Abstract
Background/Objective
Between attacks, migraine is associated with hypersensitivities to sensory stimuli. The objective of this study was to investigate hypersensitivity to pain in migraineurs between attacks.
Methods
Cutaneous heat pain thresholds were measured in 112 migraineurs, migraine free for ≥48 hours, and 75 healthy controls. Pain thresholds at the head and at the arm were compared between migraineurs and controls using two-tailed t-tests. Amongst migraineurs, correlations between heat pain thresholds and headache frequency, allodynia symptom severity, and time interval until next headache were calculated.
Results
Migraineurs had lower pain thresholds than controls at the head (43.9°C+/−3.2°C vs. 45.1°C+/−3.0°C, p=.015) and arm (43.2°C+/−3.4°C vs. 44.8°C+/−3.3°C, p<0.001). There were not significant correlations between pain thresholds and headache frequency or allodynia symptom severity. For the 41 migraineurs for whom time to next headache was known, there were positive correlations between time to next headache and pain thresholds at the head (r=.352, p=.024) and arm (r=.312, p=.047).
Conclusions
This study provides evidence that migraineurs have low heat pain thresholds between migraine attacks. Mechanisms underlying these lower pain thresholds could also predispose the migraineur to their next migraine attack, a hypothesis supported by finding positive correlations between pain thresholds and time to next migraine attack.
Keywords: Migraine, Headache, Sensitization, Pain Thresholds, Allodynia
Introduction
Hypersensitivities to sensory stimuli are most prominent during migraine attacks, manifesting as photophobia, phonophobia, osmophobia and cutaneous allodynia.1-5 However, these hypersensitivities often persist, albeit less prominently, in migraineurs between attacks.6-10 The presence of between-attack hypersensitivities has been suggested via answers to questionnaires assessing patient symptoms, physiologic studies (e.g. measuring light or auditory discomfort thresholds), and in functional magnetic imaging studies that demonstrate enhanced stimulus-induced brain activations.6-14
The central and peripheral sensitization of nociceptors that occurs during migraine attacks can lead to lowering of pain thresholds and symptoms of cutaneous allodynia within and outside of the trigeminal nerve territory.1, 3, 15-17 Although it is relatively well established that migraineurs develop cutaneous sensitization and allodynia during migraine attacks, sensitivity to potentially noxious stimuli between migraine attacks is less well established. The few studies that have investigated thermal, mechanical or electrical pain thresholds in migraineurs between migraine attacks have yielded conflicting results.9, 10, 18-20 This current study adds to the existing literature because of its larger sample size, ability to perform subgroup analyses that exclude subjects with pain at the time of testing and subjects taking migraine prophylactic medications, and ability to determine correlations between pain thresholds and headache frequency, allodynia symptom severity, and time to next migraine attack.
The objective of this study was to determine if, compared to healthy controls, migraineurs have lower heat pain thresholds between migraine attacks. The mechanisms accounting for low pain thresholds might also predispose a migraineur towards development of their next migraine and could potentially be a target for migraine prophylactic therapy.
Methods
Institutional Review Board approval for this study was obtained from Washington University School of Medicine and from Mayo Clinic. Subjects were recruited from an institutional database of research volunteers at Washington University, from headache clinics at the two institutions, and via use of advertisements in the medical centers. All subjects provided informed consent prior to participation. Subjects were adult men and women between the ages of 18 years and 65 years who did not have any medical conditions that could directly affect the results of quantitative sensory testing (e.g. peripheral neuropathy) and did not have any acute or chronic pain disorders other than the migraineurs having migraine. Control subjects could not have headaches other than tension type headache on 3 or fewer days per month. Headache diagnoses were made according to the criteria of the International Classification of Headache Disorders 2 (ICHD-2).21
All subjects were studied at a time when they had been migraine free for at least 48 hours. All episodic migraine and control subjects had been completely pain free for at least 48 hours. Chronic migraineurs were studied as long as they had not had a constellation of symptoms that met ICHD-2 diagnostic criteria for a migraine attack within 48 hours. However, since many chronic migraineurs are never completely free of migraine symptoms for 48 hours, chronic migraineurs were allowed to participate even if they were not pain free at the time of testing. All subjects were free of pain meds and abortive migraine medications for at least 48 hours. Episodic migraineurs had not used migraine prophylactic medications for at least 8 weeks at the time of testing. Chronic migraineurs taking migraine prophylactic medications were enrolled. The primary data analysis compared all migraine subjects to healthy control subjects. However, two secondary analyses were performed: 1) migraine subjects who were pain free at the time of testing and not using migraine prophylactic medications vs. healthy control subjects; and 2) episodic migraine subjects vs. healthy control subjects.
Subjects underwent structured interviews and were assigned a diagnosis as a control or migraineur by a neurologist with board certification in headache medicine (TJS). Subjects completed the Migraine Disability Assessment (MIDAS), the State-Trait Anxiety Inventory (STAI), the Beck Depression Inventory (BDI-II), and the Allodynia Symptom Checklist (ASC-12).22-25 Subjects completed the ASC-12 twice; once according to their recall of symptoms during times when they were headache free and again according to recall of symptoms during their most severe headaches.
Quantitative sensory testing was performed to determine heat pain thresholds. Standardized instructions were delivered to the subjects prior to quantitative sensory testing. ‘Pain threshold’ was defined for subjects as the first moment that the stimulus changed from feeling hot to feeling painful. Each subject underwent testing at the right and left forehead and the right and left ventral medial forearm. Thermal testing was performed using the Medoc Pathway platform with a 30 mm × 30 mm thermode. The thermode was applied to the skin and fastened with a Velcro strap. Using the method of limits, the thermode increased in temperature at a rate of 1°C per second from its starting point at 32°C until the subject pressed a button on the subject response unit indicating that the stimulus became painful. Testing was performed three times at each body location. The mean of the three trials in each body location was considered the pain threshold.
Forty-eight of the migraine subjects completed a daily headache diary for 1 week following testing. This diary was utilized to determine the interval between testing and onset of the next headache (in hours).
Demographics, anxiety, disability, depression, and allodynia scores were described using descriptive statistics and compared between subject groups using chi-squared tests or t-tests, as appropriate. Pain thresholds on the right and left were averaged in order to obtain one measure of pain threshold at the arms and one at the head. Two-sample t-tests were used to compare heat pain thresholds in migraineurs to those in controls with p<.05 being considered significant. Pearson correlations were used to investigate relationships between heat pain thresholds in migraineurs with headache frequency, allodynia symptom severity, anxiety scores, depression scores, and time to next headache. For these correlations, p<.05 were considered significant.
Results
Seventy-five healthy control subjects and 112 migraine subjects were included in this study. Subject demographics, headache frequency, MIDAS score, STAI scores, BDI-II scores, and ASC-12 scores are shown in Table 1. Migraineurs had higher trait anxiety scores, BDI scores, and cutaneous allodynia scores. However, scores for trait anxiety, BDI scores and headache free ASC-12 scores were within normal limits in the migraine group and in the control group. Mean ASC-12 score during headache was indicative of mild allodynia in the migraineurs.
Table 1.
Demographics, Headache Characteristics, and Pain Thresholds in Migraineurs and Healthy Controls. “All migraineurs” includes those with pain at the time of testing (n=19), those without pain at the time of testing (n=93), those not taking migraine prophylactic medications (n=97), and those taking prophylactic medications (n=15). All variables are reported as means followed by (standard deviations) except for female sex which is reported as absolute number followed by (percentage). MIDAS = Migraine Disability Assessment Score; BDI = Beck Depression Inventory; ASC-12 = Allodynia Symptom Checklist 12.
|
Healthy
Controls (n=75) |
All
Migraineurs (n=112) |
p-value
All Migraineurs vs. Healthy Controls |
Migraineurs
Pain free No prophylactics (n=88) |
p-value
Migraineurs Pain free No prophylactics vs. Healthy Controls |
Episodic
Migraineurs (n=75) |
p-value
Episodic Migraineurs vs. Healthy Controls |
|
|---|---|---|---|---|---|---|---|
| Age in years | 36.5 (11.4) | 34.4 (10.6) | .22 | 34.9 (10.7) | .36 | 35.4 (11.3) | .58 |
| Female | 56 (74.7) | 89 (79.5) | .44 | 68 (77.3) | .70 | 57 (77.1) | .85 |
| MIDAS | NA | 25.8 (32.0) | NA | 18.2 (21.1) | NA | 6.5 (1.8) | NA |
| State Anxiety | 26 (6.5) | 27.8 (7.4) | .085 | 27.9 (7.8) | .10 | 27.1 (7.2) | .35 |
| Trait Anxiety | 29.7 (8.1) | 33.5 (9.8) | .007* | 32.6 (9.7) | .045* | 31.7 (9.5) | .16 |
| BDI | 2.5 (3.6) | 4.9 (5.4) | <.001* | 4.4 (5.0) | .006* | 4.1 (4.1) | 0.01* |
|
ASC-12
No headache |
0.2 (0.6) | 1.1 (2.2) | <.001* | 1.1 (2.3) | <.001* | 1.2 (2.5) | 0.001* |
| ASC-12 Headache | 0.4 (1.4) | 5.4 (4.5) | <.001* | 5.3 (4.6) | <.001* | 5.5 (4.7) | 0.001* |
| Headache days/month | NA | 10.6 (7.9) | NA | 7.8 (5.3) | NA | 5.9 (2.7) | NA |
| Forehead Pain Threshold | 45.1 (3.0) | 43.9 (3.2) | .017* | 44.0 (3.3) | .028* | 44.0 (3.5) | 0.041* |
| Forearm Pain Threshold | 44.8 (3.3) | 43.2 (3.4) | <.001* | 43.1 (3.4) | .003* | 43.3 (3.4) | 0.008* |
P < 0.05 versus control subjects.
Heat pain thresholds were lower in migraineurs, both at the forehead (43.9°C +/− 3.2°C vs. 45.1°C +/− 3.0°C, p=.017) and at the forearm (43.2°C +/− 3.4°C vs. 44.8°C +/− 3.3°C, p<0.001). Amongst the migraineurs, there were no significant correlations between pain thresholds and headache frequency (n=112), allodynia symptom severity (n= 112), anxiety scores (n=112), or depression scores (n=111). Forty-one of the 48 migraine subjects who completed a headache diary for 1 week following measurement of pain thresholds had a headache during that week. There were significant positive correlations between number of hours until the next headache and heat pain thresholds at the head (r = .352, p = .024) and at the forearm (r = .312, p=.047). [Figure 1]
Figure 1. Correlations Between Heat Pain Thresholds and Time Interval until Next Migraine Attack.
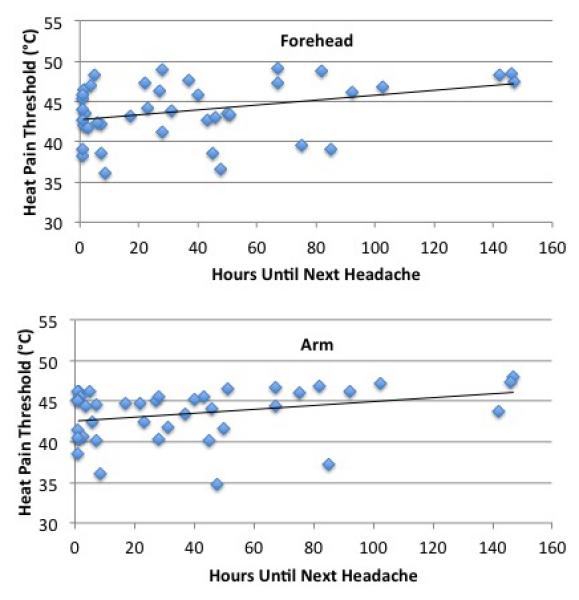
There were positive correlations between heat pain thresholds and the number of hours until the next migraine attack at the forehead (r=.352, p=.024) and at the forearm (r=.312, p=.047). Thus, subjects who were closer to their next migraine attack had lower pain thresholds.
The first post-hoc analysis excluded migraineurs who were in pain at the time of testing (n=19) and excluded migraineurs who were using migraine prophylactic medications (n=15) (10 subjects were in pain at the time of testing and were using migraine prophylactic medications). After excluding these subjects, migraineurs (n=88) still had significantly lower heat pain thresholds at the forehead (44.0°C +/− 3.3°C vs. 45.1 °C +/− 3.0°C, p= .028) and at the forearm (43.1°C +/− 3.4°C vs. 44.8°C +/− 3.3°C, p=.003). For the 38 migraineurs who completed a headache diary following QST, 31 of whom had a headache during that week, significant positive correlations remained between number of hours until next headache and heat pain thresholds at the head (r =.325, p=.046) but not at the forearm (r=.299, p=.068).
A second post-hoc analysis interrogated differences in heat pain thresholds for episodic migraineurs only (n=74) compared to those of healthy controls (n=75). None of these episodic migraineurs were using migraine prophylactic medications and all were pain-free at the time of testing. Compared to healthy controls, episodic migraineurs had lower heat pain thresholds at the forehead (44.0°C +/− 3.5°C vs. 45.1°C +/− 3.0°C, p=.041 ) and at the forearm ( 43.3°C +/− 3.4°C vs. 44.8°C +/− 3.3°C, p=.008). For the 36 episodic migraineurs who maintained a headache diary following QST, 29 of whom had a headache during that week, there were significant positive correlations between number of hours until the next headache and heat pain thresholds at the head (r=.344, p=.040) but not at the forearm (r=.315, p=.061).
Discussion
In this study heat pain thresholds within and outside of the trigeminal nerve territory were measured in a relatively large cohort of migraineurs who were at least 48 hours free from their last migraine attack. Compared to healthy controls, migraineurs had lower heat pain thresholds at the forehead and at the forearm. Although the absolute differences in pain thresholds found between migraineurs and healthy controls were relatively small and did not associate with symptoms of cutaneous allodynia, the mechanisms underlying these lower pain thresholds in migraineurs could also predispose the migraineur to their next migraine attack or the lower pain thresholds could be an early manifestation of the migraine attack itself. The findings of positive correlations between pain thresholds and number of hours until the next migraine attack support these theories.
Previously published studies that have compared pain thresholds within and outside of trigeminal innervated territory in migraineurs between migraine attacks to those of healthy controls have had mixed results. A few studies have found interictal migraineurs to be more sensitive to mechanical/pressure stimuli and to have lower thermal pain thresholds.9, 10, 18-20 However, others have found no differences in electrical or thermal pain thresholds between interictal migraineurs and healthy controls.10, 18 A study from our lab that was published in 2011 and included 60 of the subjects (20 episodic migraineurs, 20 chronic migraineurs, and 20 controls) also included in the study reported herein found that interictal episodic and chronic migraineurs had lower thermal pain thresholds and thermal pain tolerance thresholds than controls.9 Similar to the current study that investigated an additional 72 migraine subjects, there were no correlations between headache frequency and pain thresholds, a relationship suggested by a few other studies.26, 27 Overall, prior publications addressing interictal pain thresholds in migraineurs have had conflicting results, likely due to small sample sizes, the use of different types of stimuli (thermal, pressure, electrical), testing at different body sites, differing definitions of “interictal”, and different inclusion/exclusion criteria (e.g. pain level at the time of testing, use of migraine prophylactic medications, inclusion of patients with different headache types). The study that we report herein has attempted to clarify some of these issues by using a strict definition of no migraine within 48 hours prior to testing, performing secondary analyses that exclude patients who had any pain at the time of testing, those taking migraine prophylactic medications, and chronic migraineurs, and by exploring correlations between pain thresholds with headache frequency and time interval until the next headache.
In this study, there were positive correlations between heat pain thresholds and number of hours until the next migraine attack. These correlations support the notion that there could be a lowering of pain thresholds prior to the onset of migraine attack symptoms. A relationship between pain thresholds and time interval until the next migraine attack has been suggested in a study by Sand and colleagues.28 In that study, 11 migraineurs who were studied in the pre-attack phase (within 24 hours of their next migraine attack) and during the interictal period (more than 24 hours from last attack and more than 24 hours from next attack) were found to have lower pain thresholds during their pre-attack phase. This increasing sensitivity to painful stimuli might correlate with a lower threshold for full activation of the trigeminocervical system and thus lead to or predispose the migraineur to their next migraine attack. Alternatively, increasing sensitivity to painful stimuli might be an early manifestation of the migraine attack itself, beginning well before the onset of migraine attack symptoms.
The absolute difference in heat pain thresholds in the migraineurs between attacks compared to healthy controls in this study was relatively small. For example, the 1.2°C to 1.5°C difference in pain thresholds found in this study are much smaller than the differences seen when comparing migraineurs between migraine attacks to themselves during a migraine attack. 16, 29 The lower pain thresholds of migraineurs in this study are still within the normal range for non-sensitized c-fibers and as one would expect, they were not associated with symptoms of cutaneous allodynia. 16, 30, 31 However, we believe that the lower pain thresholds detected in these migraineurs are meaningful as they might be a very early manifestation of a migraine attack or they might be indicative of an underlying process that also allows for initiation of a migraine attack. For example, inadequate nociceptive inhibition at the level of the spinal dorsal horn and trigeminal subnucleus caudalis could account for the lower pain thresholds found in migraineurs. Since lower pain thresholds were found at the forehead and at the forearm, this lack of inhibition would likely be mediated centrally by brainstem regions of the descending pain system, such as the rostral ventral medulla, periaqueductal gray, and/or nucleus cuneiformis. In support of this theory, functional imaging studies of migraineurs have shown these brainstem regions to have atypical pain-induced activation (e.g. hypoactivation of the nucleus cuneiformis) and atypical resting state functional connectivity, and functional imaging studies have implicated these brainstem regions in central sensitization. 32-36 The correlation between pain thresholds and time interval until next headache found in this study suggests that the extent of inadequate inhibition might increase as the migraineur nears their next migraine. In addition to causing increased sensitivity to cutaneous thermal stimuli, a progressive lack of inhibition by brainstem nuclei responsible for central modulation of pain could lead to increasing excitability of the trigeminovascular system and thus to the start of the next migraine attack. 37 An even greater lack of pain inhibition, perhaps combined with enhanced pain facilitation, during a migraine attack could then lead to the sensitization and allodynia that are typical during a migraine attack. 32, 38
Limitations of this study include the finding that migraineurs had higher trait anxiety and depression scores compared to controls. Anxiety and depression can have an impact on the determination of pain thresholds, typically being associated with lower pain thresholds.39, 40 However, although anxiety and depression scores in the migraineurs were statistically higher, average scores for migraineurs were within normal limits. Furthermore, there were no correlations between pain thresholds and anxiety scores or depression scores in this study, suggesting that any effect of anxiety or depression on pain thresholds was minimal, if at all present. This finding is consistent with prior investigations that show anxiety and depression to have an effect on determination of pain tolerance thresholds and ratings of pain intensity, but not on pain thresholds.41 The ability to draw conclusions about a possible relationship between decreasing pain thresholds and time to next migraine attack is limited by the cross-sectional nature of this study. Future investigations should assess pain thresholds within individuals at numerous time points in order to more conclusively determine if pain thresholds drop prior to migraine attack onset.
Conclusions
This study shows that migraine patients have lower heat pain thresholds between migraine attacks compared to healthy controls. The mechanisms underlying these lower pain thresholds might also predispose the migraine patient to their next migraine attack. It is also possible that a lowered pain threshold is a very early sign of a migraine attack itself. The positive correlation between heat pain thresholds and time interval to the next migraine attack that was identified in this study supports these possibilities.
Acknowledgments
Funding: NIH K23NS070891 and NIH KL2RR024994
References
- 1.Bigal ME, Ashina S, Burstein R, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70:1525–33. doi: 10.1212/01.wnl.0000310645.31020.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache. 2004;44:1019–23. doi: 10.1111/j.1526-4610.2004.04197.x. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–58. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell MB, Rasmussen BK, Fenger K, et al. Migraine without aura and migraine with aura are distinct clinical entities: a study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 1996;16:239–45. doi: 10.1046/j.1468-2982.1996.1604239.x. [DOI] [PubMed] [Google Scholar]
- 5.Wober-Bingol C, Wober C, Karwautz A, et al. Clinical features of migraine: a cross-sectional study in patients aged three to sixty-nine. Cephalalgia. 2004;24:12–7. doi: 10.1111/j.1468-2982.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Mushtaq A, Yang I, et al. Ictal and interictal phonophobia in migraine-a quantitative controlled study. Cephalalgia. 2009;29:1042–8. doi: 10.1111/j.1468-2982.2008.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demarquay G, Royet JP, Giraud P, et al. Rating of olfactory judgements in migraine patients. Cephalalgia. 2006;26:1123–30. doi: 10.1111/j.1468-2982.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 8.Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997;37:492–5. doi: 10.1046/j.1526-4610.1997.3708492.x. [DOI] [PubMed] [Google Scholar]
- 9.Schwedt TJ, Krauss MJ, Frey K, et al. Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia. 2011;31:6–12. doi: 10.1177/0333102410365108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissman-Fogel I, Sprecher E, Granovsky Y, et al. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain. 2003;104:693–700. doi: 10.1016/S0304-3959(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 11.Antal A, Polania R, Saller K, et al. Differential activation of the middle-temporal complex to visual stimulation in migraineurs. Cephalalgia. 2011;31:338–45. doi: 10.1177/0333102410379889. [DOI] [PubMed] [Google Scholar]
- 12.Demarquay G, Royet JP, Mick G, et al. Olfactory hypersensitivity in migraineurs: a H(2)(15)O-PET study. Cephalalgia. 2008;28:1069–80. doi: 10.1111/j.1468-2982.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- 13.Schwedt TJ, Chong CD, Chiang CC, et al. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014 doi: 10.1177/0333102414526069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent M, Pedra E, Mourao-Miranda J, et al. Enhanced interictal responsiveness of the migraineous visual cortex to incongruent bar stimulation: a functional MRI visual activation study. Cephalalgia. 2003;23:860–8. doi: 10.1046/j.1468-2982.2003.00609.x. [DOI] [PubMed] [Google Scholar]
- 15.Ashkenazi A, Silberstein S, Jakubowski M, et al. Improved identification of allodynic migraine patients using a questionnaire. Cephalalgia. 2007;27:325–9. doi: 10.1111/j.1468-2982.2007.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–24. [PubMed] [Google Scholar]
- 17.Mathew NT, Kailasam J, Seifert T. Clinical recognition of allodynia in migraine. Neurology. 2004;63:848–52. doi: 10.1212/01.wnl.0000137107.27585.f7. [DOI] [PubMed] [Google Scholar]
- 18.Cooke L, Eliasziw M, Becker WJ. Cutaneous allodynia in transformed migraine patients. Headache. 2007;47:531–9. doi: 10.1111/j.1526-4610.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-de-las-Penas C, Madeleine P, Cuadrado ML, et al. Pressure pain sensitivity mapping of the temporalis muscle revealed bilateral pressure hyperalgesia in patients with strictly unilateral migraine. Cephalalgia. 2009;29:670–6. doi: 10.1111/j.1468-2982.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 20.Filatova E, Latysheva N, Kurenkov A. Evidence of persistent central sensitization in chronic headaches: a multi-method study. J Headache Pain. 2008;9:295–300. doi: 10.1007/s10194-008-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Classification Committee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II) Pscyhology Corp; San Antonio, TX. 1996 [Google Scholar]
- 23.Jakubowski M, Silberstein S, Ashkenazi A, et al. Can allodynic migraine patients be identified interictally using a questionnaire? Neurology. 2005;65:1419–22. doi: 10.1212/01.wnl.0000183358.53939.38. [DOI] [PubMed] [Google Scholar]
- 24.Spielberger C, Gorsuch R. State trait anxiety inventory for adults: sampler set, manual, test, scoring key. Mind Garden; Redwood City, California: 1983. [Google Scholar]
- 25.Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56:S20–8. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 26.Kitaj MB, Klink M. Pain thresholds in daily transformed migraine versus episodic migraine headache patients. Headache. 2005;45:992–8. doi: 10.1111/j.1526-4610.2005.05179.x. [DOI] [PubMed] [Google Scholar]
- 27.LoPinto C, Young WB, Ashkenazi A. Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine. Cephalalgia. 2006;26:852–6. doi: 10.1111/j.1468-2982.2006.01121.x. [DOI] [PubMed] [Google Scholar]
- 28.Sand T, Zhitniy N, Nilsen KB, et al. Thermal pain thresholds are decreased in the migraine preattack phase. Eur J Neurol. 2008;15:1199–205. doi: 10.1111/j.1468-1331.2008.02276.x. [DOI] [PubMed] [Google Scholar]
- 29.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123:1703–9. doi: 10.1093/brain/123.8.1703. Pt 8. [DOI] [PubMed] [Google Scholar]
- 30.Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 31.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 32.Lee MC, Zambreanu L, Menon DK, et al. Identifying brain activity specifically related to the maintenance and perceptual consequence of central sensitization in humans. J Neurosci. 2008;28:11642–9. doi: 10.1523/JNEUROSCI.2638-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011;70:838–45. doi: 10.1002/ana.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moulton EA, Burstein R, Tully S, et al. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3:e3799. doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwedt TJ, Larson-Prior L, Coalson RS, et al. Allodynia and Descending Pain Modulation in Migraine: A Resting State Functional Connectivity Analysis. Pain Med. 2014;15:154–65. doi: 10.1111/pme.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zambreanu L, Wise RG, Brooks JC, et al. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114:397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Vecchia D, Pietrobon D. Migraine: a disorder of brain excitatory-inhibitory balance? Trends Neurosci. 2012;35:507–20. doi: 10.1016/j.tins.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Edelmayer RM, Vanderah TW, Majuta L, et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–93. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bar KJ, Brehm S, Boettger MK, et al. Pain perception in major depression depends on pain modality. Pain. 2005;117:97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Jones A, Zachariae R, Arendt-Nielsen L. Dispositional anxiety and the experience of pain: gender-specific effects. Eur J Pain. 2003;7:387–95. doi: 10.1016/S1090-3801(02)00139-8. [DOI] [PubMed] [Google Scholar]
- 41.Thibodeau MA, Welch PG, Katz J, et al. Pain-related anxiety influences pain perception differently in men and women: a quantitative sensory test across thermal pain modalities. Pain. 2013;154:419–26. doi: 10.1016/j.pain.2012.12.001. [DOI] [PubMed] [Google Scholar]


