Abstract
Objective
We sought to determine the therapeutic effect of robotic-assisted step training (RAST) on neuromuscular abnormalities associated with spasticity by characterization of their recovery patterns in people with spinal cord injury (SCI).
Methods
Twenty-three motor-incomplete SCI subjects received one-hour RAST sessions three times per week for four weeks, while an SCI control group received no training. Neuromuscular properties were assessed using ankle perturbations prior to and during the training, and a system-identification technique quantified stretch reflex and intrinsic stiffness magnitude and modulation with joint position. Growth-mixture modeling classified subjects based on similar intrinsic and reflex recovery patterns.
Results
All recovery classes in the RAST group presented significant (p<0.05) reductions in intrinsic and reflex stiffness magnitude and modulation with position; the control group presented no changes over time. Subjects with larger baseline abnormalities exhibited larger reductions, and over longer training periods.
Conclusions
Our findings demonstrate that RAST can effectively reduce neuromuscular abnormalities, with greater improvements for subjects with higher baseline abnormalities.
Significance
Our findings suggest, in addition to its primary goal of improving locomotor patterns, RAST can also reduce neuromuscular abnormalities associated with spasticity. These findings also demonstrate that these techniques can be used to characterize neuromuscular recovery patterns in response to various types of interventions.
Keywords: Locomotor training, reflex, spasticity, ankle, spinal cord injury, gait rehabilitation
Introduction
Two primary clinical sequelae following with incomplete spinal cord injury (SCI) are spasticity and impaired voluntary movement (Barbeau et al., 2002, Drolet et al., 1999, Katz and Rymer, 1989, Lehmann et al., 1989, Thomas et al., 1997). Spasticity is a motor disorder associated with lesions of the nervous system (Lance, 1980), which can lead to changes in mechanical properties of the neuromuscular system (Mirbagheri et al., 2001) and lead to several forms of motor impairment including dysfunction of motor coordination (Burne et al., 2005, Gerhart et al., 1993, Thomas et al., 1998). Many gait impairments are associated with spasticity in the lower extremity, including the inability to distribute weight evenly between limbs, reduced step width and length, and abnormalities in step rhythm and function; all of these factors can negatively affect walking capacity (Field-Fote et al., 2001).
Clinical symptoms of spasticity in SCI include hypertonia, uncontrolled spasms, clonus, and the clasp-knife phenomenon (Lance, 1980). Hypertonia is defined as an abnormal increase in resistance to passive movement (Katz and Rymer, 1989, Lance, 1980). Regarded as the defining feature of spasticity (Katz and Rymer, 1989), hypertonia can arise from abnormalities in the mechanical properties of passive tissues, muscle fibers, and stretch reflexes (Dietz et al., 1981, Hufschmidt and Mauritz, 1985, Mirbagheri et al., 2001, O'Dwyer et al., 1996, Sinkjaer and Magnussen, 1994). Since hypertonia manifests as a mechanical abnormality, it is appropriate to characterize it in terms of mechanical properties of the spastic joint (Mirbagheri et al., 2000, Mirbagheri et al., 2001). The overall mechanical properties of a joint at rest are determined by the combination of i) intrinsic mechanisms arising from inertial and viscoelastic properties of the joint and ii) reflex mechanisms arising from changes in muscle activation due to afferent response to stretch. Hyperexcitable reflexes manifest as a stiffer joint, i.e., a joint more resistant to imposed motion; this quantitative characteristic is defined here as stretch reflex stiffness. This separation is useful for quantifying the nature of abnormalities associated with spasticity, as each abnormality requires its own specific treatments, and thus has a diagnostic and therapeutic significance.
One physical intervention used to promote gait recovery and improve function is body weight-supported treadmill training (BWSTT) with manual assistance (Barbeau and Fung, 2001, Behrman and Harkema, 2000, Dietz and Harkema, 2004, Wernig et al., 1995). Here, subjects are provided with partial support of body weight over a treadmill and therapists provide assistance to promote foot clearance and to prevent knee buckling during the stance phase of gait. Recently, robotic devices have been developed to assist therapists in the rehabilitation of people with neurological injury (Volpe et al., 2001). One such device is the LOKOMAT (Hocoma, Switzerland), a powered device that provides robotic-assisted step training (RAST) similar to BWSTT, but through a motorized exoskeleton that attaches to the patient’s legs, rather than manual positioning by a therapist (Colombo et al., 2000). Recent results have shown that RAST can improve walking capacity by improving gait speed, endurance, or temporal patterns of electromyographic (EMG) activity (Field-Fote et al., 2005, Hornby et al., 2005, Mazzoleni et al., 2011, Schwartz et al., 2011, Wirz et al., 2005). It has also been shown that RAST can improve spasticity as measured by the Pendulum Test (Manella et al., 2010) and reflex excitability (Manella and Field-Fote, 2013), but had no significant impact on hypertonia as measured by the Modified Ashworth Scale (MAS) (Manella and Field-Fote, 2013, Manella et al., 2010). Thus, the effect of RAST on spasticity has been inadequately studied and remains controversial.
While these studies have investigated the effects of RAST on EMGs, gait kinematics, clinical and electrophysiological measures of spasticity, the influence of such training on neuromuscular abnormalities associated with spasticity has been seldom studied, largely due to the aforementioned lack of quantitative and objective clinical tools to characterize the neuromuscular components of hypertonia. Given that alternative physical therapies have been shown to reduce hypertonia, for example passive cycling with and without functional electrical stimulation (Kakebeeke et al., 2005, Krause et al., 2008, Rayegani et al., 2011), we hypothesized that RAST would similarly have a positive effect on reducing hypertonia. One preliminary study found that a single session of RAST could yield a significant reduction in joint stiffness in children with spastic cerebral palsy (Schmartz et al., 2011). However, the long-term effect of this training on joint stiffness, particularly once training is completed, is unclear.
Typically no intervention has a uniform impact across all subjects; rather there is a marked heterogeneity in patient response. However, the standard pre- vs. post-treatment analysis, which uses group-averaging techniques, neglects the substantial heterogeneity among SCI individuals. Consequently, there is a need for the use of advanced statistical methods, such as growth mixture models, that can classify subjects by similar recovery patterns and model these patterns over time.
In this study, we addressed these deficits, for the first time, by characterizing the effects of RAST on stretch reflex and muscular mechanical properties for SCI subjects, using system identification techniques (Mirbagheri et al., 2000, Mirbagheri et al., 2001), and by identifying and modeling different recovery patterns for the muscular and reflex stiffness properties over the course of one month due to RAST. We investigated the therapeutic effects of the training on stretch reflex stiffness, which increases abnormally after SCI (Mirbagheri et al., 2001).
Methods
Subjects
Forty-six ambulatory chronic SCI subjects with incomplete motor function loss participated in this single-center, randomized study. All subjects had motor-incomplete SCI with an American Spinal Injury Association Impairment Scale (ASIA) classification of C or D (Kirshblum et al., 2011), a lesion level between C2 and T9, were ambulatory or had a passive range of motion (PROM) within functional limits for ambulation, had ankle spasticity indicated by a MAS score of 1 or greater, and were granted medical clearance to participate. Twenty-three subjects were randomly assigned to the intervention group to receive RAST, while the other half were assigned to the control group and received no intervention. The RAST group (7 females and 16 males) had mean±SD age of 46.4±12.6 years, were evaluated 10.1±8.3 years after the injury, and had median (interquartile range) MAS scores of 2 (1.8–3). The control group (8 females and 15 males) had mean±SD age of 47.9±12.2 years, were evaluated 8.9±8.2 years post-injury, and had median (interquartile range) MAS scores of 2 (2–3).
Subjects were draw from the Rehabilitation Institute of Chicago’s outpatient service. All provided informed consent and the Northwestern University Institutional Review Board approved the study.
Robotic-Assisted Step Training (RAST)
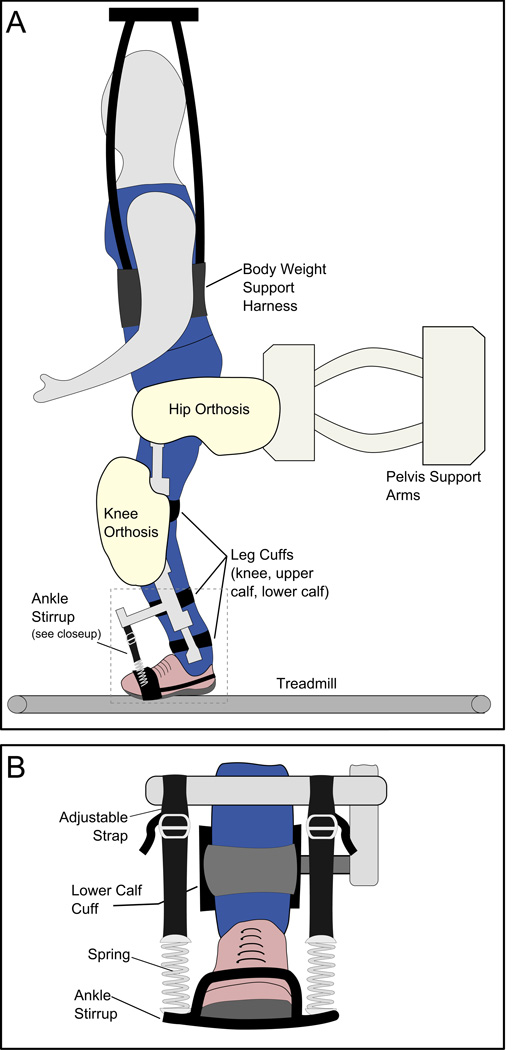
The RAST device consists of four motors aligned bilaterally at the hip and knee joints and attached to the patient’s legs by fitted cuffs (Figure 1a). These motors move through physiological gait patterns while the patient walks on a motorized treadmill. The degree to which the device assists gait can be adjusted by the therapist. Dynamic body weight support provides automatic lifting and unloading of the patient through an overhead harness (Colombo et al., 2000). Adjustable elastic stirrups attached to the motorized shank prevented foot-drop and supported the ankles in the neutral (90°) position but allowed ankle movements by the subject (Figure 1b). During training, subjects were encouraged (through therapist’s feedback and a mirror placed in front of the subject) to voluntarily contribute to their ambulation and replicate stepping behavior as much as possible, with particular attention given to activating their ankle muscles.
Figure 1.
A: Lokomat apparatus.
B: Close-up view of ankle supports on Lokomat device.
All participants in the RAST group received training three times a week for four weeks (12 sessions in total). Each 1-hour session included up to 45 minutes of training. During training, we increased the treadmill speed from 1.5 km/h to 2.8 km/h, reduced the guidance force (i.e., the level of robotic assistance provided to each leg) from full-assistance to 20% assistance, and reduced the body-weight support from 95% to 25%, customized to the individual subject’s ability.
Experimental Apparatus
To quantify the neuromuscular properties (intrinsic and reflex stiffness) of the ankle joint, ankle perturbations were applied to one of the subject’s ankles to elicit the stretch reflex. Typically, in motor-incomplete SCI subjects, the sides of the body are affected differently. The tested ankle was the one on which the higher MAS score was observed prior to RAST; this usually corresponded with the patient’s self-reported “weaker” side.
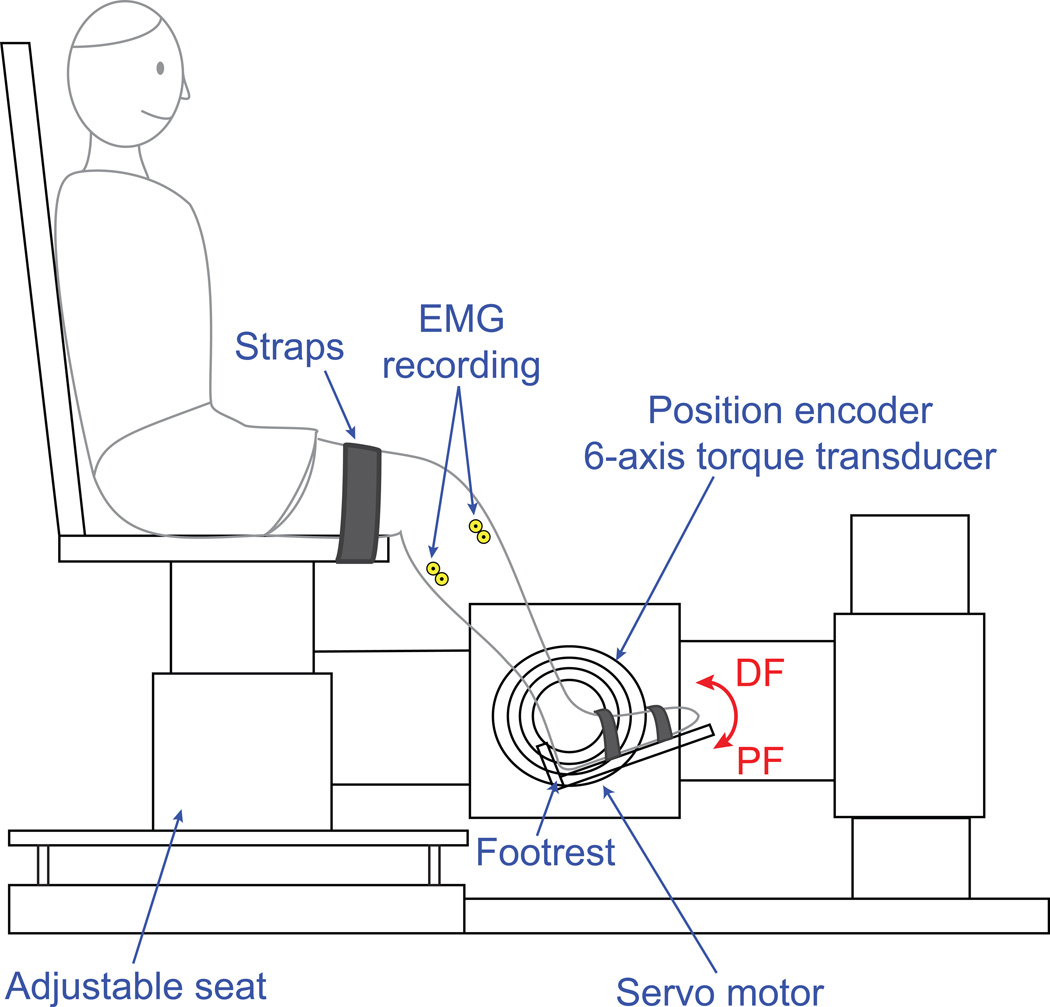
Participants were seated and secured in an experimental chair with the tested ankle strapped to a custom “footrest” mounted to the shaft of a position-controlled servo motor, aligned with the ankle joint center of rotation (Figure 2). Ankle position and torque were recorded by a rotary encoder and 6-axis torque transducer, respectively. The neutral position (NP) was defined as 90°,with plantarflexion considered negative. Electomyograms were recorded using bipolar surface electrodes (Delsys Inc., Boston MA), placed on the belly of the tibialis anterior and gastrocnemius muscles, amplified (10k gains for all channels) and low-pass filtered (8 dB/decade rolloff) using a 16-channel Delsys Bagnoli amplifier. All data were subsequently sampled by a 16-bit A/D converter (National Instruments, Austin, TX) and anti-aliased filtered on-line at 230 Hz.
Figure 2.
Experimental apparatus.
Experimental Procedure
Prior to the experiment, the PROM was determined by an examiner manually stretching the patient’s ankle in both directions at a very slow speed (to prevent muscle activation) until maximal resistance or pain was reached. The motor then applied pseudo-random binary sequence perturbations to the ankle, with 0.03 rad amplitude and 150 ms switching rate, at 5° position increments over its PROM, while the knee was held at 60° flexion.
These experiments were conducted while the subject was passive, i.e., no active contractions of the muscles were performed (verified by real-time monitoring of EMG activity; trials with active contractions were repeated).
Evaluations were performed at baseline (prior to training), and after 1, 2, and 4 weeks of RAST.
Analytical Method: Identification of Neuromuscular Properties
Intrinsic and reflex contributions to the ankle stiffness dynamics were separated using a parallel-cascade system identification technique (Mirbagheri et al., 2000, Mirbagheri et al., 2001). We have previously used this technique to demonstrate that ankle stiffness is strongly dependent on ankle angle in both healthy and spastic subjects (Mirbagheri et al., 2001, Mirbagheri et al., 2007). This method is valid (Alibiglou et al., 2008) and sensitive enough to detect the nature of mechanical abnormalities associated with spasticity throughout the progression of disease and following specific treatments (Mirbagheri et al., 2011, Mirbagheri et al., 2012, Mirbagheri et al., 2009). This method separates the measured joint torque due to perturbations into intrinsic (musculotendinous) and reflex contributions. The intrinsic pathway is modeled as a second-order dynamic system with intrinsic stiffness K (the amount of torque per unit change in ankle position), while the reflex pathway consists of a differentiator (to convert position to velocity), a time delay, a half-wave rectifier in velocity, and a third-order dynamic system with reflex stiffness G (which describes the amount of torque per unit change in perturbation velocity). This analysis was performed separately for each of the evaluated ankle positions, yielding stiffness vs. joint angle curves for each subject.
Modulation of Intrinsic and Reflex Stiffness with Ankle Joint
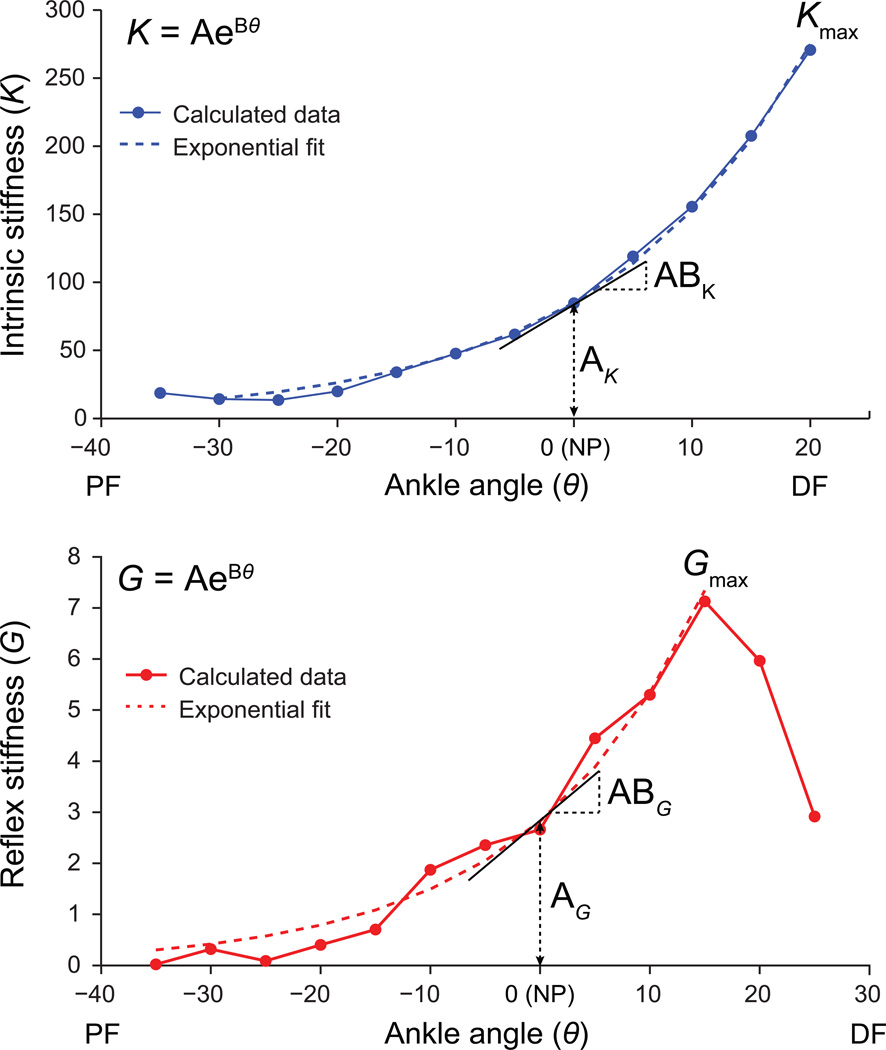
For each test, the increasing portion of the stretch reflex stiffness vs. angle (θ) curve was fit to an exponential model:
| (1) |
where coefficient A represents stiffness at the neutral position (θ=0), and B is the exponential factor (Figure 3). Differentiating (1) with respect to θ gives:
| (2) |
Figure 3.
Illustration of exponential model fit to reflex and intrinsic stiffness data.
Thus, the product of A and B represents the slope of the exponential curve through the neutral position. This model was fit to the same θ-range for a given subject for all four evaluations. Parameters A (offset or intercept) and AB (slope) were used to quantify how the stiffness-angle curve changed over time. Further, the maximum value of G over the PROM (i.e. Gmax) was tracked over time. The same exponential model was fit to the intrinsic stiffness K vs. angle curves; for clarity, a subscript G indicates reflex fit parameters, while a subscript K indicates intrinsic fit parameters.
Statistical Analysis: Identification of Neuromuscular Recovery Patterns
Growth Mixture Modeling (GMM) was then used to characterize the recovery patterns of neuromuscular parameters identified above for both RAST and control groups. GMM is a longitudinal mixture model which assumes that a population can be partitioned into distinct classes based on similar recovery patterns, and has been widely used to analyze heterogeneity in developmental pathways (Hagenaars and McCutcheon, 2002, Jung and Wickrama, 2008, Muthen, 2004, Muthen and Muthen, 2000). GMM was applied separately for six key parameters of interest: AG, ABG, and Gmax (reflex fit), and AK, ABK, and Kmax (intrinsic fit). The quality of the resulting classification was evaluated by the posterior probabilities of class membership and the Bayesian Information Criterion. We have previously demonstrated the validity of this model (Mirbagheri et al., 2008b) for tracking recovery during rehabilitation.
Random Coefficient Regression (RCR) was then used within each class to model the recovery pattern as an exponential function of time, and to identify whether the change in the parameter value over time was significant:
| (3) |
where t = (0, 1, 2, 4) to represent the training week (t=0 is the baseline). The coefficient C0 has the same units as the parameter (AK, ABK, etc.), while β has units of 1/week. This equation was then transformed to a linear form as:
| (4) |
RCR determined whether ln C0 and β were significantly different from zero (p < 0.05). A significant value of β indicates that the given parameter changed over time. This approach provides insight into whether the RAST intervention provided a statistically-significant effect on the intrinsic and reflex stiffness parameters.
Results
To review, for each discrete angular position over the ankle PROM, the system identification technique was used to quantify the intrinsic and reflex stiffness properties. The stiffness vs. angle curves were then modeled using exponential trends, and GMM and RCR techniques were used to model the changes in the exponential parameters over time. This analysis allowed us to determine whether the RAST provided a significant reduction in neuromuscular properties over the course of four weeks of training.
Effects of Robotic-Assisted Step Training on Neuromuscular Properties
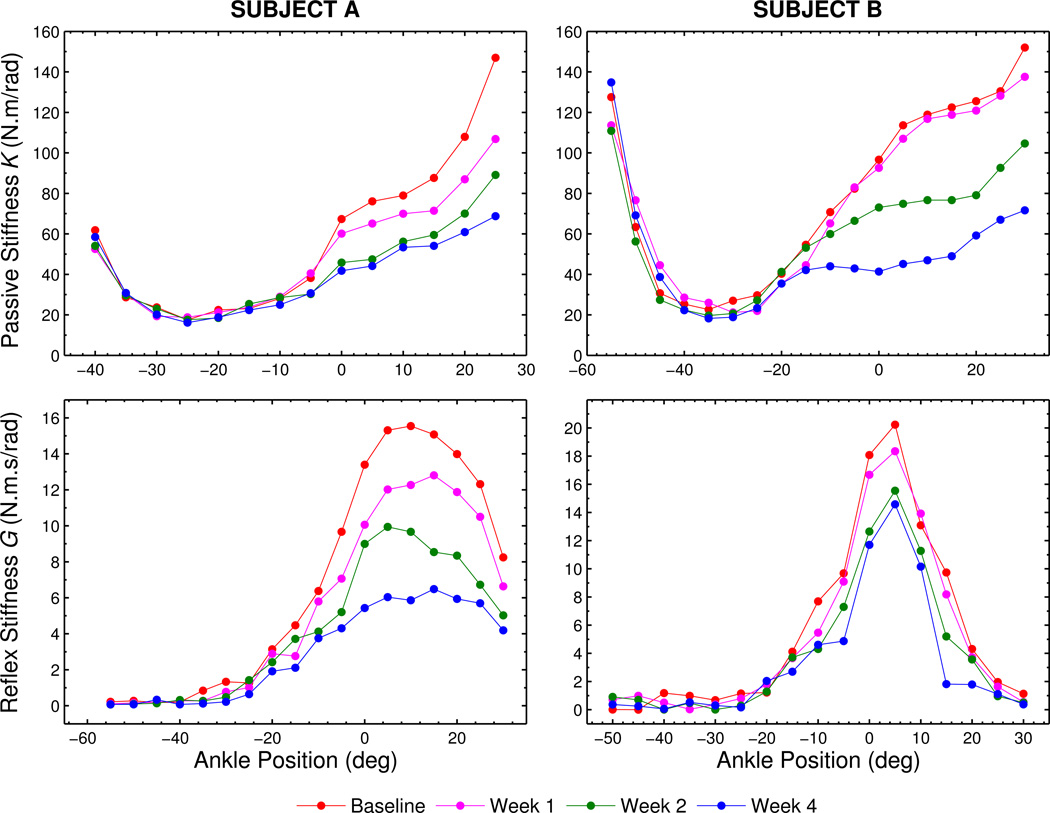
Our earlier studies showed that both intrinsic and reflex stiffness abnormally increase and modulate with the ankle joint position after SCI (Mirbagheri et al., 2001, 2002). In the current study, we observed that subjects exhibited different recovery patterns of these abnormalities over time. Consider two typical subjects (Figure 4): Subject A experiences an approximately-uniform change in stiffness over time, while subject B exhibits minimal changes in intrinsic and reflex stiffness between the baseline and week 1 observations but marked changes arose between weeks 1 and 2. This finding shows there is no single recovery pattern, which underscores the need to delineate the subjects into multiple recovery patterns.
Figure 4.
Illustration of stiffness trends with ankle position, for two representative subjects. Note that subjects A and B exhibit different recovery patterns over time.
Therapeutic Effects: Identification of Recovery Patterns of Intrinsic and Reflex Stiffness
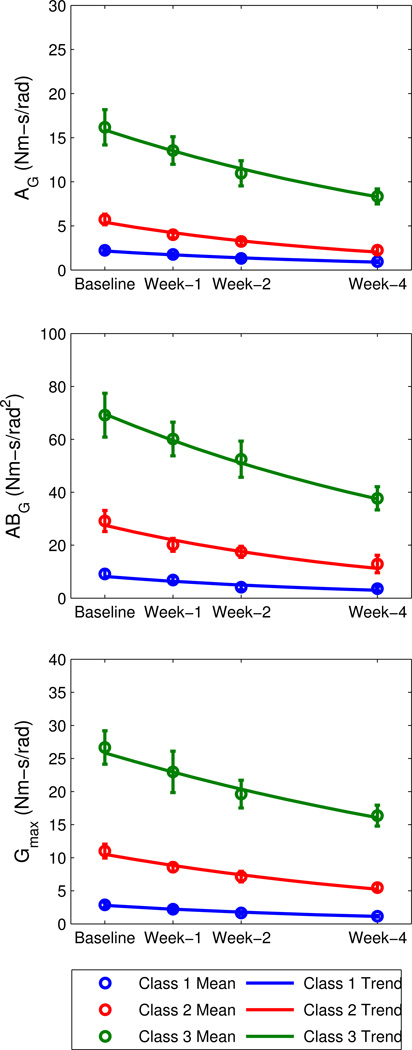
For the RAST group, GMM was used to identify three classes for each of the reflex parameters. For consistency, classes were numbered in order of increasing values of the mean baseline parameter within each class, such that the group with the lowest mean baseline was termed ‘Class 1’, and the group with the highest mean baseline was termed ‘Class 3’ (See Figure 5 and Table 1).
Figure 5.
Reflex stiffness trends, by class, for the robotic-assisted step training (RAST) group.
Table 1.
Results from GMM class separation and exponential trend fitting for LOKOMAT group.
| Parameter (units) |
GMM Classification |
Class Mean ± Std. Error | Exponential Trend Parameters | ||||
|---|---|---|---|---|---|---|---|
| Class | N | Baseline | Week-4 | C0 | β (1/week) |
Rate (C0* β) |
|
|
AG (Nm-s/rad) |
1 | 5 (22%) | 2.22±0.18 | 0.95±0.12 | 2.15*** | −0.220** | −0.5 |
| 2 | 7 (30%) | 5.74±0.60 | 2.25±0.45 | 5.41**** | −0.245** | −1.3 | |
| 3 | 11 (48%) | 16.18±2.00 | 8.34±0.85 | 15.89**** | −0.162*** | −2.6 | |
|
ABG (Nm-s/rad2) |
1 | 6 (26%) | 9.17±1.58 | 3.57±0.72 | 8.02** | −0.253*** | −2.0 |
| 2 | 8 (35%) | 29.18±3.98 | 12.91±3.31 | 27.50**** | −0.224** | −6.2 | |
| 3 | 9 (39%) | 69.14±8.27 | 37.72±4.37 | 69.33**** | −0.155** | −10.7 | |
|
Gmax (Nm-s/rad) |
1 | 6 (26%) | 2.87±0.41 | 1.15±0.13 | 2.79**** | −0.226** | −0.6 |
| 2 | 11 (48%) | 11.01±1.05 | 5.49±0.57 | 10.51**** | −0.174*** | −1.8 | |
| 3 | 6 (26%) | 26.66±2.52 | 16.36±1.58 | 25.84**** | −0.119* | −3.1 | |
|
AK (Nm/rad) |
1 | 11 (50%) | 58.74±2.79 | 46.92±2.94 | 57.19**** | −0.057** | −3.3 |
| 2 | 11 (50%) | 99.57±5.13 | 74.05±4.48 | 99.15**** | −0.077**** | −7.6 | |
|
ABK (Nm/rad2) |
1 | 7 (32%) | 116.75±6.13 | 78.04±9.27 | 112.87**** | −0.109* | −12.3 |
| 2 | 15 (68%) | 218.28±13.04 | 171.81±11.61 | 218.87**** | −0.063**** | −13.8 | |
|
Kmax (Nm/rad) |
1 | 12 (55%) | 152.28±2.97 | 130.81±4.12 | 151.37*** | −0.039** | −5.9 |
| 2 | 10 (45%) | 225.77±13.35 | 159.57±13.68 | 223.10**** | −0.097*** | −21.6 | |
Significance level:
:p < 0.05,
:p < 0.01,
:p < 0.001,
:p < 0.0001
For the reflex parameters, subjects in all classes exhibited continuous, significant decreases in AG, ABG, and Gmax during the training period (Figure 4). This was evident because β was negative and significantly different from zero (p<0.05) for all classes (Table 1). (The intercept C0 was also significantly different from zero, with p<0.01). As the exponential trends were close to linear during the time range of interest (0–4 weeks), the baseline slope (i.e. C0*β) was used to estimate the rate of change over the training period.
For all reflex parameters (AG, ABG, and Gmax), the rates of change (Table 1) became more negative with increasing class number (higher baseline mean value). As a result, subjects in Class 3 exhibited a reduction in their reflex stiffness and slope that was about five times higher than subjects in Class 1, and 1.7–2 times higher than subjects in Class 2 over the same time period. In fact, the mean baseline value correlated strongly with the rate of change (r2 =0.94, p<0.0001), indicating that subjects with higher baseline values of stiffness or slope tended to exhibit greater reductions in reflex stiffness or slope in response to the RAST training.
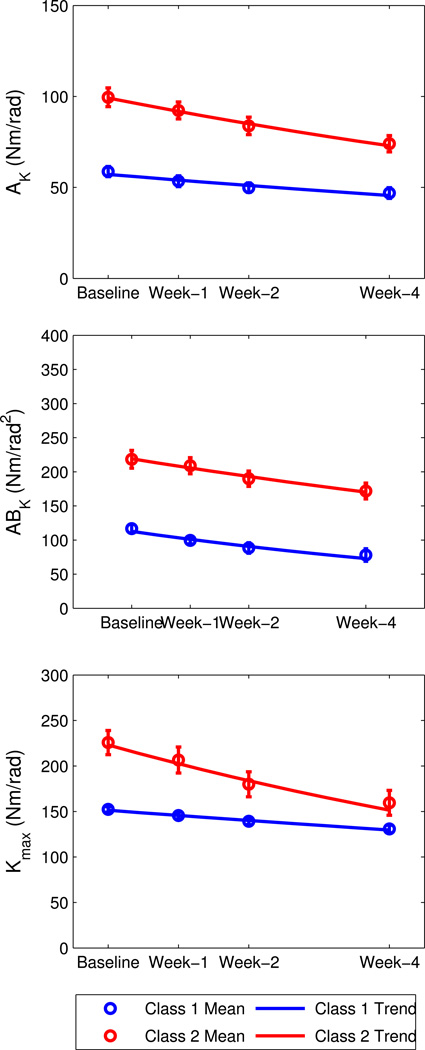
For the intrinsic parameters, subjects were classified into two classes; again, classes were ranked in order of ascending baseline value, such that Class 1 had the lower baseline mean and Class 2 had the higher baseline mean (Figure 6). Again, for all classes β<0 (p<0.05), indicating that subjects in the class exhibited significant reductions in stiffness over time.
Figure 6.
Intrinsic stiffness trends, by class, for the robotic-assisted step training (RAST) group.
As with reflex stiffness, the reduction rate for intrinsic stiffness parameters became more negative (a greater reduction in stiffness) as the baseline value increased (correlation was r2 =0.84, p=0.01), indicating that subjects with initially higher intrinsic stiffness and/or slope tended to present larger reductions in stiffness and/or slope after RAST training. However the changes over training were less consistent between the three parameters. For AK and Kmax, Class 2 rate was 2.7–3.4 times higher than Class 1, while for ABK, the reduction rate was only 12% higher than Class 1.
For the control group, GMM and RCR were used to identify and model three classes for both reflex and intrinsic parameters (Table 2). It was found that β was not significantly different from zero for any of the classes (p>0.05 for all), indicating no significant change in time for any of the six parameters.
Table 2.
Results from GMM class separation and exponential trend fitting for Control group.
| Parameter (units) |
GMM | Class Mean ± Std. Error | Exponential Trend Parameters | |||
|---|---|---|---|---|---|---|
| Class | N | Baseline | Week-4 | C0 | β (1/week) | |
|
AG (Nm-s/rad) |
1 | 10 (43%) | 0.98±0.15 | 0.96±0.14 | 0.88 | −0.002 |
| 2 | 6 (26%) | 4.00±0.35 | 3.93±0.35 | 3.89**** | −0.002 | |
| 3 | 7 (30%) | 17.99±2.89 | 17.50±2.76 | 16.41**** | −0.005 | |
|
ABG (Nm-s/rad2) |
1 | 16 (70%) | 8.61±1.62 | 8.65±1.68 | 6.15**** | −0.002 |
| 2 | 3 (13%) | 53.26±8.04 | 51.73±7.09 | 50.58† | 0.001 | |
| 3 | 4 (17%) | 145.11±25.77 | 128.00±17.46 | 132.03* | −0.018 | |
|
Gmax (Nm-s/rad) |
1 | 9 (39%) | 2.05±0.28 | 2.04±0.30 | 1.91*** | −0.002 |
| 2 | 9 (39%) | 8.08±0.42 | 8.17±0.42 | 7.93**** | 0.004 | |
| 3 | 5 (22%) | 17.90±2.60 | 17.97±2.74 | 17.21**** | 0.001 | |
|
AK (Nm/rad) |
1 | 6 (27%) | 35.07±1.12 | 35.95±1.45 | 35.43**** | 0.004 |
| 2 | 7 (32%) | 52.10±1.02 | 53.60±1.44 | 52.36**** | 0.006 | |
| 3 | 9 (41%) | 88.85±7.08 | 87.54±7.40 | 87.09**** | −0.004 | |
|
ABK (Nm/rad2) |
1 | 11 (50%) | 112.75±4.11 | 113.02±4.40 | 111.88**** | −0.001 |
| 2 | 4 (18%) | 160.20±9.11 | 167.07±4.61 | 157.53*** | 0.014 | |
| 3 | 7 (32%) | 263.16±8.42 | 262.92±9.48 | 260.85**** | 0.001 | |
|
Kmax (Nm/rad) |
1 | 5 (23%) | 117.50±5.48 | 110.07±3.71 | 115.09**** | −0.015 |
| 2 | 10 (45%) | 164.82±4.98 | 169.62±4.39 | 164.13**** | 0.007 | |
| 3 | 7 (32%) | 234.20±7.05 | 232.78±5.11 | 231.53**** | 0.001 | |
Significance level:
:p < 0.05,
:p < 0.01,
:p < 0.001,
:p < 0.0001
The lack of significance for this C0 parameter is likely due to the low number of subjects (N=3) in this class.
Discussion
Therapeutic Effects of Robotic-Assisted Step Training on Neuromuscular Abnormalities
Our results reveal several major findings, which provide insight into the therapeutic benefits of RAST on SCI subjects. To our knowledge this is the first study to quantitatively characterize the therapeutic effects of RAST on the mechanical properties of the neuromuscular system, with a specific focus on the effects of amplitude (offset) and modulation of reflex and intrinsic stiffness. In addition, GMM and RCR statistical techniques were successfully used to identify and model distinct classes of recovery for each key parameter of intrinsic and reflex stiffness. All recovery classes showed significant, continuous decreases over the 4-week training period; classes with higher levels of baseline abnormality presented larger rates of improvement over longer periods of training.
We found that RAST had therapeutic effects in reducing stiffness offset and peak magnitude, as well as its modulation with joint angle (slope), for both reflex and intrinsic parameters, both of which abnormally increase after SCI (Mirbagheri et al., 2001, 2002). The intrinsic parameter levels at baseline was 130–350% higher compared to able bodied individuals in our earlier study (Mirbagheri et al., 2008a) and these differences altered after 4 weeks of training, indicating a substantial reduction in intrinsic stiffness parameters. The RAST effects were even stronger on reflex stiffness parameters. Reflex stiffness offset and peak magnitude were over 10 times higher compared to able bodied individuals, and reflex slope was over 30 times higher; these differences reduced to just over 4 times higher after 4 weeks. This demonstrates a considerable reduction in these parameters (Mirbagheri et al., 2008a), although the stiffness measure levels were still much higher than normal indicating that more intense therapy for longer periods may be required to achieve better results. The training provides greater benefit to those subjects who initially presented greater abnormalities; further these subjects appear to benefit from longer training periods. Our findings demonstrate that these techniques can be used to characterize and predict the progress of changes to neuromuscular properties due to various types of intervention.
The Nature of Stretch Reflex Recovery
Our findings indicated that robotic-assisted step training significantly reduced the magnitude and modulation of reflex stiffness with ankle position. Our results are consistent with significant decreased spasticity as measured by the Pendulum Test (Manella et al., 2010), and by decreased plantarflexor reflex excitability (Manella and Field-Fote, 2013). Although these studies have investigated the effects of RAST on clinical and electrophysiological measures of spasticity, there are very limited similar studies with which to compare our results regarding the influence of RAST on neuromuscular mechanical abnormalities associated with SCI. This is largely due to the aforementioned lack of a quantitative and objective technique for neuromuscular quantification. Currently, it is unclear as to which factor of the stretch reflex activation is responsible for increased muscle tone. Abnormal enhancement of stretch reflexes could be due to increased motoneuronal excitability and/or increased stretch-evoked synaptic excitation of motoneurons (Katz and Rymer, 1989), which are related to reflex threshold and gain, respectively. Thus future work is required to fully understand the mechanisms by which RAST training modifies reflex threshold and gain. Distinguishing the role of threshold and gain may have both scientific and clinical significance, because their underlying mechanisms (Dietrichson, 1971, Katz and Rymer, 1989, Matthews, 1966, Stein, 1995, Young and Shahani, 1980) are different and require different treatments.
Robotic-Assisted Step Training Mechanisms
We chose to focus on the ankle joint because of its pivotal role in locomotion (Lin et al., 2006, Nadeau et al., 1999) and because modifications to intrinsic and reflex properties in the ankle can play a significant role in gait (Barbeau et al., 2002). In this study, we demonstrated RAST significantly reduced the abnormal increase in both intrinsic and reflex stiffness as well as modified their abnormal modulations with the ankle position.
What mechanisms may explain these improvements? Spastic hypertonia causes hyperactivity of spastic ankle extensor muscles, which is usually concurrent with hypoactivity of ankle flexor muscles (Katz and Rymer, 1989, Sehgal and McGuire, 1998). Prolongation of these abnormalities may lead to structural changes in the spastic muscle including reduction in the number and resting length of sarcomeres and shortening of spastic muscles (as has been found in cerebral palsy, Friden and Lieber (2003)) and changes in fiber size and type (as has been found in SCI, Rochester et al. (1995)). These changes can result in contracture and alteration of the muscle length-tension relationship, ultimately leading to impaired movement and function (Hufschmidt and Mauritz, 1985, Lieber et al., 2003, McDonald et al., 2005, Tabary et al., 1972, Tang and Rymer, 1981, Williams and Goldspink, 1978), which can include foot-drop and impaired gait. During training a strap is needed to reduce foot-drop, which stretches the ankle extensor muscles toward neutral position and therefore could modify the spastic ankle extension pattern during training sessions. This modify the functional condition under which the ankle is acting, by promoting more natural movements of the lower extremity joints, and could promote improvement in the mechanical properties of the spastic joint and muscles and the sensory mechanisms, observed in this study.
In addition to altering the neuromuscular properties of the ankle, RAST could improve walking impairment through alternative mechanisms. The repetitive normal walking pattern during locomotor training might lead to the development of an increasingly ‘normal’ locomotor pattern (Dietz et al., 1994, Visintin and Barbeau, 1989) as well as the noted improvements in spastic symptoms (including hypertonia) by the activation of spinal locomotor centers (Dietz and Sinkjaer, 2011). RAST is assumed to provide similar afferent input to that induced by normal overground locomotion.
Interactions between Neuromuscular Abnormalities and Walking Impairment
In showing that both stretch reflex stiffness magnitude and modulation were reduced by RAST training, our study underscores the conflicting views regarding the link between spasticity and impaired voluntary movement. Some studies have demonstrated a relationship between spastic hypertonia and impaired function (Corcos et al., 1986, Eyre and Miller, 1993, Gottlieb and Myklebust, 1993), while other studies reject this relationship, based on clinical observations that reducing reflex hyperexcitability does not always promote functional improvement (Little et al., 1994, Norman et al., 1998). This discrepancy in the literature may be due to two major points (i) the hypothetical mechanism that stretch reflex hyperexcitability may not directly induce impaired function, but rather, may promote abnormal modulation of stretch reflexes during a voluntary movement (Dietz et al., 2002, Faist et al., 1996), and may also cause impairments (Eyre and Miller, 1993), and (ii) the debate regarding the origins of spasticity.
Regarding the first point, this proposed mechanism is supported by the present quantitative study demonstrating the therapeutic effects of RAST on the modulation of stretch reflex stiffness with the ankle angle (quantified here as ABG). However, further investigations are needed to confirm this relationship because of the limitation of this study, which is the inability to separate short- and long-latency stretch reflex torque responses. While there is common agreement that short-latency stretch reflexes increase abnormally after spinal cord injury, their contribution of these early responses to functional impairment is controversial, as discussed above. There is no doubt that the neural activity described as long-latency stretch reflex is highly adaptable to meet functional needs (Shemmell et al., 2009) and its suppression may play a major role in spastic movement disorders; however, the role of stretch reflex components has not been fully characterized due to a lack of appropriate tools for quantifying their mechanical properties in meaningful behavioral situations. This limitation is due to the fact that reflex torque responses, elicited in response to a rapid pulse or PRBS perturbation position, appear after 40 ms and last for a few hundred ms (Mirbagheri et al., 2000, Mirbagheri et al., 2001), due to muscle dynamic characteristics, which make it difficult to separate short- and long-latency reflex torques.
As a second point, the discrepancy regarding the relationship between spasticity and functional impairment could also arise from uncertainty regarding the nature and origin of spasticity. Although most studies have attributed spastic hypertonia to hyperexcitability of stretch reflexes (Lance, 1980), some studies have attributed it to an increase in intrinsic stiffness due to changes in the visco-elastic properties of passive tissues and active muscle fibers (Hufschmidt and Mauritz, 1985, Sinkjaer et al., 1993). However, our results demonstrated that, in addition to reflex stiffness, intrinsic stiffness was reduced significantly during RAST. Our findings address this controversy by showing that hypertonia is due to abnormality in both intrinsic and reflex mechanisms, in support of our previous study (Mirbagheri et al., 2001). Furthermore, a significant reduction in AK and ABK during RAST shows that the training acts both to reduce the magnitude of intrinsic stiffness and to modify the functional condition (e.g. muscle length-tension curve) under which the ankle acts. Both of these modifications may contribute to the development and progression of gait impairment. Our functional outcomes showed that the subjects with initially higher walking capacity benefited more from RAST than subjects with low initial capacity, and these high-capacity subjects gain significant improvements in speed and functional mobility assessed by 10-Meter-Walking speed and Timed-Up-and-Go clinical measures (Niu et al., 2014). This disparity could be due to the fact that subjects with lower walking capacity require training for a longer period than subjects with higher walking capacity, consistent with neuromuscular outcomes of this study.
Advantages of GMM and RCR Statistical Analysis: Identification of Neuromuscular Recovery Patterns
Typically, studies evaluate the efficacy of a particular intervention by comparing evaluations taken before and after the intervention using a group-means comparison (e.g,. t-test or signed-rank test). This approach is insufficient for patient populations as it does not consider the often significant inter-subject variability and does not contain longitudinal information about the intervention effect. For example, the response to a medication may be nonlinear, with different rates of change at different time-points during the intervention period. In this study, we overcame these limitations by using two advanced statistical techniques, GMM and RCR, to first classify and then model recovery patterns. We used GMM to stratify subjects into distinct classes, assuming that subjects’ recovery falls into multiple patterns. Further, this classification is performed based on recovery patterns determined at multiple time points; thus it is more powerful than clustering techniques which are performed only at a single point in time. We then used RCR to mathematically model the recovery pattern; this indicated that recovery patterns were rather non-linear, with larger changes between the baseline and Week-1 observations than between the Week-3 and Week-4 observations. These two powerful and robust techniques incorporate both heterogeneity and longitudinal information into the recovery trajectory, and can be useful in other studies for tracking recovery processes from neurological disease and the efficacy of a particular intervention.
Conclusions/Implications
The present study suggests that RAST had a beneficial effect for reducing neuromuscular abnormalities associated with spasticity. Not all subjects will respond equally to the same training protocol; rather, subjects with high levels of neuromuscular abnormality prior to the start of training are more likely to experience greater benefits from RAST in terms of reducing neuromuscular abnormalities, and further are expected to present improvements beyond four weeks of training. By contrast, subjects with low levels of neuromuscular abnormality prior to training will present less improvement and will attain their maximal benefit in fewer than four weeks of training. The next step in this study is to identify predictors for these levels of neuromuscular abnormality that can be quickly and easily evaluated in the clinic. Such information can be used by clinicians to help determine which subjects are pre-disposed to receive substantial improvements in neuromuscular abnormality due to RAST, and how much training is appropriate, prior to assigning such a treatment, thereby maximizing results while minimizing efforts and costs. Further, this study can help researchers to gain insight into the mechanisms by which the RAST can be used to modify reflex and intrinsic stiffness, offering a potential secondary benefit of this training regimen, in addition to its primary intended outcome of improved walking.
Highlights.
The ability of robotic-assisted step training (RAST) to reduce neuromuscular abnormalities associated with spasticity was determined.
RAST reduced the abnormalities in both stiffness magnitude and modulation with position, proportional to the initial level of abnormality.
These techniques can be used to predict the therapeutic effects of different interventions on neuromuscular abnormalities.
Acknowledgements
We would like to thank Deborah Varoqui, Petra Conaway, Chirag Patel, and Lanitia Ness for their assistance with running the experiments and operating the robotic-assisted step training. The National Institute of Health [5R01HD059895], and the Craig H. Neilsen Foundation supported this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None.
References
- Alibiglou L, Rymer WZ, Harvey RL, Mirbagheri MM. The relation between Ashworth scores and neuromechanical measurements of spasticity following stroke. J Neuroeng Rehabil. 2008;5(18):1–15. doi: 10.1186/1743-0003-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, Fung J. The role of rehabilitation in the recovery of walking in the neurological population. Curr Opin Neurol. 2001;14:735–740. doi: 10.1097/00019052-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Ladouceur M, Mirbagheri MM, Kearney RE. The effect of locomotor training combined with functional electrical stimulation in chronic spinal cord injured subjects: walking and reflex studies. Brain Res Rev. 2002;40:274–291. doi: 10.1016/s0165-0173(02)00210-2. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80:688–700. [PubMed] [Google Scholar]
- Burne JA, Carleton VL, O'Dwyer NJ. The spasticity paradox: movement disorder or disorder of resting limbs? J Neurol Neurosurg Psych. 2005;76:47–54. doi: 10.1136/jnnp.2003.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Joerg M, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev. 2000;37:693–700. [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Penn RD, Myklebust B, Agarwal GC. Movement deficits caused by hyperexcitable stretch reflexes in spastic humans. Brain. 1986;109:1043–1058. doi: 10.1093/brain/109.5.1043. [DOI] [PubMed] [Google Scholar]
- Dietrichson P. Phasic ankle reflexes in spasticity and Parkinsonian rigidity. Possible role of the fusimotor system. Acta Neurol Scand. 1971;47:22–51. doi: 10.1111/j.1600-0404.1971.tb07462.x. [DOI] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L. Locomotor activity in spinal man. Lancet. 1994;344:1260–1263. doi: 10.1016/s0140-6736(94)90751-x. [DOI] [PubMed] [Google Scholar]
- Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl Physio. 2004;96:1954–1960. doi: 10.1152/japplphysiol.00942.2003. [DOI] [PubMed] [Google Scholar]
- Dietz V, Müller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity: evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104:431–439. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spasticity. In: Aminoff M, Boller F, Swaab D, editors. Handbook of Clinical Neurology. Amsterdam: Netherlands: Elsevier; 2011. pp. 197–211. [Google Scholar]
- Drolet M, Noreau L, Vachon J, Moffet H. Muscle strength changes as measured by dynamometry following functional rehabilitation in individuals with spinal cord injury. Arc Phys Med Rehabil. 1999;80:791–800. doi: 10.1016/s0003-9993(99)90229-0. [DOI] [PubMed] [Google Scholar]
- Eyre J, Miller S. Disturbance of Voluntary Movement. In: Thilman AF, Burke DJ, Rymer WZ, editors. Spasticity: Mechanisms and Management. Berlin Heidelberg: Springer-Verlag; 1993. pp. 1970–1973. [Google Scholar]
- Faist M, Dietz V, Pierrot-Deseilligny E. Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Exp Brain Res. 1996;109:441–449. doi: 10.1007/BF00229628. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Fluet GG, Schafer SD, Schneider EM, Smith R, Downey PA, et al. The spinal cord injury functional ambulation inventory (SCI-FAI) J Rehabil Med. 2001;33:177–181. doi: 10.1080/165019701750300645. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Lindley SD, Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther. 2005;29:127–137. doi: 10.1097/01.npt.0000282245.31158.09. [DOI] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;27:157–164. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- Gerhart KA, Bergstrom E, Charlifue SW, Menter RR, Whiteneck GG. Long-term spinal cord injury: functional changes over time. Arc Phys Med Rehabil. 1993;74:1030–1034. doi: 10.1016/0003-9993(93)90057-h. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Myklebust BM. Hyper-reflexia and disordered voluntary movement. In: Thilman AF, Burke DJ, Rymer WZ, editors. Spasticity: Mechanisms and Managment. Springer-Verlag; 1993. pp. 155–169. [Google Scholar]
- Hagenaars JA, McCutcheon AL. Applied latent class analysis. Dordrecht, Netherlands: Kluwer; 2002. p. 476. [Google Scholar]
- Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther. 2005;85:52–66. [PubMed] [Google Scholar]
- Hufschmidt A, Mauritz KH. Chronic transformation of muscle in spasticity: peripheral contribution to increased tone. J Neurol Neurosurg Psych. 1985;48:676–685. doi: 10.1136/jnnp.48.7.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social Personality Psych Compass. 2008;2:302–317. [Google Scholar]
- Kakebeeke T, Lechner H, Knapp P. The effect of passive cycling movements on spasticity after spinal cord injury: preliminary results. Spinal Cord. 2005;43:483–488. doi: 10.1038/sj.sc.3101747. [DOI] [PubMed] [Google Scholar]
- Katz RT, Rymer WZ. Spastic hypertonia: Mechanisms and measurement. Arch Phys Med Rehabil. 1989;70:144–155. [PubMed] [Google Scholar]
- Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (Revised 2011) J Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P, Szecsi J, Straube A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin Rehabil. 2008;22:627–634. doi: 10.1177/0269215507084648. [DOI] [PubMed] [Google Scholar]
- Lance JW. Symposium synopsis. In: Feldman RGYR, Koella WP, editors. Spasticity: Disordered Motor Control. Chicago: Year Book Publishers; 1980. pp. 485–494. [Google Scholar]
- Lehmann JF, Price R, deLateur BJ, Hinderer S, Traynor C. Spasticity: Quantitative measurements as a basis for assessing effectiveness of therapeutic intervention. Arch Phys Med Rehabil. 1989;70:6–15. [PubMed] [Google Scholar]
- Lieber RL, Runesson E, Einarsson F, Friden J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve. 2003;28:464–471. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- Lin P-Y, Yang Y-R, Cheng S-J, Wang R-Y. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch Phys Med Rehabil. 2006;87:562–568. doi: 10.1016/j.apmr.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Little JW, Powers RK, Michelson P, Moore D, Robinson LR, Goldstein B. Electrodiagnosis of upper limb weakness in acute quadriplegia. Am J Phys Med Rehabil. 1994;73:15–22. [PubMed] [Google Scholar]
- Manella KJ, Field-Fote EC. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci. 2013;31:633–646. doi: 10.3233/RNN-120255. [DOI] [PubMed] [Google Scholar]
- Manella KJ, Torres J, Field-Fote EC. Restoration of walking function in an individual with chronic complete (AIS A) spinal cord injury. J Rehabil Med. 2010;42:795–798. doi: 10.2340/16501977-0593. [DOI] [PubMed] [Google Scholar]
- Matthews W. Ratio of maximum H-reflex to maximum M-response as measure of spasticity. J Neurol Neurosurg Psych. 1966;29:201–204. doi: 10.1136/jnnp.29.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoleni S, Boldrini E, Laschi C, Carrozza M, Stampacchia G, Rossi B. Changes on EMG activation in healthy subjects and incomplete SCI patients following a robot-assisted locomotor training. IEEE Int Conf Rehabil Robot: ICORR. 2011:1–6. doi: 10.1109/ICORR.2011.5975467. [DOI] [PubMed] [Google Scholar]
- McDonald MF, Kevin Garrison M, Schmit BD. Length–tension properties of ankle muscles in chronic human spinal cord injury. J Biomech. 2005;38:2344–2353. doi: 10.1016/j.jbiomech.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Alibiglou L, Thajchayapong M, Rymer WZ. Muscle and reflex changes with varying joint angle in hemiparetic stroke. J Neuroeng Rehabil. 2008a;5:1–16. doi: 10.1186/1743-0003-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbagheri MM, Barbeau H, Kearney RE. Intrinsic and reflex contributions to human ankle stiffness: Variation with activation level and position. Exp Brain Res. 2000;135:423–436. doi: 10.1007/s002210000534. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Ladouceur M, Barbeau H, Kearney RE. Intrinsic and reflex stiffness in normal and spastic spinal cord injured subjects. Exp Brain Res. 2001;141:446–459. doi: 10.1007/s00221-001-0901-z. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Ladouceur M, Barbeau H, Kearney RE. The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured subjects. IEEE Trans Neural Sys Rehabil Eng. 2002;10:280–289. doi: 10.1109/TNSRE.2002.806838. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Lilaonitkul T, Rymer WZ. Prediction of natural history of neuromuscular properties after stroke using Fugl-Meyer scores at 1 month. Neurorehabil Neural Repair. 2011;25:458–468. doi: 10.1177/1545968310390222. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Niu X, Kindig M, Varoqui D. The effects of locomotor training with a roboticgait orthosis (Lokomat) on neuromuscular properties in persons with chronic SCI. Conf Proc IEEE Eng Med Biol Soc: IEEE. 2012:3854–3857. doi: 10.1109/EMBC.2012.6346808. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Settle K, Harvey R, Rymer WZ. Neuromuscular abnormalities associated with spasticity of upper extremity muscle in hemiparetic stroke. J Neurophysiol. 2007;98:629–637. doi: 10.1152/jn.00049.2007. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Tsao C, Rymer WZ. Natural history of neuromuscular properties after stroke: A longitudinal study. J Neurol Neurosurgery Psych. 2009;90:1212–1217. doi: 10.1136/jnnp.2008.155739. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Tsao CC, Rymer WZ. Changes of elbow kinematics and kinetics during 1 year after stroke. Muscle Nerve. 2008b;37:387–395. doi: 10.1002/mus.20965. [DOI] [PubMed] [Google Scholar]
- Muthen B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Muthen B, Muthen LK. Integrating person-centered and variable-centered analysis: growth mixtue modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24:882–891. [PubMed] [Google Scholar]
- Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech. 1999;14:125–135. doi: 10.1016/s0268-0033(98)00062-x. [DOI] [PubMed] [Google Scholar]
- Niu X, Varoqui D, Kindig M, Mirbagheri MM. Prediction of gait recovery in spinal cord injured individuals trained with robotic gait orthosis. J Neuroeng Rehabil. 2014;11:42. doi: 10.1186/1743-0003-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman K, Pepin A, Barbeau H. Effects of drugs on walking after spinal cord injury. Spinal Cord. 1998;36:699–715. doi: 10.1038/sj.sc.3100674. [DOI] [PubMed] [Google Scholar]
- O'Dwyer NJ, Ada L, Nieilson PD. Spasticity and muscle contracture following stroke. Brain. 1996;119:1737–1749. doi: 10.1093/brain/119.5.1737. [DOI] [PubMed] [Google Scholar]
- Rayegani SM, Shojaee H, Sedighipour L, Soroush MR, Baghbani M, Amirani OB. The effect of electrical passive cycling on spasticity in war veterans with spinal cord injury. Front Neurol. 2011;2:39. doi: 10.3389/fneur.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester L, Barron M, Chandler C, Sutton R, Miller S, Johnson M. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Spinal Cord. 1995;33:514–522. doi: 10.1038/sc.1995.112. [DOI] [PubMed] [Google Scholar]
- Schmartz AC, Meyer-Heim AD, Müller R, Bolliger M. Measurement of muscle stiffness using robotic assisted gait orthosis in children with cerebral palsy: a proof of concept. Disability & Rehabil: Assistive Tech. 2011;6:29–37. doi: 10.3109/17483107.2010.509884. [DOI] [PubMed] [Google Scholar]
- Schwartz I, Sajina A, Neeb M, Fisher I, Katz-Luerer M, Meiner Z. Locomotor training using a robotic device in patients with subacute spinal cord injury. Spinal Cord. 2011;49:1062–1067. doi: 10.1038/sc.2011.59. [DOI] [PubMed] [Google Scholar]
- Sehgal N, McGuire JR. Beyond Ashworth. Electrophysiologic quantification of spasticity. Phys Med Rehabil Clin N Am. 1998;9:949–979. [PubMed] [Google Scholar]
- Shemmell J, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci. 2009;29:13255–13263. doi: 10.1523/JNEUROSCI.0892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain. 1994;117:355–363. doi: 10.1093/brain/117.2.355. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Toft E, Larsen K. Non-reflex and reflex mediated ankle joint stiffness in multiple sclerosis patients with spasticity. Muscle Nerve. 1993;16:69–76. doi: 10.1002/mus.880160112. [DOI] [PubMed] [Google Scholar]
- Stein R. Presynaptic inhibition in humans. Prog Neurobiol. 1995;47:533–544. doi: 10.1016/0301-0082(95)00036-4. [DOI] [PubMed] [Google Scholar]
- Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat soleus muscle due to immobilization at different lengths by plaster casts. J Physio (London) 1972;224:231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Rymer WZ. Abnormal force EMG relations in paretic limbs of hemiparetic human subjects. J Neuorl Neurosurg Psych. 1981;44:690–698. doi: 10.1136/jnnp.44.8.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CK, Tucker ME, Bigland-Ritchie BR. Voluntary muscle weakness and coactivation after chronic cervical spinal cord injury. J Neurotrauma. 1998;15:149–161. doi: 10.1089/neu.1998.15.149. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Zaidner EY, Calancie B, Broton JG, Bigland-Ritchie BR. Muscle weakness, paralysis and atrophy after human cervical spinal cord injury. Exp Neurol. 1997;148:414–423. doi: 10.1006/exnr.1997.6690. [DOI] [PubMed] [Google Scholar]
- Visintin M, Barbeau H. The effects of body weight support on the locomotor pattern of spastic paretic patients. Canadian J Neuro Sci. 1989;16:315–325. doi: 10.1017/s0317167100029152. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N. Is robot-aided sensorimotor training in stroke rehabilitation a realistic option? Curr Opin Neurol. 2001;14:745–752. doi: 10.1097/00019052-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Wernig A, Müller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur J Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. J Anat. 1978;127:459–468. [PMC free article] [PubMed] [Google Scholar]
- Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil. 2005;86:672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Young R, Shahani B. Clinical neurophysiological analysis of single motor unit discharge patterns in spasticity. In: Feldman R, Young RR, Koella WP, editors. Spasticity: Disordered Motor Control. Chicago: Year Book Publishers; 1980. pp. 219–231. [Google Scholar]