA, Sundvik M, Rozov S, Chen YC, Panula P. Acute ethanol treatment upregulates Th1, Th2, and Hdc in larval zebrafish in stable networks. Front Neural Circuits. 2013;7:102. doi: 10.3389/fncir.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride WJ, Murphy JM, Lumeng L, Li TK. Serotonin, dopamine and GABA involvement in alcohol drinking of selectively bred rats. Alcohol. 1990;7:199–205. doi: 10.1016/0741-8329(90)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2014 doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–9. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leibowitz SF, Avena NM, Chang GQ, Karatayev O, Chau DT, Hoebel BG. Ethanol intake increases galanin mRNA in the hypothalamus and withdrawal decreases it. Physiol Behav. 2003;79:103–11. doi: 10.1016/s0031-9384(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 21.Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010;34:886–96. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belfer I, Hipp H, McKnight C, Evans C, Buzas B, Bollettino A, et al. Association of galanin haplotypes with alcoholism and anxiety in two ethnically distinct populations. Mol Psychiatry. 2006;11:301–11. doi: 10.1038/sj.mp.4001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von der Goltz C, Koopmann A, Dinter C, Richter A, Grosshans M, Fink T, et al. Involvement of orexin in the regulation of stress, depression and reward in alcohol dependence. Horm Behav. 2011;60:644–50. doi: 10.1016/j.yhbeh.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Chang GQ, Karatayev O, Liang SC, Barson JR, Leibowitz SF. Prenatal ethanol exposure stimulates neurogenesis in hypothalamic and limbic peptide systems: possible mechanism for offspring ethanol overconsumption. Neuroscience. 2012;222:417–28. doi: 10.1016/j.neuroscience.2012.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barson JR, Fagan SE, Chang GQ, Leibowitz SF. Neurochemical heterogeneity of rats predicted by different measures to be high ethanol consumers. Alcohol Clin Exp Res. 2013;37 (Suppl 1):E141–51. doi: 10.1111/j.1530-0277.2012.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karatayev O, Baylan J, Leibowitz SF. Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol. 2009;43:571–80. doi: 10.1016/j.alcohol.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Pedro N, Cespedes MV, Delgado MJ, Alonso-Bedate M. The galanin-induced feeding stimulation is mediated via alpha 2-adrenergic receptors in goldfish. Regul Pept. 1995;57:77–84. doi: 10.1016/0167-0115(95)91255-4. [DOI] [PubMed] [Google Scholar]
- 28.Yokobori E, Kojima K, Azuma M, Kang KS, Maejima S, Uchiyama M, et al. Stimulatory effect of intracerebroventricular administration of orexin A on food intake in the zebrafish, Danio rerio. Peptides. 2011;32:1357–62. doi: 10.1016/j.peptides.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda K, Azuma M, Kang KS. Orexin system in teleost fish. Vitam Horm. 2012;89:341–61. doi: 10.1016/B978-0-12-394623-2.00018-4. [DOI] [PubMed] [Google Scholar]
- 30.Panula P. Hypocretin/orexin in fish physiology with emphasis on zebrafish. Acta Physiol (Oxf) 2010;198:381–6. doi: 10.1111/j.1748-1716.2009.02038.x. [DOI] [PubMed] [Google Scholar]
- 31.Woods IG, Schoppik D, Shi VJ, Zimmerman S, Coleman HA, Greenwood J, et al. Neuropeptidergic signaling partitions arousal behaviors in zebrafish. J Neurosci. 2014;34:3142–60. doi: 10.1523/JNEUROSCI.3529-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elbaz I, Yelin-Bekerman L, Nicenboim J, Vatine G, Appelbaum L. Genetic ablation of hypocretin neurons alters behavioral state transitions in zebrafish. J Neurosci. 2012;32:12961–72. doi: 10.1523/JNEUROSCI.1284-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokogawa T, Marin W, Faraco J, Pezeron G, Appelbaum L, Zhang J, et al. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007;5:e277. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podlasz P, Sallinen V, Chen YC, Kudo H, Fedorowska N, Panula P. Galanin gene expression and effects of its knock-down on the development of the nervous system in larval zebrafish. J Comp Neurol. 2012;520:3846–62. doi: 10.1002/cne.23131. [DOI] [PubMed] [Google Scholar]
- 35.Bisaga A, Kostowski W. Individual behavioral differences and ethanol consumption in Wistar rats. Physiol Behav. 1993;54:1125–31. doi: 10.1016/0031-9384(93)90336-e. [DOI] [PubMed] [Google Scholar]
- 36.van Erp AM, Miczek KA. Increased aggression after ethanol self-administration in male resident rats. Psychopharmacology (Berl) 1997;131:287–95. doi: 10.1007/s002130050295. [DOI] [PubMed] [Google Scholar]
- 37.Harper C, Lawrence C. The Laboratory Animal Pocket Reference Series. New York: CRC Press; 2011. The Laboratory Zebrafish. [Google Scholar]
- 38.Francis-Floyd R, Reed P. Management of hexamita in ornamental cichlids (VM 67) 1994 [cited 2014 July 23]; Available from: http://www.extension.org/mediawiki/files/0/02/Management_of_hexamita_in_ornamental_cichlids.pdf.
- 39.Royes J-AB, Chapman F. Preparing your own fish needs (Cir97/FA097) 2009 [cited 2014 June 23]; Available from: http://edis.ifas.ufl.edu/fa097.
- 40.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–10. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- 42.Cachat J, Kyzar EJ, Collins C, Gaikwad S, Green J, Roth A, et al. Unique and potent effects of acute ibogaine on zebrafish: the developing utility of novel aquatic models for hallucinogenic drug research. Behav Brain Res. 2013;236:258–69. doi: 10.1016/j.bbr.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Gerlai R, Ahmad F, Prajapati S. Differences in acute alcohol-induced behavioral responses among zebrafish populations. Alcohol Clin Exp Res. 2008;32:1763–73. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 2013;10:70–86. doi: 10.1089/zeb.2012.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosemberg DB, Rico EP, Mussulini BH, Piato AL, Calcagnotto ME, Bonan CD, et al. Differences in spatio-temporal behavior of zebrafish in the open tank paradigm after a short-period confinement into dark and bright environments. PLoS One. 2011;6:e19397. doi: 10.1371/journal.pone.0019397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong K, Stewart A, Gilder T, Wu N, Frank K, Gaikwad S, et al. Modeling seizure-related behavioral and endocrine phenotypes in adult zebrafish. Brain Res. 2010;1348:209–15. doi: 10.1016/j.brainres.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Gupta T, Mullins MC. Dissection of organs from the adult zebrafish. J Vis Exp. 2010 doi: 10.3791/1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaslin J, Nystedt JM, Ostergard M, Peitsaro N, Panula P. The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J Neurosci. 2004;24:2678–89. doi: 10.1523/JNEUROSCI.4908-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–19. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang GQ, Karatayev O, Leibowitz SF. Prenatal exposure to nicotine stimulates neurogenesis of orexigenic peptide-expressing neurons in hypothalamus and amygdala. J Neurosci. 2013;33:13600–11. doi: 10.1523/JNEUROSCI.5835-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010;208:450–7. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 52.Eames SC, Philipson LH, Prince VE, Kinkel MD. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish. 2010;7:205–13. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pedroso GL, Hammes TO, Escobar TD, Fracasso LB, Forgiarini LF, da Silveira TR. Blood collection for biochemical analysis in adult zebrafish. J Vis Exp. 2012:e3865. doi: 10.3791/3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerlai R. A small fish with a big future: zebrafish in behavioral neuroscience. Rev Neurosci. 2011;22:3–4. doi: 10.1515/RNS.2011.002. [DOI] [PubMed] [Google Scholar]
- 55.Echevarria DJ, Jouandot DJ, Toms CN. Assessing attention in the zebrafish: Are we there yet? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1416–20. doi: 10.1016/j.pnpbp.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 56.NIAAA. NIAAA Newsletter. National Institute of Alcohol Abuse and Alcoholism; Rockville: 2004. NIAAA council approves definition of binge drinking. [Google Scholar]
- 57.Lemon CH, Wilson DM, Brasser SM. Differential neural representation of oral ethanol by central taste-sensitive neurons in ethanol-preferring and genetically heterogeneous rats. J Neurophysiol. 2011;106:3145–56. doi: 10.1152/jn.00580.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scinska A, Koros E, Habrat B, Kukwa A, Kostowski W, Bienkowski P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol Depend. 2000;60:199–206. doi: 10.1016/s0376-8716(99)00149-0. [DOI] [PubMed] [Google Scholar]
- 59.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duke AA, Giancola PR, Morris DH, Holt JC, Gunn RL. Alcohol dose and aggression: another reason why drinking more is a bad idea. J Stud Alcohol Drugs. 2011;72:34–43. doi: 10.15288/jsad.2011.72.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakamachi T, Matsuda K, Maruyama K, Miura T, Uchiyama M, Funahashi H, et al. Regulation by orexin of feeding behaviour and locomotor activity in the goldfish. J Neuroendocrinol. 2006;18:290–7. doi: 10.1111/j.1365-2826.2006.01415.x. [DOI] [PubMed] [Google Scholar]
- 62.Novak CM, Jiang X, Wang C, Teske JA, Kotz CM, Levine JA. Caloric restriction and physical activity in zebrafish (Danio rerio) Neurosci Lett. 2005;383:99–104. doi: 10.1016/j.neulet.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 63.Unniappan S, Cerda-Reverter JM, Peter RE. In situ localization of preprogalanin mRNA in the goldfish brain and changes in its expression during feeding and starvation. Gen Comp Endocrinol. 2004;136:200–7. doi: 10.1016/j.ygcen.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Volkoff H, Bjorklund JM, Peter RE. Stimulation of feeding behavior and food consumption in the goldfish, Carassius auratus, by orexin-A and orexin-B. Brain Res. 1999;846:204–9. doi: 10.1016/s0006-8993(99)02052-1. [DOI] [PubMed] [Google Scholar]
- 65.Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–65. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 66.Moller C, Sommer W, Thorsell A, Heilig M. Anxiogenic-like action of galanin after intra-amygdala administration in the rat. Neuropsychopharmacology. 1999;21:507–12. doi: 10.1016/S0893-133X(98)00102-X. [DOI] [PubMed] [Google Scholar]
- 67.Silote GP, Rosal AB, de Souza MM, Beijamini V. Infusion of galanin into the mid-caudal portion of the dorsal raphe nucleus has an anxiolytic effect on rats in the elevated T-maze. Behav Brain Res. 2013;252:312–7. doi: 10.1016/j.bbr.2013.06.023. [DOI] [PubMed] [Google Scholar]