Abstract
A subset of T cells defined by the cell surface expression of MCAM (CD146) has been identified in the peripheral circulation of healthy individuals. These cells comprise approximately 3% of the pool of circulating T cells, have an effector memory phenotype, and are capable of producing several cytokines. Notably, the MCAM positive cells are enhanced for IL-17 production compared to MCAM negative effector memory T cells. These call are committed for IL-17 production and do not require in vitro polarization with exogenous cytokines. MCAM positive T cells also demonstrate an increased ability to bind to endothelial monolayers. In numerous autoimmune diseases these cells are found at increased proportions in the peripheral circulation, and at the sites of active inflammation in patients with autoimmune disease, these cells appear in large numbers are major contributors to IL-17 production. Studies to date have been performed with human subjects and it is uncertain if appropriate mouse models exist for this cells type. These cells could represent early components of the adaptive immune response and serve as targets of therapy in these diseases, although much work remains to be performed in order to discern the exact nature and function of these cells.
Keywords: CD146, MCAM Th17, Tc17, Rheumatoid arthritis, Psoriasis, Multiple sclerosis
Introduction
In the past decade, a population of T cells identified by cell surface expression of the melanoma cell adhesion molecule, MCAM (CD146), has been reported to be associated with a number of human autoimmune diseases. These cells have the ability to bind to endothelial monolayers, secrete cytokines, and have a pro-inflammatory phenotype and genotype; hence we have called these EPIC T Cells (Endothelial-binding, ProInflammatory, with Cytokine secretion). MCAM is found on other cell types, notably on both endothelial cells and melanoma cells and was once thought to be specific for the former type of cells in healthy individuals. Endothelial MCAM has been well characterized and is expressed primarily at endothelial junctions, playing an important role in maintaining the integrity of endothelial monolayers. In this review we will discuss the discovery of EPIC T cells and subsequent studies alluding to their potential role in various autoimmune diseases, as well as some of the basic features and functions of CD146 in studies of this molecule on endothelial cells. Current data, such as higher levels of these cells in the blood of patients with autoimmune diseases and the enhanced secretion of IL-17A by these cells, strongly argue for a role of MCAM expressing T cells in the pathogenesis of numerous inflammatory autoimmune disease, although studies of EPIC T cells are hampered by the lack of a widely accepted mouse model.
Distribution and biological properties of CD146
MCAM (CD146) has an interesting history in that it has been the subject of intense investigation in two areas, melanomas and endothelial biology, but has received scant attention for its role in lymphocyte biology. In the study of melanoma, MCAM was first reported by Lehmann and colleagues (1) as a surface marker to discriminate between melanoma cells and benign melanocytes. Subsequently it was recognized to be an adhesion molecule with a potential role in tumor growth (2). Nearly a decade after its first description in melanoma, Bardin and associates found expression of MCAM on endothelial cells, leading to its use for as a marker for endothelial cells and circulating endothelial cells (3,4). In endothelial cells CD146 is found primarily at the endothelial junctions and is thought to play a major role in tightly binding these cells together (5). Much of the work concerning CD146 structure and expression on various tissues has been reviewed by several groups (6-8). MCAM has also been recognized to be expressed on a number cell types including mesenchymal stem cells (MSC) and dental pulp (9-12). In the former expression of CD146 has been suggested to differentiate between perivascular and endosteal localization of non-hematopoietic bone marrow-MSC populations (13) and may be developmentally related (14).
Binding partners for MCAM
Initially CD146 was postulated to have a homotypic ligand-receptor interaction (15), a mechanism which has neither been proven nor discredited. However, several studies have provided data suggesting that CD146 has heterophilic ligands in addition to, or instead of, homophilic binding (Table 1). Recent reports suggest that a ligand for MCAM is laminin-411 (α4-chain, a β1-chain and a γ1 chain, also known as laminin-8) (16, 17). Schneider-Hohendorf et al extended these findings by demonstrating that adhesion of MCAM positive cells was independent of very late antigen-4 (VLA-4) but may work in concert with p-selectin glycoprotein ligand-1 (PSGL-1)-mediated rolling of these cells (18). In endothelial cells galectin-1, but not galectin-2, has been shown to bind to CD146 and may help to protect against apoptosis in these cells (19). CD146 has also been demonstrated to be a coreceptor for VEGFR2 on endothelial cells, to interact directly with VEGFR2, and that this binding enhances VEGFR2 signaling (20). Wnt5a has also been proposed as a binding partner for CD146 (21). The reports of multiple ligands for CD146 create a degree of confusion regarding the mechanisms of adherence and migration of EPIC T cells. Unfortunately the reports describing these ligands for CD146 have not been widely confirmed by subsequent studies and it is unclear if these ligands are competitive or cooperative. More studies are needed to elucidate the binding ligands of MCAM and the precise mechanisms of migration and signaling in EPIC T cells.
TABLE 1. Ligands reported for CD146.
| Ligand | Cell type | Reference |
|---|---|---|
| Galectin-1 | CD146 transfected Fibroblast | Jouve et al, 2013. |
| Laminin-411 | CD146 transfected Chinese hamster ovary (CHO) cells |
Flanagan et al, 2012. |
| Wnt5a | CD146 transfected human embryonic kidney cell line HEK293T cells |
Ye et al, 2013 |
| Vegfr2 | CD146 transfected human embryonic kidney cell line HEK293T and Endothelial cells for VEGFR-2 |
Jiang et al, 2012 |
CD146 engagement and signaling
Signaling resulting from MCAM engagement has been widely studied in a number of non-leukocytic cells types, however a comprehensive, coherent picture of CD146 has not been offered. In endothelial cells, engagement of CD146 was found to recruit p59fyn to CD146, triggering a calcium flux through phospholipase C-γ activation and initiate a protein tyrosine kinase (PTK)-dependent signaling pathway, with tyrosine phosphorylation of the focal adhesion kinase, p125FAK and paxillin (22,23). Engagement of CD146 in endothelial cells also has been reported to cause actin redistribution to an activated form as well as translocation of NF-κB to the nucleus (24). In human melanoma cell lines Li and colleagues (25) described a reciprocal regulation of MCAM and protein kinase B (PKB) (also known as Akt), leading to inactivation of the Bcl-2-associated death promoter (BAD) and increased survival of the melanoma cells.
There are several reports in the literature associating Wnt5a non-canonical signaling with CD146 expression and function. Witze et al (26) demonstrated that brief treatment of melanoma cells with Wnt5a led to redistribution of MCAM from a uniformly distributed pattern to a highly polarized structure in concert with actin-myosin rearrangements. This mechanism could thereby control directional movement in response to chemokine gradients. Subsequent data using human umbilical cord endothelial cells as well as zebrafish embryos indicated that CD146 binds to Wnt5a with high affinity and is essential for endothelial cell migration and activity of c-jun amino-terminal kinase (JNK) via non-canonical signaling (21). CD146 was reported to do this through phosphorylation of Dishevelled (Dvl). Insulin-like growth factor binding protein 4 (IGFBP4), an antagonist of the Wnt/β-catenin signaling, was found to activate Wnt/β-catenin signaling pathway and to induce the expression of MCAM in renal carcinoma cells (27). To date, no studies have been performed in human T cells concerning the signaling pathways associated with CD146 engagement.
Early description on lymphocytes
The first description of MCAM expression on lymphocytes appeared in 1997 in a report by Pickl et al (28). Here, MCAM was described as an activation marker of T cells, ‘not significantly’ expressed on the leukocytes of healthy donors. It was, however, found on T cells in synovial fluid from patients with rheumatoid arthritis. Furthermore, skin specimens from contact dermatitis patients demonstrated that 50-80% of the CD3+ cells in tissue sections were MCAM+. These authors suggested, without any supporting data, that MCAM might facilitate extravasation or homing of these cells. This initial observation lay dormant for nearly a decade until a report identified MCAM+ T cells in the peripheral circulation of healthy donors (29). The MCAM positive T cells could be found in both the CD4+ and CD8+ subpopulations and demonstrated no clonality in the peripheral blood of healthy donors based on TCRVβ analysis. CD146 is also expressed on a low percentage of B cells in the peripheral circulation of healthy donors but is only rarely expressed on the NK population. MCAM could be upregulated on B cells by mitogen stimulation and by activation with a combination of CD40L and IL-4 (29). Seftalioğlu and Karakoç used immunohistochemistry to demonstrate the presence of MCAM on immature cortical thymocytes, supporting the concept that this antigen was expressed on T cells at an early stage (30). A subsequent study by Elshal and colleagues (31) revealed that MCAM positive T cells had an enhanced ability to bind the endothelial monolayers in vitro compared to CD146 negative cells.
Immunophenotyping
Although MCAM was initially described as an activation antigen of T cells, the distribution of this antigen is distinct from other common markers of activation such as CD25, CD38, CD69, OX-40, and HLA-Dr in freshly isolated cells, (31), although there are varying degrees of in the expression of MCAM with many of the other activation markers (32). The MCAM+ T cells were also found to be CD45RA−, CD45RO+, CD28+and CCR7−, indicative of effector memory T cells, and displayed no primitive markers such as CD133 or CD34 and no other markers associated with endothelial cells (31, 33). Markers associated with Th17 cells, CD26 and CD58 were coexpressed on MCAM positive cells, while additional markers associated with Th17 cells, including CD161, CCR6, and CCR4, were partially co-expressed with MCAM (33, 34).
Role in adherence and extravasation
The initial speculation that MCAM expression on T cells might facilitate extravasation was based on MCAM-based adherence in other cells types but without any direct evidence on human T cells. The renewed attention to MCAM a decade later included studies that provided direct evidence for the role in MCAM in lymphocyte-endothelial interactions. In addition to the study mentioned above (31), Guezguez and coworkers reported that a human NK cell line transfected with MCAM decreases rolling velocity and increases firm adhesion of these cells to endothelial cells in vitro and that antibodies against MCAM could reverse these findings (15). MCAM transfection also increases the microvilli of the transfected NK cells compared to non-transfected controls. Furthering this association between MCAM expression on T cells and cell adherence, Brucklacher-Waldert et al demonstrated that clones of MCAM positive Th17 cells isolated from peripheral blood could adhere to endothelial cells ex vivo better than corresponding MCAM negative Th17 cells (35). Additional, direct evidence showing a role for MCAM in extravasation of T cells per se are data showing MCAM+ lymphocytes migrate more efficiently across human blood-brain endothelial cells than do the corresponding MCAM negative cells, and that this migration could be blocked by anti-MCAM antibodies (36). In a similar context of MCAM positive cells migrating through the blood-brain barrier, Duan and coworkers (37) extended this work by using knockout mice lacking endothelial CD146 (MCAM) to demonstrate that extravasation of MCAM positive T cells to the central nervous system (CNS) diminished in comparison to wild type mice, suggesting that endothelial MCAM as well as lymphocytic MCAM plays a role in this process. Interestingly, HEMCAM, the avian orthologue of MCAM/CD146, downregulates β1 integrin expression at the cell surface and with high levels of HEMCAM there is reduced cellular adhesion to laminin 1 (38). These data, together with the reports of various binding partners for MCAM, suggest that the role of this molecule in cell adhesion may be quite complex.
Cytokine production by EPIC T cells
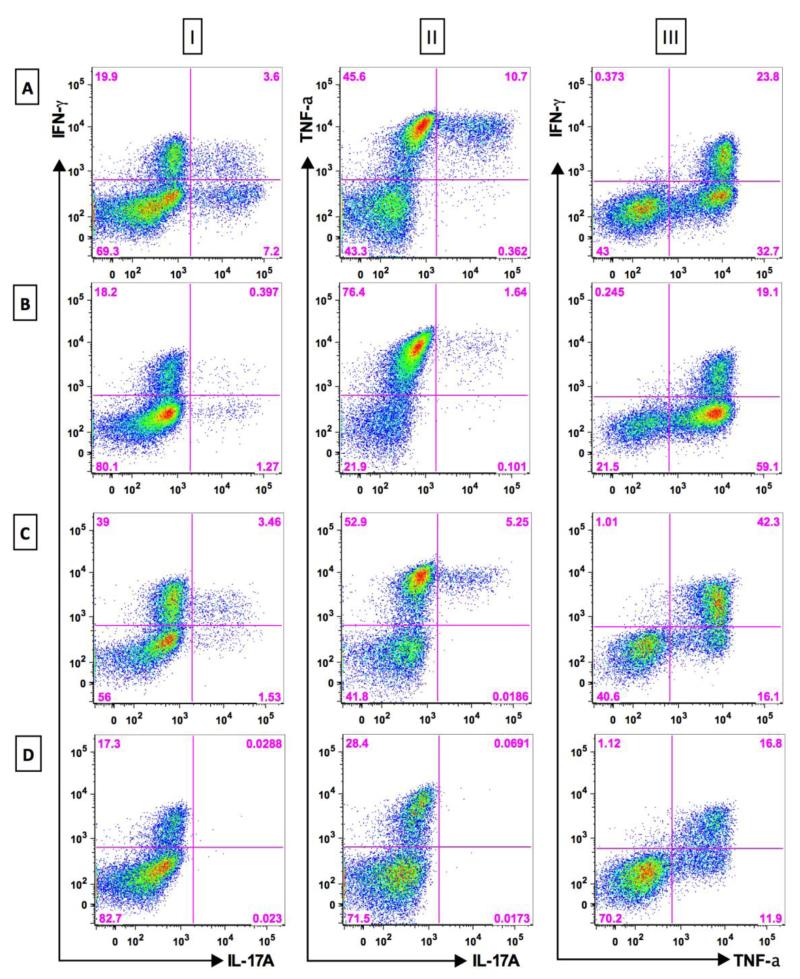
The initial reports of MCAM-expressing T cells made no mention of cytokine production by these cells. The first hint of cytokine production from these cells was in a report by Elshal et al (31) which presented a gene list showing IL-8 and RORC2 upregulation associated with CD146 expression on T cells; however there was no direct proof of actual cytokine production. The first association of cytokine production by MCAM positive T cells was a finding that sixty nine percent of clones of Th17 cells expressed MCAM whereas no Th1 clones did so (35). More proof of cytokine secretion from the MCAM positive cells was presented in a study of peripheral blood CD4 T cells isolated on the basis of MCAM expression demonstrated pronounced intracellular IL-17 and INF-γ (34) whereas their MCAM-negative counterparts showed minimal IL-17 secretion but retained INF-γ production. Furthermore, supernatants from sorted, short-term cultured MCAM positive cells revealed increased IL-17 compared to their MCAM negative counterparts. Gene expression analysis also revealed increased mRNA for IL17A, IL-22, and IL-26 in CD146 positive CD4 T cells relative to the MCAM negative cells, as well as increased RORC2. It is extremely important to note that this cytokine secretion could be seen without in vitro polarization, such as that used previously by Veldhoen et al to induce Th17 cells (39). Later Kamiyama and colleagues (33) demonstrated that CCR6+ CD146+ CD4+ cells had a greater capacity to produce IL-17 than did cells negative for one or both of these markers. In contrast to the previous study which did not use polarization, here the secretion of IL-17 was demonstrated after in vitro polarization with IL-1β and IL-23. It has also been found that human CD8+ T cells expressing MCAM can also produce IL-17 and INF-γ without the need for in vitro polarization in a manner very similar to the CD4+ T cells, and both can also produce TNFα (FIG 1) (40).
Figure 1. Secretion of IL-17, IFN-γ, and TNF-α by MCAM+ and MCAM− T cell subsets.
Subpopulations of T cells from a healthy donor were sorted by flow cytometry to separate CD146+ cells from their CD146− counterparts were subsequently cultured for 5 days with CD3/28 stimulation. The cells were then stained for CD146 and three intracellular cytokines, and then analyzed by flow cytometry. Row A: CD4+CD45RO+CD146+ T cells; Row B: CD4+CD45RO+CD146− T cells; Row C: CD8+CD146+ T cells; Row D: CD8+CD146− T cells; Column I: IFN-γ vs IL-17A; Column II: TNF-α vs IL-17A; Column III: IFN-γ vs TNF-α. These data show a marked, but not unique, association of IL-17A with CD146+ T cells. The other two cytokines are abundantly secreted by both CD146+ and CD146− T cells.
EPIC T cells in autoimmune diseases
The initial observations concerning the expression of MCAM on T cells included demonstrating MCAM on T cells in the synovial fluid of patients with rheumatoid arthritis (RA), a finding leading this group to speculate that expression of CD146 might be involved in the migration of T cells to sites of inflammation (31). A subsequent study by Dagur and coworkers (41) confirmed finding MCAM+ T cells in the synovial fluids of RA patients, reaching as high as 26% of the T cells in some patients, and extended this observation by showing that increases, albeit less than seen in the synovial fluids, of CD4+ MCAM+ T cells could also be found in the peripheral circulation of patients with arthritis and other musculoskeletal diseases. This observation raised the likelihood that the mediators of local inflammation could be detected systemically. The EPIC T cells in this study displayed an effector memory phenotype and a distinctly pro-inflammatory profile. A subsequent study again confirmed these findings in RA amd extended these to ankylosing spondylitis (AS) (42).
A number of studies have also reported the presence of MCAM positive T cells in multiple sclerosis, and suggested their potential role in the inflammatory process in this disease (35-37). Larochelle et al (36) found elevated levels of MCAM+ T cells that secrete IL17 in the circulation of MS patients compared to controls as well as MCAM expressing T lymphocytes in perivascular infiltrates in active multiple sclerosis lesions. Furthermore MCAM expression in the brain endothelium was also upregulated, suggesting homotypic binding of CD146 in the migration of these cells. In vitro experiments revealed that the MCAM+ T cells migrated more efficiently through human blood brain barrier endothelial cells than did their MCAM negative counterparts and this was inhibited by anti-MCAM antibodies. Duan and colleagues (37) extended these findings using a mouse model of experimental autoimmune encephalomyelitis (EAE) and demonstrated that anti-CD146 antibody administered in vivo reduced the severity and clinical score of EAE. Yet another group (16) found the same role for MCAM+ T cells in EAE but presented evidence that laminin 411 was the binding partner for MCAM rather than homotypic binding of endothelial MCAM. Later work identified MCAM as an exclusive pathway for Th17 cells to migrate through the blood brain barrier in patients undergoing anti-VLA-4 therapy (18).
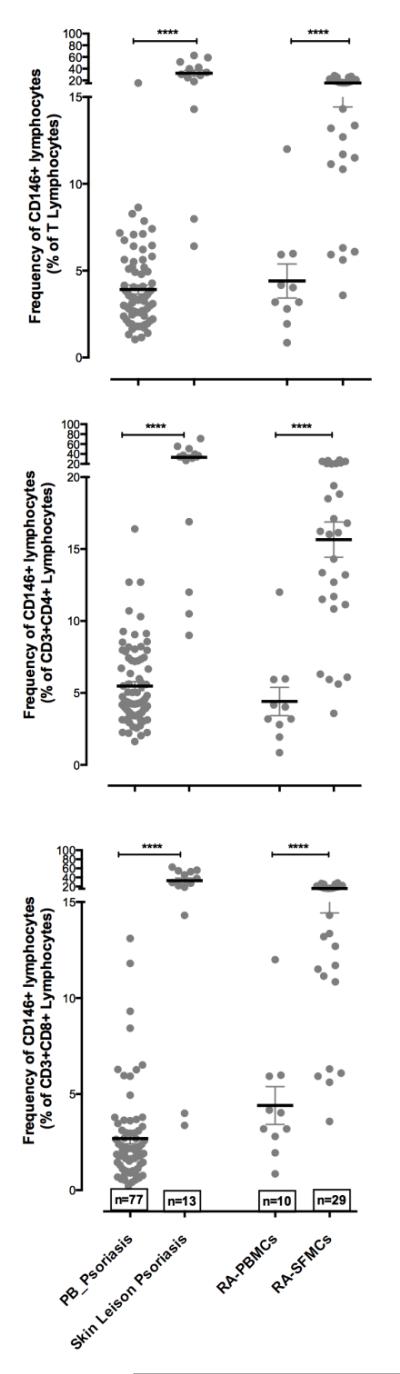
In studies of additional autoimmune diseases, patients with Sjögren’s syndrome (32) and psoriasis vulgaris (33) were found to have increased percentages of CD146+ T cells in peripheral blood. A landmark study identified EPIC T cells in active psoriatic skin lesions and revealed that these are prominent producers of IL-17 among the T cells in the active lesion (43); strongly implicating MCAM-expressing T cells in the pathogenesis of this disease, as psoriasis is an IL-17-driven disease (44, 45). In the skin lesions cytokine production was enhanced in MCAM+ T cells compared to circulating EPIC T cells. Furthermore, increased production of chemokines such as CXCL13 and CCL20 was also observed in these lesional MCAM+ T cells (Dagur and McCoy, unpublished), suggesting that this type of cell may play a role in attracting other cells to the site of inflammation.
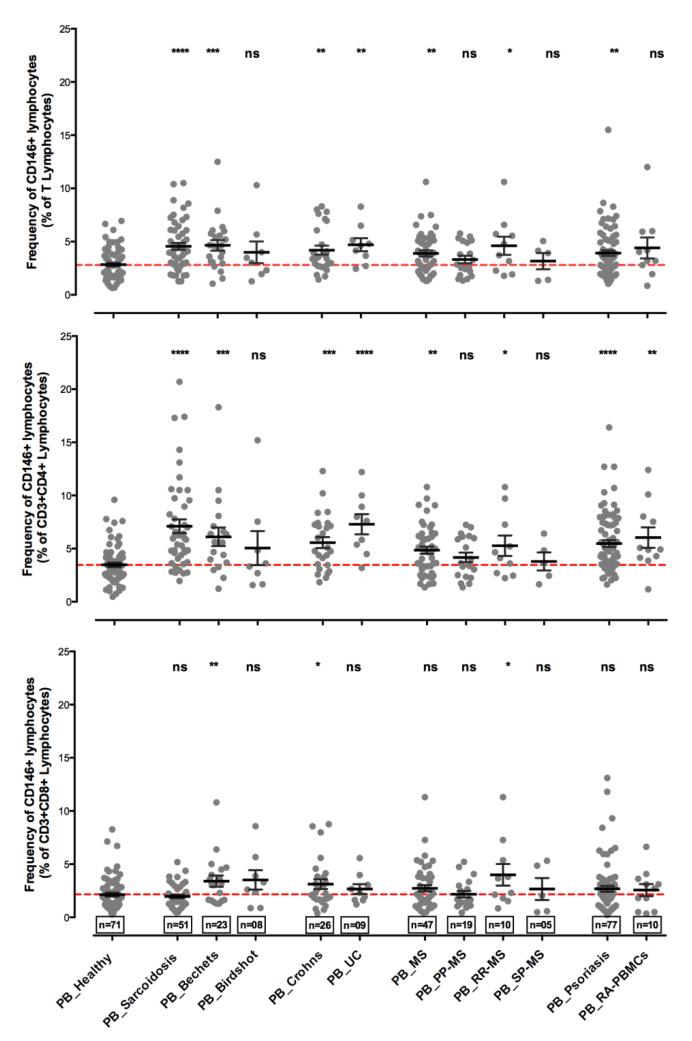
Increases in circulating MCAM positive T cells have been identified in several additional autoimmune diseases such as sarcoidosis, Behcet’s disease, Crohn’s disease, and birdshot retinochoroidopathy (34, 40). Figures 2 and 3 summarize the findings from our laboratory of CD146+ T cells in healthy subjects and in the blood and sites of inflammation in autoimmune diseases. As mentioned above, MCAM is not only expressed by CD4+ T cells but also by the CD8+ populations, which also secrete IL-17, consistent with a proposed effector role for CD8+ T cells in autoimmunity (46). It is of interest that some of these data suggest that MCAM+ CD8+ T cells are elevated in autoimmune diseases with HLA class 1 associations while MCAM+ CD4+ T cells are elevated in those autoimmune diseases without such an association. It has been suggested that this putative association of CD146+ CD8+ T cells may be of use in the differential diagnosis of autoimmune diseases with a class I association.
Figure 2. The frequencies of CD146+ T cells are elevated in peripheral circulation and are enriched at the site of inflammation in human autoimmune diseases.
The frequency of CD146+ T lymphocytes and T cells subsets in the peripheral circulation of healthy volunteers (n=71), non-infectious inflammatory uveitis of sarcoidosis (n=51); Bechet’s Disease (n=23); birdshot retinochoriodopathy (n=8)); inflammatory bowl diseases (Crohn’s (n=26) and ulcerative colitis (n=09), autoimmune neuroinflammatory disorder multiple sclerosis (n=47) and its sub categories (primary progressive or PPMS (n=19); secondary progressive or SPMS (n=10); and relapse and remitting or RRMS (n=05)); psoriasis (n=77) and rheumatoid arthritis (n=10). The scatter plots show the means and error bars representing standard error. (top) percent positive CD146+ cells in CD3+ T lymphocyte gate, (middle) percent positive CD4+CD146+ cells in T lymphocyte gate and (bottom) percent positive events in CD8+CD146+ cells in T lymphocyte gate. Red dashed line shows the mean frequency of CD146+ cells in healthy donors.
Figure 3.
(top) Scatter plots showing the mean with error bars representing standard error of the percent positive CD146+ cells in T lymphocyte gate in peripheral blood and at the site of inflammation (lesional skin for psoriasis and synovial fluid for RA); (middle) the percent positive CD4+CD146+ cells in T lymphocyte gate in the peripheral blood and at the site of inflammation (lesional skin for psoriasis (n=13) and synovial fluid for RA (n=29)); and (bottom) the percent positive events in CD8+CD146+ cells in T lymphocyte gate in peripheral blood and at the site of inflammation (lesional skin for psoriasis and synovial fluid for RA). Data were analyzed using Mann Whitney students T test with 95% confidence interval on Prism 6.0 software. P values were calculated. *= p<0.05; **=p<0.001; ***=p<0.0001; ****=p<0.00001; ns = not significant. For figure A, B and C values were compared between healthy and disease; for figure D, E and F values were compared between blood and the local site of inflammation.
A mouse model for EPIC T cells?
It is most common for novel immune subsets to be studied in a mouse model to perform various mechanistic studies in order to better understand the nature and function of those cells. Unfortunately, as pointed out by Davis, this is not always possible due to inherent differences between humans and inbred mice (47). In human leukocytes, MCAM binding is found predominantly on T lymphocytes, less on B lymphocytes, and only trace amounts are found on NK cells (31). In contrast, studies with mice have shown that MCAM binds primarily to NK cells with little, if any, binding to T cells. (34, 48). Conflicting data show CD146 expression on IL-17-secreting T cells in the mouse, similar to what is observed in humans (36, 37). Unfortunately both of the studies which demonstrated MCAM expression on murine T cells used monoclonal antibodies developed in their own laboratories rather than commercially available reagents, making replication of these findings difficult. However, this suggests that the commercially available antibodies to murine CD146 recognize epitopes found on endothelial cells and NK cells but not T cells. Further work is needed to resolve this discrepancy, and to determine of appropriate mouse models can be found for the study of EPIC T cells in vivo.
Discussion and future efforts
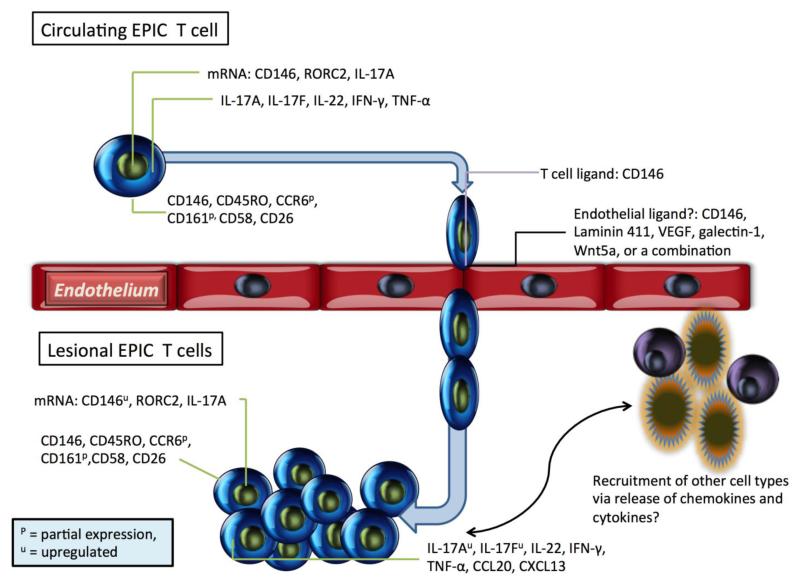
The EPIC T cells described here are interesting multi-functional cells having the abilities to bind to endothelium through a unique cell surface moiety and to produce cytokines such as interleukin-17 without ex vivo polarization using exogeneous cytokines (Figure 4). It would seem that these cells are positioned to be early adaptive responders due to their enhanced abilities to transmigrate and to increase cytokine production with only TCR engagement with CD28 costimulation. The increased numbers of these cells at the site of inflammation in psoriasis and rheumatoid arthritis as well as in the peripheral circulation of these patients suggests their role in the pathogenesis of these diseases, although at which stage remains to be determined through time course experimentation. Validation of the suggested mouse models would permit direct experimentation to observe EPIC T cell trafficking, however, to date, these mouse models have been difficult to replicate. Th17 cells have been demonstrated to orchestrate the pathogenesis of numerous autoimmune diseases (49) and thus the association of IL-17-secreting MCAM positive cells with autoimmunity comes as no surprise; however the identification of this cell surface MCAM is highly suggestive of a mechanism by which Th17 cells can transit from the peripheral circulation to sites of active inflammation. It is noteworthy that a recent publication concerning CD8αα innate-type lymphocytes revealed strong expression of CD146 on these cells (50), suggesting the expression of MCAM extends beyond traditional Th17 and Tc17 cells.
Figure 4. Features of MCAM+ T cells.
T cells expressing MCAM display an effector memory phenotype with the expression of IL-17, ILN-γ, and TNF-α. The presence of MCAM on the cell surface has been demonstrated to facilitate the binding and transmigration of these cells through the endothelium to the site of active inflammation by a mechanism that has yet to be fully elucidated.
To date there is no evidence to suggest a genetic link between MCAM expression and IL-17 secretion. Indeed, some IL-17 secreting T cells can be found in healthy individuals without concomitant MCAM expression on those cells.
The increased presence of the EPIC T cells in numerous IL-17 mediated autoimmune disorders and their prominence at the sites of inflammation in these diseases could have therapeutic implication. Early studies with targeted IL-17 therapy are showing promising results in psoriasis and rheumatoid arthritis (51-53) and it is certainly plausible that targeting EPIC T cells may be a viable alternative therapy. This could be direct targeting of the EPIC T cells via MCAM or, once the ligand for these cells is definitively identified, through interfering with the migration of these cells to sites of inflammation.
Clearly the work revealing the full nature and function of MCAM-expressing T cells is in the initial stages but this effort is gaining momentum as these cells are indentified in a myriad of autoimmune diseases. Preliminary data to date strongly indicate that these cells are primed for extravasation and cytokine secretion and their prevalence at the site of inflammation argue for a pathogenic role of EPIC T cells in inflammatory autoimmune diseases.
Take Home Points.
A small population of T lymphocytes can be identified by the expression of the adhesion molecule MCAM (CD146) in the peripheral circulation of healthy humans.
These MCAM+ cells secrete IL-17A, IFN-γ, and TNF-α without the need for ex vivo polarization and have an enhanced ability to bind to endothelial monolayers.
MCAM+ T cells are elevated in the circulation of patients with various autoimmune diseases such as rheumatoid arthritis, Behcet’s disease, sarcoidosis, inflammatory bowel disease, and psoriasis, and have been shown to be abundant in psoriatic skin lesions and are prominent producers of IL-17.
ACKNOWLEDGEMENTS
This work was supported by the intramural research program of the National Heart, Lung, and Blood Institute of NIH. The authors would like to thank numerous collaborators for providing clinical samples as well as valuable advice and insight: Robert S Nussenblatt, H Nida Sen, Warren Strober, Michael Yao, Bibi Bielekova, Mark Gourley, Nehal N Mehta, Angelique Biancotto, and Shawn Rose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lehmann JM, Holzmann B, Breitbart EW, Schmiegelow P, Riethmüller G, Johnson JP. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 1987;47(3):841–5. [PubMed] [Google Scholar]
- 2.Johnson JP, Rummel MM, Rothbächer U, Sers C. MUC18: A cell adhesion molecule with a potential role in tumor growth and tumor cell dissemination. Curr Top Microbiol Immunol. 1996;213(Pt 1):95–105. doi: 10.1007/978-3-642-61107-0_7. [DOI] [PubMed] [Google Scholar]
- 3.Bardin N, Francès V, Lesaule G, Horschowski N, George F, Sampol J. Identification of the S-Endo 1 endothelial-associated antigen. Biochem Biophys Res Commun. 1996;218(1):210–6. doi: 10.1006/bbrc.1996.0037. [DOI] [PubMed] [Google Scholar]
- 4.Khan SS, Solomon MA, McCoy JP. Detection of Circulating Endothelial Cells and Endothelial Progenitors Cells by Flow Cytometry. Cytometry. 2005;64B(1):1–8. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 5.Bardin N, Anfosso F, Massé JM, Cramer E, Sabatier F, Le Bivic A, Sampol J, Dignat-George F. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood. 2001;98(13):3677–84. doi: 10.1182/blood.v98.13.3677. [DOI] [PubMed] [Google Scholar]
- 6.Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189(1):4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Ouhtit A, Gaur RL, Abd Elmageed ZY, Fernando A, Thouta R, Trappey AK, Abdraboh ME, El-Sayyad HI, Rao P, Raj MG. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim Biophys Acta. 2009;1795(2):130–6. doi: 10.1016/j.bbcan.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330(2):150–62. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 9.Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, Peschle C, Valtieri M. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp Hematol. 2008;36(8):1035–46. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Wang W, Kapila Y, Lotz J, Kapila S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009;18(3):487–96. doi: 10.1089/scd.2008.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–19. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 12.Gruenloh W, Kambal A, Sondergaard C, McGee J, Nacey C, Kalomoiris S, Pepper K, Olson S, Fierro F, Nolta JA. Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells. Tissue Eng Part A. 2011;17(11-12):1517–25. doi: 10.1089/ten.tea.2010.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tormin A, Li O, Brune JC, Walsh S, Schütz B, Ehinger M, Ditzel N, Kassem M, Scheding S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117(19):5067–77. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maijenburg MW, Kleijer M, Vermeul K, Mul EP, van Alphen FP, van der Schoot CE, Voermans C. The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica. 2012;97(2):179–83. doi: 10.3324/haematol.2011.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guezguez B, Vigneron P, Lamerant N, Kieda C, Jaffredo T, Dunon D. Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor. J Immunol. 2007;179(10):6673–85. doi: 10.4049/jimmunol.179.10.6673. [DOI] [PubMed] [Google Scholar]
- 16.Flanagan K, Fitzgerald K, Baker J, Regnstrom K, Gardai S, Bard F, Mocci S, Seto P, You M, Larochelle C, Prat A, Chow S, Li L, Vandevert C, Zago W, Lorenzana C, Nishioka C, Hoffman J, Botelho R, Willits C, Tanaka K, Johnston J, Yednock T. Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS. PLoS One. 2012;7(7):e40443. doi: 10.1371/journal.pone.0040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa T, Wondimu Z, Oikawa Y, Ingerpuu S, Virtanen I, Patarroyo M. Monoclonal antibodies to human laminin α4 chain globular domain inhibit tumor cell adhesion and migration on laminins 411 and 421, and binding of α6β1 integrin and MCAM to α4-laminins. Matrix Biol. 2014;36:5–14. doi: 10.1016/j.matbio.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Schneider-Hohendorf T, Rossaint J, Mohan H, Böning D, Breuer J, Kuhlmann T, Gross CC, Flanagan K, Sorokin L, Vestweber D, Zarbock A, Schwab N, Wiendl H. VLA-4 blockade promotes differential routes into human CNS involving PSGL-1 rolling of T cells and MCAM-adhesion of TH17 cells. J Exp Med. 2014;211(9):1833–46. doi: 10.1084/jem.20140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jouve N, Despoix N, Espeli M, Gauthier L, Cypowyj S, Fallague K, Schiff C, Dignat-George F, Vély F, Leroyer AS. The involvement of CD146 and its novel ligand Galectin-1 in apoptotic regulation of endothelial cells. J Biol Chem. 2013;288(4):2571–9. doi: 10.1074/jbc.M112.418848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang T, Zhuang J, Duan H, Luo Y, Zeng Q, Fan K, Yan H, Lu D, Ye Z, Hao J, Feng J, Yang D, Yan X. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood. 2012;120(11):2330–9. doi: 10.1182/blood-2012-01-406108. [DOI] [PubMed] [Google Scholar]
- 21.Ye Z, Zhang C, Tu T, Sun M, Liu D, Lu D, Feng J, Yang D, Liu F, Yan X. Wnt5a uses CD146 as a receptor to regulate cell motility and convergent extension. Nat Commun. 2013;4:2803. doi: 10.1038/ncomms3803. [DOI] [PubMed] [Google Scholar]
- 22.Anfosso F, Bardin N, Francès V, Vivier E, Camoin-Jau L, Sampol J, Dignat-George F. Activation of human endothelial cells via S-endo-1 antigen (CD146) stimulates the tyrosine phosphorylation of focal adhesion kinase p125(FAK) J Biol Chem. 1998;273(41):26852–6. doi: 10.1074/jbc.273.41.26852. [DOI] [PubMed] [Google Scholar]
- 23.Anfosso F, Bardin N, Vivier E, Sabatier F, Sampol J, Dignat-George F. Outside-in signaling pathway linked to CD146 engagement in human endothelial cells. J Biol Chem. 2001;276(2):1564–9. doi: 10.1074/jbc.M007065200. [DOI] [PubMed] [Google Scholar]
- 24.Solovey AN, Gui L, Chang L, Enenstein J, Browne PV, Hebbel RP. Identification and functional assessment of endothelial P1H12. J Lab Clin Med. 2001;138(5):322–31. doi: 10.1067/mlc.2001.118519. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Kalabis J, Xu X, Meier F, Oka M, Bogenrieder T, Herlyn M. Reciprocal regulation of MelCAM and AKT in human melanoma. Oncogene. 2003;22(44):6891–9. doi: 10.1038/sj.onc.1206819. [DOI] [PubMed] [Google Scholar]
- 26.Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320(5874):365–9. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno K, Hirata H, Majid S, Tabatabai ZL, Hinoda Y, Dahiya R. IGFBP-4 activates the Wnt/beta-catenin signaling pathway and induces M-CAM expression in human renal cell carcinoma. Int J Cancer. 2011;129(10):2360–9. doi: 10.1002/ijc.25899. [DOI] [PubMed] [Google Scholar]
- 28.Pickl WF, Majdic O, Fischer GF, Petzelbauer P, Faé I, Waclavicek M, Stöckl J, Scheinecker C, Vidicki T, Aschauer H, Johnson JP, Knapp W. MUC18/MCAM (CD146), an activation antigen of human T lymphocytes. J Immunol. 1997;158(5):2107–15. [PubMed] [Google Scholar]
- 29.Elshal MF, Khan SS, Takahashi Y, Solomon MA, McCoy JP., Jr. CD146 (Mel-CAM), an adhesion marker of endothelial cells, is a novel marker of lymphocyte subset activation in normal peripheral blood. Blood. 2005;106(8):2923–4. doi: 10.1182/blood-2005-06-2307. [DOI] [PubMed] [Google Scholar]
- 30.Seftalioğlu A, Karakoç L. Expression of CD146 adhesion molecules (MUC18 or MCAM) in the thymic microenvironment. Acta Histochem. 2000;102(1):69–83. doi: 10.1078/0065-1281-00544. [DOI] [PubMed] [Google Scholar]
- 31.Elshal MF, Khan SS, Raghavachari N, Takahashi Y, Barb J, Bailey JJ, Munson PJ, Solomon MA, Danner RL, McCoy JP., Jr. A unique population of effector memory lymphocytes identified by CD146 having a distinct immunophenotypic and genomic profile. BMC Immunol. 2007;8:29. doi: 10.1186/1471-2172-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadjinicolaou AV, Wu L, Fang B, Watson PA, Hall FC, Busch R. Relationship of CD146 expression to activation of circulating T cells: exploratory studies in healthy donors and patients with connective tissue diseases. Clin Exp Immunol. 2013;174(1):73–88. doi: 10.1111/cei.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamiyama T, Watanabe H, Iijima M, Miyazaki A, Iwamoto S. Coexpression of CCR6 and CD146 (MCAM) is a marker of effector memory T-helper 17 cells. J Dermatol. 2012;39(10):838–42. doi: 10.1111/j.1346-8138.2012.01544.x. [DOI] [PubMed] [Google Scholar]
- 34.Dagur PK, Biancotto A, Wei L, Sen HN, Yao M, Strober W, Nussenblatt RB, McCoy JP., Jr. MCAM-expressing CD4(+) T cells in peripheral blood secrete IL-17A and are significantly elevated in inflammatory autoimmune diseases. J Autoimmun. 2011;37(4):319–27. doi: 10.1016/j.jaut.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132(Pt 12):3329–41. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 36.Larochelle C, Cayrol R, Kebir H, Alvarez JI, Lécuyer MA, Ifergan I, Viel É , Bourbonnière L, Beauseigle D, Terouz S, Hachehouche L, Gendron S, Poirier J, Jobin C, Duquette P, Flanagan K, Yednock T, Arbour N, Prat A. Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain. 2012;135(Pt 10):2906–24. doi: 10.1093/brain/aws212. [DOI] [PubMed] [Google Scholar]
- 37.Duan H, Xing S, Luo Y, Feng L, Gramaglia I, Zhang Y, Lu D, Zeng Q, Fan K, Feng J, Yang D, Qin Z, Couraud PO, Romero IA, Weksler B, Yan X. Targeting endothelial CD146 attenuates neuroinflammation by limiting lymphocyte extravasation to the CNS. Sci Rep. 2013;3:1687. doi: 10.1038/srep01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alais S, Allioli N, Pujades C, Duband JL, Vainio O, Imhof BA, Dunon D. HEMCAM/CD146 downregulates cell surface expression of beta1 integrins. J Cell Sci. 2001;114(Pt 10):1847–59. doi: 10.1242/jcs.114.10.1847. [DOI] [PubMed] [Google Scholar]
- 39.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206(1):43–9. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dagur PK, Biancotto A, Stansky E, Sen HN, Nussenblatt RB, McCoy JP. Secretion of interleukin-17 by CD8+ T cells expressing CD146 (MCAM) Clin Immunol. 2014;152(1-2):36–47. doi: 10.1016/j.clim.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dagur PK, Tatlici G, Gourley M, Samsel L, Raghavachari N, Liu P, Liu D, McCoy JP., Jr. CD146+ T lymphocytes are increased in both the peripheral circulation and in the synovial effusions of patients with various musculoskeletal diseases and display pro-inflammatory gene profiles. Cytometry B Clin Cytom. 2010;78(2):88–95. doi: 10.1002/cyto.b.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C, Goodall JC, Busch R, Gaston JS. Relationship of CD146 expression to secretion of interleukin-17, interleukin-22, and interferon-γ by CD4+ T cells in patients with inflammatory arthritis. Clin Exp Immunol. 2014 doi: 10.1111/cei.12434. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta NN, Dagur PK, Rose SM, Naik HB, Stansky E, Doveikis J, Biancotto A, Playford MP, McCoy JP., Jr. IL-17A Production in Human Psoriatic Blood and Lesions by CD146+ T Cells. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.317. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2013;44(2):183–93. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki E, Mellins ED, Eric Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496–502. doi: 10.1016/j.autrev.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gravano DM, Hoyer KK. Promotion and prevention of autoimmune disease by CD8 T cells. J. Autoimmun. 2013;45:68–79. doi: 10.1016/j.jaut.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Davis MM. A prescription for human immunology. Immunity. 2008;29(6):835–8. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Despoix N, Walzer T, Jouve N, Blot-Chabaud M, Bardin N, Paul P, Lyonnet L, Vivier E, Dignat-George F, Vély F. Mouse CD146/MCAM is a marker of natural killer cell maturation. Eur J Immunol. 2008;38(10):2855–64. doi: 10.1002/eji.200838469. [DOI] [PubMed] [Google Scholar]
- 49.Singh RP, Hasan S, Sharma S, Nagra S, Yamaguchi DT, Wong DTW, Hahn BH, Hossain A. Th17 cells in inflammation and autoimmunity. Autoimmun Rev. 2014;13:1174–1181. doi: 10.1016/j.autrev.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 50.Van Kaer L, Algood HM, Singh K, Parekh VV, Greer MJ, Piazuelo MB, Weitkamp JH, Matta P, Chaturvedi R, Wilson KT, Olivares-Villagómez D. CD8αα+ innate-type lymphocytes in the intestinal epithelium mediate mucosal immunity. Immunity. 2014;41(3):451–64. doi: 10.1016/j.immuni.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones SA, Sutton CE, Cua D, Mills KH. Therapeutic potential of targeting IL-17. Nat Immunol. 2012;13(11):1022–5. doi: 10.1038/ni.2450. [DOI] [PubMed] [Google Scholar]
- 52.Kellner H. Targeting interleukin-17 in patients with active rheumatoid arthritis: rationale and clinical potential. Ther Adv Musculoskelet Dis. 2013;5(3):141–52. doi: 10.1177/1759720X13485328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novelli L, Chimenti MS, Chiricozzi A, Perricone R. The new era for the treatment of psoriasis and psoriatic arthritis: Perspectives and validated strategies. Autoimmun Rev. 2014;13:64–69. doi: 10.1016/j.autrev.2013.08.006. [DOI] [PubMed] [Google Scholar]