Abstract
Despite extensive links between reinforcement-based learning and dopamine (DA), studies to date have not found consistent effects of acute DA reduction on reinforcement learning in both men and women. Here, we tested the effects of reducing DA on reward- and punishment-based learning using the deterministic passive avoidance learning (PAL) task We tested 16 (5 female) adults (ages 22–40) in a randomized, cross-over design to determine whether reducing global DA by administering an amino acid beverage deficient in the DA precursors, phenylalanine and tyrosine (P/T[−]), would affect performance on the PAL task. We found that P/T[−] beverage effects on PAL performance were modulated by age. In particular, we found that P/T depletion significantly improved learning from punishment with increasing participant age. Participants committed 1.49 fewer passive avoidance errors per additional year of age (95% CI, −0.71 – −2.27, r=−0.74, p=0.001). Moreover, in this small sample, P/T depletion improved learning from punishment in adults (ages 26–40) while it impaired learning from punishment in emerging adults (ages 22–25). We observed similar, but non-significant trends in learning from reward. While there was no overall effect of P/T-depletion on reaction time (RT), there was a relationship between the effect of P/T depletion on PAL performance and RT; those who responded more slowly on the P/T[−] beverage also made more errors on the P/T[−] beverage. When P/T-depletion slowed RT after a correct response, there was a worsening of PAL task performance; there was no similar relationship for the RT after an incorrect response and PAL task performance. Moreover, among emerging adults, changes in mood on the P/T[−] beverage negatively correlated with learning from reward on the P/T[−] beverage. Together, we found that both reward- and punishment-based learning are sensitive to central catecholamine levels, and that these effects of acute DA reduction vary with age.
Keywords: emerging adults, mood, punishment, reinforcement learning, reward
1. INTRODUCTION
Data from animal models have long established that dopamine (DA) signaling modulates reinforcement learning (Bayer and Glimcher, 2005, Satoh et al., 2003, Schultz, 2002). Links between DA signaling and reinforcement learning have also been established in humans, where changes in striatal DA signaling differentially affect learning from positive and negative feedback (Cools et al., 2006, Cools et al., 2009, Frank, 2005, Frank et al., 2004, Moustafa et al., 2008, Pessiglione et al., 2006, Robinson et al., 2010, Shohamy et al., 2008); however this issue remains incompletely explored. Both reward-based and punishment-based learning are adaptive, and both may depend to some degree on DA signaling. To investigate DA’s role in reinforcement learning, we used a passive avoidance learning (PAL) task, which quantifies learning from both positive and negative feedback (Newman and Kosson, 1986), in the context of acute DA precursor depletion in healthy human subjects.
Successful PAL task performance involves a learning period followed by a plateau phase. Neuroimaging data indicate that increased activation with successful PAL task learning, in response to both rewarded or punished stimuli, occurs in the rostral anterior cingulate, insula, caudate, and amygdala, which are all DA terminal fields (Kosson et al., 2006). The role of DA in PAL task performance has been investigated using acute DA-depletion with alpha-methyl-paratyrosine (AMPT), a competitive inhibitor of the rate-limiting enzyme in DA synthesis, in females with major depressive order in full remission and healthy controls (Hasler et al., 2009). All participants were less likely to respond to rewarded stimuli later in the task than in earlier in the task, but there was no effect of AMPT on responding to punished stimuli (Hasler et al., 2009).
To date, no studies in medically healthy males and females have examined the effect of acute DA-depletion on PAL task performance. Therefore, we tested whether reducing DA levels with an amino acid beverage deficient in the amino acids required for DA synthesis, phenylalanine and tyrosine, would affect PAL task performance in both medically healthy males and females. We hypothesized that the phenylalanine/tyrosine-depleted (P/T[−]) beverage would decrease responding to rewarded stimuli and increase responding to punished stimuli compared to the controlled/balanced amino acid beverage. Moreover, the lower age bound for recruitment to this study was set at 22 years based on published data showing that functional brain maturation asymptotes at ~22 years of age (Dosenbach et al., 2010). However, abundant evidence indicates that the emerging adult period, most commonly defined as ages 18–25 (Arnett, 2000), is distinct from adulthood in numerous respects. Although emerging adulthood has been defined largely based on cultural factors, emerging adulthood is also characterized by ongoing neural development in brain regions associated with self-regulation and inhibitory control (Sowell et al., 1999), which play a role in passive avoidance learning. Furthermore, recent data show impaired inhibitory control within an affective information processing context among emerging adults relative to adults (Cohen-Gilbert et al., 2014). As such, we also separated participants into emerging adult (22–25 years old) and adult (26–40 years old) groups to determine whether the response to dopamine depletion differed among emerging adults.
2. METHODS
2.1 Participants
Participants (n=16, 5 females) were recruited from the University of North Carolina at Chapel Hill (UNC) and surrounding community. Participants were 22–40 yr old native English speakers with a high school education or more. Subjects were free from psychoactive medications or illicit drug use and had no current psychiatric or neurological diagnosis. Smokers were also excluded. Females were not breastfeeding or pregnant (confirmed via urine test), and were tested during the follicular phase (d 1–10) of the menstrual cycle. Participants with phenylketonuria were also excluded. Participants gave written, informed consent in accordance with the guidelines of the UNC Office for Human Research Ethics. Participants were paid for their participation; payment did not depend upon performance. Nine additional subjects were tested, but were excluded from all analyses due to a programming error that rendered their data unusable.
2.2 Procedure
In a double-blind, placebo-controlled, within-subjects, counterbalanced design, we used acute P/T depletion to temporarily reduce central DA levels, using a previously described protocol (Kelm and Boettiger, 2013). Subjects consumed a low protein diet (<20 g) for 24h before each session and fasted from midnight until session onset, which occurred between 7–9 A.M. Following the consent procedure and urine screening, participants completed the Profile of Mood States (POMS) (McNair et al., 1971), and we collected a baseline blood sample via finger prick. Participants then consumed an amino acid beverage (balanced/control or P/T[−]). Participants waited 5h in the lab to allow sufficient time for P/T depletion to occur (Sheehan et al., 1996). We then collected a second blood sample, and the participant completed computerized cognitive testing, followed by the POMS. Participants had access to low protein snacks from 1h post-beverage consumption to 1h before the second blood sample collection. Participants were offered a high protein snack at session end. Sessions were separated by ≥72h.
2.3 Amino Acid Beverages
The amino acid mixes were prepared by SHS International (Liverpool, UK). The balanced/control beverage consisted of (in g): L-alanine, 4.1; L-arginine, 3.7; L-cysteine, 2.0; L-glycine, 2.4; L-histidine, 2.4; L-isoleucine, 6; L-leucine, 10.1; L-lysine, 6.7; L-methionine, 2.3; L-phenylalanine, 4.3; L-proline, 9,2; L-serine, 5.2; L-threonine, 4.9; L-tryptophan, 3.0; L-tyrosine, 5.2; and L-valine, 6.7. The P/T[−] beverage had the same composition except that phenylalanine and tyrosine were omitted. Because these amino acid beverages can cause nausea and emesis, individuals weighing <160lb (n=6) received a light version of each beverage that was reduced in composition by 20%. Beverages were mixed with cold water and a cherry-vanilla, grapefruit, or lemon-lime flavor packet from Nutricia (Gaithersburg, MD) in an 8oz sterile cup.
2.4 Behavioral Inventories
Participants completed questionnaires during the waiting period in session one. Demographic information was collected, including age, gender, ethnicity and socioeconomic status (SES). SES was quantified according to (Hollingshead, 1975) using the modification of (Barratt, 2006). Other standard questionnaires included: the Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al., 1993), the Barratt Impulsiveness Scale (BIS) (Barratt, 1994), part I of the Drug Use Screening inventory (DUSI) (Tarter, 1990), the Family Tree Questionnaire (FTQ) (Mann et al., 1985), part I of the Future Time Perspective Inventory (FTPI) (Wallace, 1956), Rotter’s Locus of Control Scale (LOC) (Rotter, 1966), the State-Trait Anxiety Inventory (STAI) (Spielberger, 1985), the Beck Depression Inventory (BDI) (Beck and Steer, 1987), and the Anti-social Practices Scale of the Minnesota Multiphasic Personality Inventory 2 (MMPI) (Butcher et al., 1990). Age groups did not differ on any of these measures, excepting age (Table 1).
Table 1.
A comparison of the emerging adult (22–25 years old) and adult groups (26–40 years old).
| Emerging Adults (n = 7) |
Adults (n = 9) |
t(14) | p value | |
|---|---|---|---|---|
| General | ||||
| Age | 23.14 ± 0.46 | 29.44 ± 1.58 | −3.83a | 0.004 |
| SES | 52.25 ± 1.62 | 53.64 ± 2.46 | −0.44 | ns |
| Gender (% female) | 28.6 | 33.3 | ns† | |
| Ethnicity (% non-white) | 14.3 | 33.3 | ns† | |
| Personal and Familial Substance Use | ||||
| AUDIT | 4.29 ± 0.87 | 5.56 ± 1.24 | −0.79 | ns |
| DUSI | 0.12 ± 0.04 | 0.11 ± 0.05 | 0.22 | ns |
| FTQ | 0.23 ± 0.06 | 0.13 ± 0.06 | 1.13b | ns |
| Psychometric Measures | ||||
| BIS | 59.00 ± 1.84 | 57.56 ± 1.59 | 0.60 | ns |
| FTPI-Mean Extension | 11.70 ± 3.31 | 8.78 ± 2.42 | 0.73 | ns |
| FTP1-Max Extension | 42.29 ± 9.46 | 35.44 ± 9.85 | 0.49 | ns |
| MMPI | 8.00 ± 1.23 | 6.78 ± 1.27 | 0.68 | ns |
| BDI | 3.86 ± 1.06 | 3.00 ± 0.82 | 0.65 | ns |
| STAI-Trait Score | 30.29 ± 1.39 | 34.78 ± 2.72 | −1.35 | ns |
| LOC | 11.71 ± 0.68 | 10.11 ± 1.09 | 1.25c | ns |
2.5 Amino Acid Analysis
We analyzed the pre- and post-beverage blood samples to determine the total plasma P/T levels and the ratio of P/T to other large, neutral amino acids (LNAA; tryptophan, valine, isoleucine, and leucine) to confirm P/T depletion by the P/T[−] beverage. This ratio was calculated from the total serum concentrations of P and T divided by the sum of the concentrations of the other LNAA [(P+T)/ΣLNAA]. We used this combined P/T ratio, as it correlates with DA availability within the brain (Montgomery et al., 2003). Using aseptic technique, we used a contact-activated lancet (BD Biosciences, San Jose, CA) to collect 150µL of blood from the finger. Samples were frozen at −20°C prior to analysis. Amino acid analysis was carried out via gas chromatography-mass spectroscopy as described previously (Kelm and Boettiger, 2013).
2.6 Passive Avoidance Learning Task
The PAL task was adapted from (Newman and Kosson, 1986) and implemented in E-Prime 2.0 (Psychology Software Tools, Inc. (PST), Pittsburgh, PA). Subjects were given task instructions and a short practice session prior to testing. Cognitive testing occurred within the bore of a mock MRI scanner (PST). Stimuli were presented on a color LCD screen, which subjects viewed via a head-coil mounted mirror. Participants made manual responses via a keypad. In the task, participants were presented with a series of numerical stimuli to which they either responded with, or withheld, a manual button press. Stimuli included six unique two-digit “good” numbers, and six unique two-digit “bad” numbers. Stimulus sets were counterbalanced for good versus bad designation across subjects to control for any pre-existing positive or negative associations with the number stimuli among participants. Participants completed a unique version of the task during each session. Within session, all twelve numbers were presented once in pseudorandom order during each block; each participant completed ten blocks. Each number was displayed in white Arial font on a black background for up to 3s. Participants learned through trial and error feedback which numbers were good or bad. Participants began the task with 5000 points, displayed in the bottom right-hand corner of the screen, and point total was updated in each trial. Participants received 400 points when responding to a good number and lost 400 points when responding to a bad number. There was no feedback when a response was withheld. Participants were instructed to accrue as many points as possible. Therefore, following learning, participants should respond to all good numbers, and withhold responding to all bad numbers. Response type (press or omission) and press reaction time (RT) were recorded for all trials.
2.7 Statistical analysis
Performance on the PAL task was assessed by analysis of omission errors (OEs, failures to respond to rewarded stimuli) and passive avoidance errors (PAEs, responses to punished stimuli). For single factor statistical comparisons within and between groups, we used paired and unpaired two-tailed t-tests, respectively. For multi-factorial comparisons, we used repeated measures ANOVA. Linear regression was used to determine if any of the demographic information, behavioral inventories, or reaction times correlated with the effect of P/T depletion on OEs and PAEs. All statistical tests were conducted using Excel (Microsoft Corp., Redmond, WA) or SPSS (SPSS Inc., Chicago, IL). Values reported as mean ± SEM, unless otherwise stated. Effect sizes for ANOVA are reported as η2; effect sizes for t-tests are reported as Cohen’s d.
3. RESULTS
3.1 Effects of P/T depletion on blood concentrations
Repeated measures ANOVA revealed significant two-way interactions between beverage (P/T[−], placebo) and time point (baseline, +5 h) on serum phenylalanine levels (F(1,15)=78.6; p<0.001, η2=0.35), serum tyrosine levels (F(1,15)=87.8; p<0.001, η2=0.33), and the P/T/ΣLNAA ratio (F(1,15)=101.8; p<.001, η2=0.14), reflecting greater reductions for the P/T[−] beverage relative to control. Simple effects analyses demonstrated that consumption of the P/T[−] beverage reduced the serum concentration of phenylalanine by an average of 45.6% (95% CI: 42.2–48.9%; t(15)=12.91, p<.001) and the serum concentration of tyrosine by an average of 47.9% (95% CI: 45.7–50.1%; t(15)=13.26, p<.001). In contrast, consumption of the control beverage increased the serum concentration of phenylalanine by an average of 32.6% (95% CI: 25.4–39.8%; t(15)=−4.79, p<.001) and the serum concentration of tyrosine by an average of 89.5% (95% CI: 75.2–1.04%; t(15)=−6.41, p<.001) in the blood. Finally, consumption of the P/T[−] beverage decreased the P/T/ΣLNAA ratio by an average of 79.2% (95% CI: 76.7–81.7%; t(15)=20.2, p<0.001), while the control beverage decreased the P/T/ΣLNAA ratio by an average of 27% (95% CI: 22.6–31.4%; t(15)=5.57, p<0.001). These data demonstrate the efficacy of our P/T-depletion protocol, and based on the relationship between the peripheral P/T/ΣLNAA ratio and DA availability within the brain (Montgomery et al., 2003), we infer successful reduction of central DA.
3.2 Effects of P/T depletion on blood pressure and mood state
P/T depletion has been reported to cause decreases in systolic and diastolic blood pressure (Moja et al., 1996). However, we observed no significant effect on either systolic blood pressure (time × beverage interaction, F(1,15)=0.47; p=0.50, η2=0.01) or diastolic blood pressure (time × beverage interaction, F(1,15)=0.08; p=0.79, η2=0). The amino acid beverage formulation in Moja and colleagues’ study included only seven amino acids, while the formulation used here includes sixteen amino acids. Thus, differences in beverage formulations could account for the differing effects on blood pressure.
A recent meta-analysis found that P/T depletion does not alter mood in healthy controls (Ruhe et al., 2007), which we confirmed here. Participants completed the POMS questionnaire before consuming the amino acid beverage and at session end. A time by beverage repeated measures ANOVA found no significant interacting effect on POMS scores (F(1,15)=0.17; p=0.69, η2=0). We also found no main effects of time or beverage (max. F=0.48, min. p=0.5).
3.3 Age-dependent effects of P/T depletion on learning from punishment
To quantify participants’ ability to learn from punishment, we calculated incorrect responses to “bad” stimuli, so called “passive avoidance errors” (PAEs). A mixed measured ANOVA (beverage × task block), found no significant effect of P/T depletion on PAEs (F(1,15) = 0.68, p = 0.42; Fig. 1a). We did find a significant effect of task block (F(8,120)=33.6; p<0.001, η2=0.45), reflecting a decrease in PAEs across blocks, indicating successful learning to avoid punishment (Fig. 1b). We detected no significant beverage × block interaction (F(8,120)=0.86; p=0.55, η2=0.01; Fig. 1b), indicating that P/T depletion did not affect the rate at which participants learned from punishment.
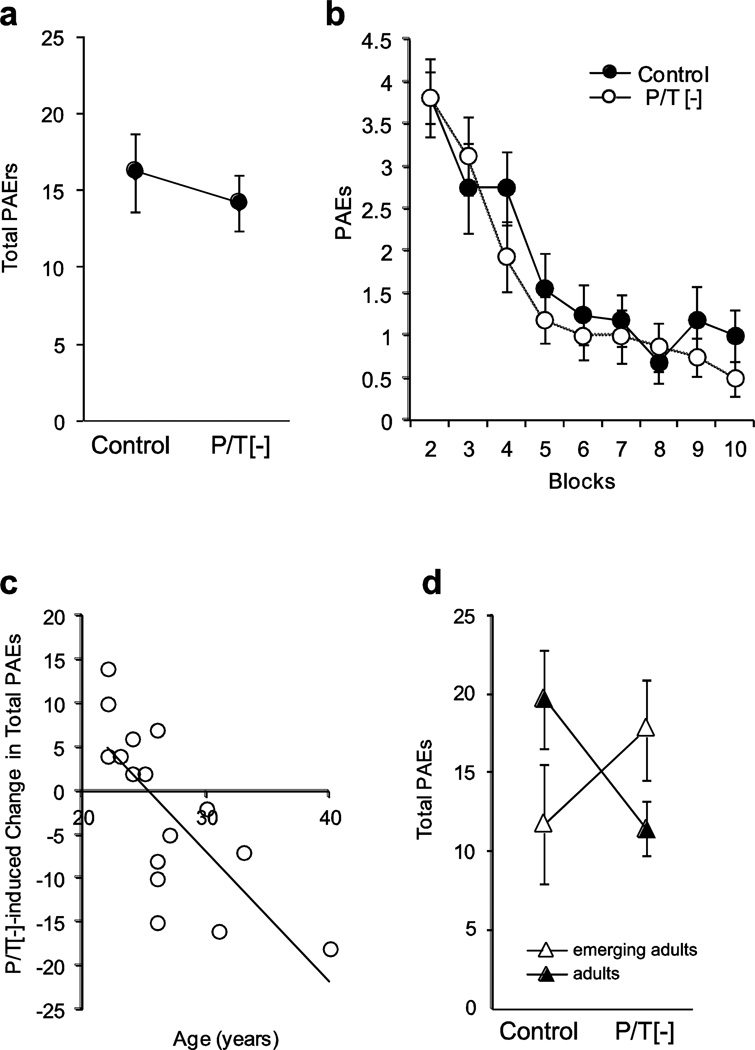
Figure 1.
Effect of P/T depletion on PAEs is age dependent. a) There is no effect of P/T depletion on total PAEs b) There is no effect of P/T depletion on PAEs when the data are separated by block. c) There is a significant correlation between the age of the participant and the effect of P/T depletion on PAEs (95% CI, −0.71– −2.27, r=−0.74, p=0.001). d) When participants are separated into emerging adult (22–25 years old) and adult (26–40 years old) groups, there is a significant beverage×group interaction (F(1,14)=18.35; p=0.001, η2=0.07), where the adult participants (n = 9) perform better on the P/T[−] beverage (t(8)=3.2, p=0.007, Cohen’s d=−0.88), and the emerging adult participants (n = 7) perform worse (t(6)=−3.6, p=0.006, Cohen’s d=−0.60). PAEs, Passive Avoidance Errors; P/T[-], phenylalanine/tyrosine-depleted beverage.
A variety of evidence suggests age-dependent changes in DA signaling in emerging adults relative to “full” adults (Smith and Boettiger, 2012, Tunbridge et al., 2007, Wahlstrom et al., 2010). Therefore, we evaluated whether P/T depletion effects on PAE interacted with age. First, considering age as a continuous variable, we found that P/T depletion reduced the rate of PAEs with increasing age by 1.49 PAEs/year (95% CI, −0.71–−2.27, r=−0.74, p=0.001, Fig. 1c). In other words, P/T depletion tended to reduce the PAEs of older participants, while it tended to increase the PAE among emerging adult participants. When age was included as a covariate in our repeated measures ANOVA (beverage × block), we found a significant effect of beverage on PAEs (F(1,14) = 14.66, p = .002, η2=0.09), as well as a significant beverage*age interaction (F(1,14)=16.75, p=0.001, η2=0.10). Examining PAEs in the control beverage session as a function of age, we found 1.22 additional PAEs with each additional year of age (95% CI, 0.24–2.21, r=0.58, p=0.019). In other words, on the control beverage, increasing age was associated with decreased ability to learn from punishment.
We next separated participants into emerging adult (22–25 years old) and adult (26–40 years old) groups to determine whether the response to dopamine depletion differed among emerging adults. We found a significant beverage by age group interaction (FS=18.35; p=0.001, η2=0.07; Fig. 1d), which reflected the fact that P/T depletion reduced PAEs among the adults (t(8)=3.2, p=0.007, Cohen’s d=−0.88), while it increased PAEs among the emerging adults (t(6)=−3.6, p=0.006, Cohen’s d=−0.60). A comparison of the emerging adult and adult groups found no significant differences (aside from age) in their demographics, personal and familial substance use, or psychometric measures (Table 1).
3.4 Age-dependent effects of P/T depletion on reward learning
In the PAL task, in addition to learning from punishment, participants also learn from reward, which is quantified in terms of omission errors (OEs). Taking OEs as our dependent measure, a mixed measured ANOVA (beverage × task block) found no significant main effect of beverage (F(1,15)= 0.82, p=0.38, η2=0.03; Fig. 2a) or task block (F(2.97, 56.07)=1.19, p=0.32, η2=0.02), and no significant beverage by block interaction (F(3.76,56.37)=1.26; p=0.30, η2=0.02; Fig. 2b).
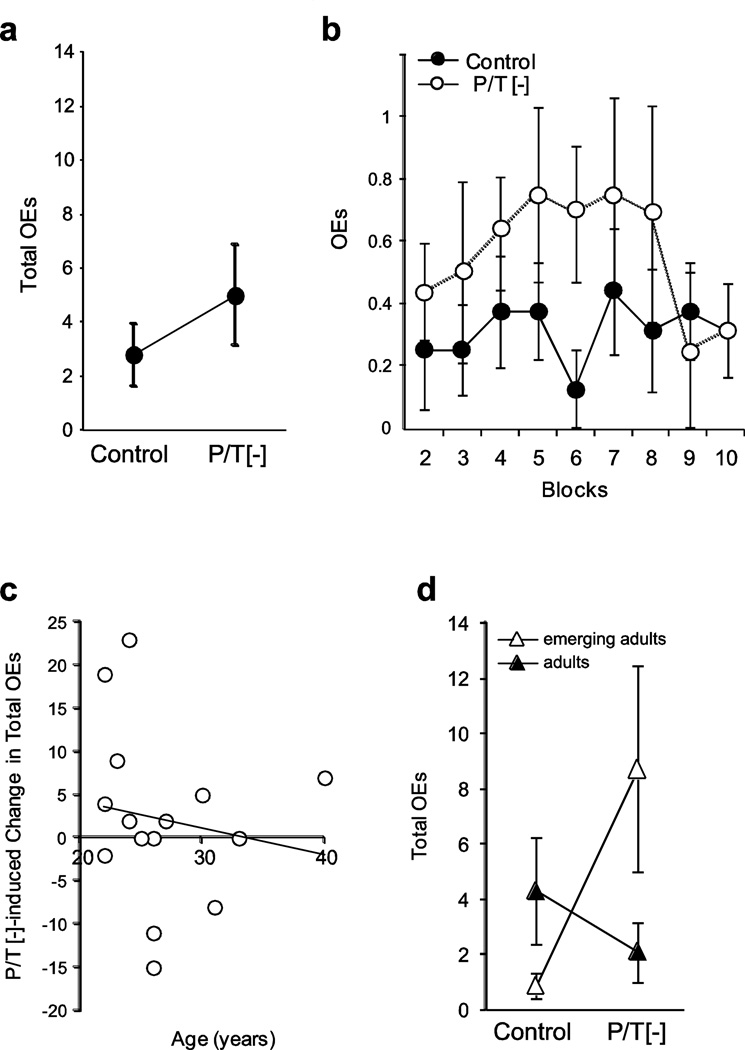
Figure 2.
Effect of P/T depletion on OEs is age dependent. a) There is no effect of P/T depletion on total OEs b) There is no significant beverage × block interaction on OEs. c) There is not a significant correlation between the age of the participant and the effect of P/T depletion on PAEs. d) When participants are separated into emerging adult (22–25 years old) and adult (26–40 years old) groups, there is a significant beverage × age group interaction (F(1,14)=5.57; p=0.033, η2=0.26). OEs, Omission Errors; P/T[−], phenylalanine/tyrosine-depleted beverage.
Given the age moderation of P/T depletion effects on PAEs, we examined whether the effect of P/T depletion on OE performance also varied with age. In considering age as a continuous variable, we found a small, non-significant decline in OEs with increasing age (0.30 errors/year; 95% CI: −1.45–0.84, r=−0.15, p=0.58; Fig. 2c). When we included age as a covariate in our beverage × block ANOVA, we found no significant main or interacting effects of age on OEs (max. F=1.07; min. p=0.38). Examining control session performance as a function of age, in contrast to the PAE data, we found no significant correlation between OEs and age (r=0.093, p=0.73). When participants were separated into emerging adult and adult groups (as for the PAE analysis in the previous section), we found a large and significant beverage by age group interaction effect on total OEs (F(1,14)=5.57; p=0.033, η2=0.26; Fig. 2d), which reflected a trend toward 10-fold greater OEs after P/T depletion among emerging adults (t(6)=−2.45, p=0.075, Cohen’s d=6.5). In contrast, adults showed a small, statistically insignificant decrease in OEs following P/T depletion (t(8)=0.90, p=0.40, Cohen’s d=−0.39).
3.5 Effects of P/T depletion on reaction times
Profound DA depletion should induce Parkinsonian motor deficits, which could confound detection of cognitive effects. Thus, we assessed the effect of P/T depletion on aspects of motor function (Table 2). We found no significant main effect of P/T depletion on overall task RT (F(1,15)=0.05; p=0.83, η2=0); this finding was not altered by including age as either a covariate (F(1,14)=0.46; p=0.51, η2=0.03) or a factor (F(1,14)=0.01; p=0.94, η2=0). We also observed no main or interacting effects of age on overall RT (max. F=1.39, min. p=0.26). We next investigated whether individual differences in P/T depletion effects on RT correlated with effects on PAL task performance. We found that P/T depletion effects on RT were positively correlated with effect on OEs (r=0.60, p=0.01). In other words, more slowing after P/T depletion was associated with more OEs. A similar trend was seen in the relationship between P/T depletion effects on PAEs and RT (r=0.43, p=0.10).
Table 2.
The effect of P/T depletion on RT
| Control | P/T[−] | |||
|---|---|---|---|---|
| Full Sample | t(15) | p value | ||
| Overall Reaction Time (RT) | 953.2 ± 32.9 | 945.8 ± 27.7 | 0.22 | 0.83 |
| Post Error Response RT | 995.4 ± 52.0 | 943.8 ± 45.5 | 0.75a | 0.46 |
| Post Correct Response RT | 951.9 ± 32.9 | 965.9 ± 32.1 | −0.31 | 0.76 |
| Post Error Slowing | 30.7 ± 46.8 | −31.3 ± 51.9 | 0.98 | 0.35 |
| Emerging Adults | t(12) | p value | ||
| Overall RT | 917.2 ± 34.3 | 954.3 ± 36.6 | −0.74 | 0.47 |
| Post Error Response RT | 1004.9 ± 90.2 | 948.9 ± 74.1 | 0.48 | 0.64 |
| Post Correct Response RT | 911.4 ± 34.1 | 990.7 ± 44.3 | −1.42 | 0.18 |
| Post Error Slowing | 93 ± 85.6 | −41.9 ± 109.3 | 1.24 | 0.26 |
| Adults | t(16) | p value | ||
| Overall RT | 981.2 ± 51.9 | 939.1 ± 42.0 | 0.63 | 0.54 |
| Post Error Response RT | 987.0 ± 63.3 | 939.3 ± 60.5 | 0.55b | 0.59 |
| Post Correct Response RT | 983.3 ± 51.4 | 946.6 ± 46.8 | 0.53 | 0.60 |
| Post Error Slowing | −24.3 ± 42.3 | −22.0 ± 69.9 | −0.03 | 0.97 |
Values are reported as mean ± standard deviation. Reported p-values reflect the results of paired two-tailed comparisons for the effect of the beverage. Exact p-values reported unless p < 0.001.
df=28,
df=14.
In addition to investigating overall RT, we also examined effects of P/T depletion on post-error slowing, a marker of response-monitoring (Dutilh et al., 2012, Rabbitt, 1966). We found that P/T depletion had no significant effects on post-error response RT, post-correct response RT, or post-error slowing, either in the sample as a whole or within the adult or emerging adult groups (Table 2). Likewise, an age group by beverage ANOVA found no significant main or interacting effects on post-error slowing (max. F=1.19, min. p=0.30). After accounting for effects of age, we found no relationship between P/T depletion effects on post-error slowing and OEs (β= −0.33, t=−1.21, p=0.25); however, P/T depletion-induced change in post-error slowing negatively correlated with the change in PAEs (β= −0.38, t=−2.44, p=0.031). Examining post-error and post-correct RTs separately, after accounting for effects of age, we observed a relationship between the effect of P/T depletion on OEs and on the post-correct response RT (Table 3). We also found a positive relationship between the effect of P/T depletion on PAEs and on the post-correct response RT (Table 3). Thus, the more that P/T depletion slowed RT after correct responses, the more P/T depletion increased errors. In contrast, we found no relationship between the effect of P/T depletion on post-error response RT and either OEs (β=0.06, t=0.2, p=0.85) or PAEs (β= −0.15, t=−0.81, p=0.44).
Table 3.
Factors Predicting the Effect of P/T-depletion on PAL Task Performance
| B | SE B | β | p | |
|---|---|---|---|---|
| Omission Errors | ||||
| Full sample model | ||||
| Constant | 2.10 | 10.8 | 0.849 | |
| Age | −0.001 | 0.398 | −0.001 | 0.997 |
| Post-correct RT change | 0.046 | 0.014 | 0.705 | 0.006 |
| POMS change | −0.085 | 0.237 | −0.075 | 0.726 |
| Adults | ||||
| Constant | −15.7 | 9.95 | 0.176 | |
| Age | 0.522 | 0.332 | .399 | 0.176 |
| Post-correct RT change | 0.025 | 0.011 | .568 | 0.067 |
| POMS change | 0.226 | 0.197 | .293 | 0.302 |
| Emerging Adults | ||||
| Constant | −27.1 | 32.4 | 0.464 | |
| Age | 1.54 | 1.43 | .193 | 0.360 |
| Post-correct RT change | 0.040 | 0.015 | .454 | 0.075 |
| POMS change | −1.02 | 0.239 | −.780 | 0.023 |
| Passive Avoidance Errors | ||||
| Full sample model | ||||
| Constant | 31.9 | 8.60 | 0.003 | |
| Age | −1.29 | 0.316 | −0.640 | 0.002 |
| Post-correct RT change | 0.029 | 0.011 | 0.403 | 0.026 |
| POMS change | 0.141 | 0.188 | 0.115 | 0.470 |
| Adults | ||||
| Constant | 20.1 | 13.9 | 0.208 | |
| Age | −0.915 | 0.464 | −0.556 | 0.106 |
| Post-correct RT change | 0.035 | 0.015 | 0.624 | 0.070 |
| POMS change | 0.130 | 0.275 | 0.134 | 0.656 |
| Emerging Adults | ||||
| Constant | 54.8 | 38.3 | 0.248 | |
| Age | −2.07 | 1.69 | −0.562 | 0.307 |
| Post-correct RT change | −0.005 | 0.018 | −0.111 | 0.815 |
| POMS change | −0.150 | 0.283 | −0.248 | 0.632 |
Results from multiple linear regression analysis of predictors of the P/T-depletion effect on PAL task performance.
B: beta value; SE B: beta value standard error; β: standardized beta.
3.6 Relationship between P/T depletion effects on mood state on and PAL task performance
Based on our finding that P/T depletion appears to diminish reward-learning, possibly by dampening normal responses to positive feedback, we investigated whether P/T depletion effects on mood state could also predict learning performance. When the effect of P/T depletion on POMS scores was added as a regressor in a model predicting change in OEs in the whole sample, it did not predict significant variance (β= −0.08, t=−0.36, p=0.73) beyond that predicted by age and change in post-correct RT. We also found no significant relationship between the effect of P/T depletion on PAEs and on POMS scores (β=0.12, t=0.75, p=0.47). When considering the age groups separately, among adults, we again found no significant relationships between changes in mood and changes in errors (min. p=0.3). In contrast, within the emerging adult group, we found that change in POMS scores predicted substantial variance in P/T depletion effect on OEs (β= −0.78, t=−4.28, p=0.023), after accounting for age and change in post-correct RT change. Thus, among emerging adults, greater declines in mood following P/T depletion resulted in more OE following P/T depletion. No such relationship was observed between POMS scores and PAEs among emerging adults (β= −0.25, t=−0.53, p=0.63).
4. DISCUSSION
4.1 Dopamine depletion and sensitivity to punishment
A variety of evidence supports the idea that reducing striatal DA signaling improves learning from negative feedback, or punishment, whereas increases in striatal DA signaling may improve learning from positive feedback, or reward (Cools et al., 2006, Cools et al., 2009, Frank, 2005, Frank et al., 2004, Moustafa et al., 2008, Pessiglione et al., 2006, Robinson et al., 2010, Shohamy et al., 2008). Given the decline in DA signaling following late adolescence (Wahlstrom et al., 2010), one would expect to find age-dependent improvement in learning from punishment, which is what we observed here: the ability to learn from negative feedback in the control session correlated with age: the older participants were, the better they learned from punishment. In addition, acute reduction in central DA via P/T depletion improved learning from punishment among adults. Here again we observed age-dependent effects, where the older participants were, the more DA depletion improved their ability to learn from punishment. Paradoxically, however, among emerging adults, acute DA depletion actually impaired learning from punishment. The degree to which learning from punishment was impaired by DA depletion was predicted by the degree to which DA depletion slowed RT after correct responses. Such trial-to-trial changes in RT during feedback-based learning may reflect DA depletion effects in extrastriatal sites, such as the prefrontal cortex (Moustafa et al., 2008). Why emerging adults would be more sensitive to DA depletion in extrastriatal sites remains unclear. One possibility is that baseline differences in frontal DA tone may differ between adults and emerging adults. While data from non-human primates indicate that DA enervation of the PFC peaks in adolescence (Lambe et al., 2000, Rosenberg and Lewis, 1995), and DA receptor expression reaches adult levels in adolescence (Lidow and Rakic, 1992), expression of the catechol-o-methyltransferase (COMT) enzyme increases from emerging adulthood to adulthood (Tunbridge et al., 2007). As COMT catabolism of DA regulates tonic DA levels in the PFC (Gogos et al., 1998, Karoum et al., 1994, Tunbridge et al., 2004, Wu et al., 2012), emerging adults may have elevated levels of tonic DA in the PFC relative to adults. To our knowledge, this hypothesis has not been tested in human subjects. Moreover, while P/T depletion decreases both tonic DA and DA release in the striatum of adults (Leyton et al., 2004, Montgomery et al., 2003), no data is available regarding its effect on either tonic or phasic DA in the PFC, and no data is available regarding P/T depletion effects in emerging adults.
4.2 Dopamine depletion and sensitivity to reward
It is unclear why P/T depletion causes an improvement in PAL performance in adults and a worsening in PAL performance in emerging adults. Among emerging adults, we found that those who experienced a more negative mood state following P/T-depletion were also more likely to fail to respond to rewarding stimuli following P/T-depletion. We observed no such relationship between P/T-depletion effects on mood and reward-sensitivity in adults. Therefore, it is possible that the effect of reducing DA in emerging adults is not purely cognitive and also involves an affective aspect. Given data showing developmental changes in DA signaling (Kaasinen et al., 2002, Kaasinen et al., 2000), our data suggest that age-related differences in DA signaling underlie developmental changes in reward-sensitivity (Jarcho et al., 2012). Our data suggest that emerging adults are more sensitive to dysphoric effects of acute P/Tdepletion, which induces a reduction in DA signaling. As dopamine depletion has long been hypothesized to serve as the physiological basis of cocaine dependence (Dackis and Gold, 1985), this may have implications for substance abuse risk. Indeed, loss of phasic dopamine has recently been proposed as a marker of addiction (Caprioli et al., 2014) on the basis of recent studies in rodents (Willuhn et al., 2014). Notably, emerging adulthood is associated with both peak experimental drug use (Kandel and Logan, 1984) and the peak of substance use disorder onset (Kessler et al., 2005). It is tempting to speculate that peculiarities of the emerging adult dopamine system render this age cohort more vulnerable to the dopamine depleting effects of drugs of abuse, which may in turn drive the compulsive use that can lead to addiction.
4.3 Motor effects of DA depletion
There was a relationship between the effect of the reduction in DA on PAL task performance and RT, where the individuals who performed poorly following DA-depletion also slowed their RT following DA-depletion. This RT effect appears to be specifically driven by the post-correct response RT, where individuals slowed down after making a correct response following DA-depletion. One possible explanation for this effect is that reduced DA levels rendered participants more distracted by rewarding feedback, which in turn could slow their RT on the next trial. These motor effects provide a confirmation that central DA is being depleted by P/T-depletion.
4.4 Study limitations
We acknowledge some limitations of the present study. First, our sample size is rather modest, so although some of effect sizes were rather large, these findings bear replicating. Moreover, any negative findings here may result from lack of power. In addition, we acknowledge some limitations to the method we used to manipulate DA, acute P/T-depletion. Although studies in rats (McTavish, Cowen, & Sharp, 1999) and humans (Leyton et al., 2004) show that P/T-depletion reduces DA release in the striatum by 30% or more, lowering DA levels in the brainstem could theoretically reduce DRD2-mediated autoinhibition of DA neurons, thereby increasing DA release in other projection regions. However, human PET studies demonstrate that the degree to which P/T-depletion reduces DA receptor occupancy in the striatum predicts the level of executive function impairment (Mehta, Gumaste, Montgomery, McTavish, & Grasby, 2005); P/T-depletion effects on extrastriatal targets have not been adequately investigated. Beyond changes in DA within target regions, P/T-depletion reduces frontostriatal connectivity (Nagano-Saito et al., 2008). While both rodent and human studies suggest that P/T-depletion does not affect norepinephrine (NE) dependent processes (Leyton et al., 2004; McTavish, Callado, Cowen, & Sharp, 1999; McTavish et al., 2001; B. Sheehan et al., 1996), one study found that P/T depletion reduces levels of a NE metabolite (Palmour, Ervin, Baker, & Young, 1998). However, acute P/T-depletion does not affect NE-regulated melatonin levels, while it does alter DA-regulated prolactin levels (Harmer et al., 2001; B. Sheehan et al., 1996). Therefore, while P/T-depletion effects on NE cannot be completely ruled out, available evidence suggests that any such effects are relatively minimal. Another limitation of P/T-depletion is that it is subtler than the profound global forebrain DA depletions used in animals. Given the profound dependence of motor function on DA, the subtlety of the effects of P/T-depletion on cognition are advantageous in that it allows detection of behavioral effects without confounding motor impairment.
CONCLUSIONS
In a sample of healthy adults, the effects of acute reduction in DA signaling on positive- and negative-reinforcement based learning in the deterministic PAL task was modulated by age. Specifically, with DA depletion had increasingly beneficial effects on learning from punishment with increasing age. In this small sample, DA depletion impaired PAL performance in emerging adults (ages 22–25), but improved PAL performance in more mature adults (ages 26–40). Effects on learning from reward were qualitatively similar, but did not reach statistical significance; this negative finding may reflect a lack of statistical power to detect an effect. In addition, while we failed to detect a global slowing of RT’s, those who responded more slowly after DA depletion also made more errors in the DA depletion condition. Moreover, when DA depletion slowed RT after correct responses, PAL task performance was degraded; this was specific to the positive feedback condition. Finally, among emerging adults, changes in mood following DA depletion negatively correlated with learning from positive feedback. Together, these data indicate that both positive- and negative-feedback based learning are sensitive to central catecholamine levels, and that these effects of acute DA reduction vary with age.
Highlights.
Dopamine depletion effects on passive avoidance learning are modulated by age.
Dopamine depletion improves learning from punishment with increasing age.
In emerging adults, depleting dopamine affects reward learning together with mood.
ACKNOWLEDGEMENTS
This work was supported by NIH awards KL2RR025746, UL1RR025747, and P60AA011605, and an IBM Junior Faculty Award (CAB), and by F32AA019838 and T32AA007573 (MKK), and by the UNC Nutrition Obesity Research Center (P30DK056350). We thank M. Beck, C. Green, Q. Shi, and E. Steel and for their assistance on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests in relation to the work described. Authors declare no conflict of interest.
REFERENCES
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American psychologist. 2000;55:469. [PubMed] [Google Scholar]
- Barratt ES. Violence and mental disorder: Developments in risk assessment. Chicago: University of Chicago Press; 1994. Impulsiveness and aggression; pp. 61–79. [Google Scholar]
- Barratt W. The Barratt Simplified Measure of Social Status (BSMSS) measuring SES. Indiana State University; 2006. Unpublished manuscript. [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Revised Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Butcher J, Graham J, Williams C, Ben-Porath Y. Development and Use of the MMPI-2 Content Scales. Minneapolis: University of Minnesota Press; 1990. p. 196. [Google Scholar]
- Caprioli D, Calu D, Shaham Y. Loss of phasic dopamine: a new addiction marker? Nature neuroscience. 2014;17:644–646. doi: 10.1038/nn.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gilbert J, Killgore W, White C, Schwab Z, Crowley D, Covell M, et al. Differential influence of safe versus threatening facial expressions on decision-making during an inhibitory control task in adolescence and adulthood. Developmental science. 2014;17:212–223. doi: 10.1111/desc.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Altamirano L, D'Esposito M. Reversal learning in Parkinson's disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Gold MS. New concepts in cocaine addiction: the dopamine depletion hypothesis. Neuroscience & Biobehavioral Reviews. 1985;9:469–477. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilh G, Vandekerckhove J, Forstmann BU, Keuleers E, Brysbaert M, Wagenmakers EJ. Testing theories of post-error slowing. Attention, perception & psychophysics. 2012;74:454–465. doi: 10.3758/s13414-011-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. Journal of cognitive neuroscience. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Mondillo K, Drevets WC, Blair JR. Impairments of probabilistic response reversal and passive avoidance following catecholamine depletion. Neuropsychopharmacology. 2009;34:2691–2698. doi: 10.1038/npp.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. New Haven: Yale University; 1975. [Google Scholar]
- Jarcho JM, Benson BE, Plate RC, Guyer AE, Detloff AM, Pine DS, et al. Developmental effects of decision-making on sensitivity to reward: an fMRI study. Dev Cogn Neurosci. 2012;2:437–447. doi: 10.1016/j.dcn.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Kemppainen N, Någren K, Helenius H, Kurki T, Rinne JO. Age-related loss of extrastriatal dopamine D2-like receptors in women. Journal of neurochemistry. 2002;81:1005–1010. doi: 10.1046/j.1471-4159.2002.00895.x. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Logan JA. Patterns of drug use from adolescence to young adulthood: I. Periods of risk for initiation, continued use discontinuation. Am J Public Health. 1984;74:660–666. doi: 10.2105/ajph.74.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. Journal of neurochemistry. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Boettiger CA. Effects of Acute Dopamine Precusor Depletion on Immediate Reward Selection Bias and Working Memory Depend on Catechol-O-methyltransferase Genotype. Journal of cognitive neuroscience. 2013 doi: 10.1162/jocn_a_00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Budhani S, Nakic M, Chen G, Saad ZS, Vythilingam M, et al. The role of the amygdala and rostral anterior cingulate in encoding expected outcomes during learning. Neuroimage. 2006;29:1161–1172. doi: 10.1016/j.neuroimage.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Dagher A, Boileau I, Casey K, Baker GB, Diksic M, et al. Decreasing amphetamine-induced dopamine release by acute phenylalanine/tyrosine depletion: A PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2004;29:427–432. doi: 10.1038/sj.npp.1300328. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Rakic P. Scheduling of monoaminergic neurotransmitter receptor expression in the primate neocortex during postnatal development. Cereb Cortex. 1992;2:401–416. doi: 10.1093/cercor/2.5.401. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug and alcohol dependence. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Moja EA, Lucini V, Benedetti F, Lucca A. Decrease in plasma phenylalanine and tyrosine after phenylalanine-tyrosine free amino acid solutions in man. Life sciences. 1996;58:2389–2395. doi: 10.1016/0024-3205(96)00242-1. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, McTavish SF, Cowen PJ, Grasby PM. Reduction of brain dopamine concentration with dietary tyrosine plus phenylalanine depletion: an [11C]raclopride PET study. The American journal of psychiatry. 2003;160:1887–1889. doi: 10.1176/appi.ajp.160.10.1887. [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Cohen MX, Sherman SJ, Frank MJ. A role for dopamine in temporal decision making and reward maximization in parkinsonism. J Neurosci. 2008;28:12294–12304. doi: 10.1523/JNEUROSCI.3116-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J Abnorm Psychol. 1986;95:252–256. [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt PM. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Standing HR, DeVito EE, Cools R, Sahakian BJ. Dopamine precursor depletion improves punishment prediction during reversal learning in healthy females but not males. Psychopharmacology. 2010;211:187–195. doi: 10.1007/s00213-010-1880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. The Journal of comparative neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychological monographs. 1966;80:1–28. [PubMed] [Google Scholar]
- Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Satoh T, Nakai S, Sato T, Kimura M. Correlated coding of motivation and outcome of decision by dopamine neurons. J Neurosci. 2003;23:9913–9923. doi: 10.1523/JNEUROSCI.23-30-09913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction (Abingdon, England) 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Sheehan BD, Tharyan P, McTavish SF, Campling GM, Cowen PJ. Use of a dietary manipulation to deplete plasma tyrosine and phenylalanine in healthy subjects. J Psychopharmacol. 1996;10:231–234. doi: 10.1177/026988119601000309. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neuroscience and biobehavioral reviews. 2008;32:219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Boettiger CA. Age modulates the effect of COMT genotype on delay discounting behavior. Psychopharmacology. 2012;222:609–617. doi: 10.1007/s00213-012-2653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Assessment of state and trait anxiety: conceptual and methodological issues. South Psychol. 1985;2:6–16. [Google Scholar]
- Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. The American journal of drug and alcohol abuse. 1990;16:1–46. doi: 10.3109/00952999009001570. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2007;17:1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72:146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. Future time perspective in schizophrenia. Journal of abnormal psychology. 1956;52:240–245. doi: 10.1037/h0039899. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nature neuroscience. 2014 doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, O’Keeffe D, Politis M, O’Keeffe GC, Robbins TW, Bose SK, et al. The catechol-O-methyltransferase Val158Met polymorphism modulates fronto-cortical dopamine turnover in early Parkinson’s disease: a PET study. Brain. 2012;135:2449–2457. doi: 10.1093/brain/aws157. [DOI] [PubMed] [Google Scholar]