Abstract
Purpose
A pilot study was conducted to assess whether renal shock wave lithotripsy (SWL) influences the onset and severity of metabolic syndrome (MetS).
Materials and Methods
Three-month-old juvenile female Ossabaw miniature pigs were treated with SWL (2000 SWs, 24 kV, 120 SWs/min using the HM3 lithotripter; n=2) or sham-SWL (no SWs; n=2). SWs were targeted to the upper pole of the left kidney so as to model treatment that would also expose the pancreatic tail to SWs. Pigs were then instrumented for direct measurement of arterial blood pressure via an implanted radiotelemetry device, and later fed a hypercaloric atherogenic diet for ~7 months. The development of MetS was assessed from intravenous glucose tolerance tests (IVGTTs).
Results
The progression and severity of MetS was similar in the sham-treated and SWL-treated groups. The only exception was with respect to arterial blood pressure, which remained relatively constant in the sham-treated pigs but began to rise at ~2 months towards hypertensive levels in SW-treated pigs. Metabolic data from both groups were pooled to provide a more complete assessment of the development and progression of MetS in this juvenile pig model. IVGTTs revealed substantial insulin resistance with impaired glucose tolerance within 2 months on the hypercaloric atherogenic diet with signs of further metabolic impairment at 7 months.
Conclusions
These preliminary results suggest that renal SWL is not a risk factor for worsening of glucose tolerance or the onset of diabetes mellitus, but does appear to be a risk factor for early onset hypertension in MetS.
Keywords: Swine, shock wave lithotripsy, hypertension, metabolic syndrome, kidney
Introduction
Shock wave lithotripsy (SWL) has become the standard of care for uncomplicated adult and pediatric kidney stones,1,2 largely because SWL is effective, noninvasive and typically is performed on an outpatient basis. However, most patients that undergo SWL show some degree of acute renal trauma3,4 that in ~0.5% of cases will result in clinically significant hematomas.5,6 The long-term implication of acute SWL-induced tissue injury are less clear with conflicting clinical reports of reduced renal function,7,8 higher rates of hypertension7–12 and diabetes.9,12
In 2006, Krambeck and colleagues from the Mayo Clinic published the results of a 19-year follow-up study of SWL-treated adults and reported an increased risk of developing hypertension and diabetes.9 They argued that SWL-induced damage to the kidney and adjacent pancreas might be responsible for the development of these complications. Due to limitations of their study and subsequent clinical reports failing to uncover such associations,10,12,13 the urological community has been highly critical of the view that renal SWL can give rise to diabetes in adults, including those patients with predisposing risk factors such as obesity.
The pediatric population in SWL is understudied, and the literature on the long-term complications in this cohort is limited.2 The lithotripter focal zone has a fixed dimension and a greater fraction of a child’s small kidney and surrounding organs will be exposed to SWs compared to an adult.14 Therefore, it is conceivable that SWL-treatment of pediatric stone patients could place them at a greater risk of complications—one example being diabetes given the increasing prevalence of kidney stone disease in the pediatric population15 and the alarming rise in childhood obesity, MetS and diabetes.16–18 We tested this possibility in an Ossabaw miniature swine model that develops similar features of human MetS19—a cluster of conditions that includes central (intra-abdominal) obesity, insulin resistance, impaired glucose tolerance, dyslipidemia and hypertension, and their presence increases the risk for diabetes, cardiovascular disease and death.20
Materials and Methods
Studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Indiana University School of Medicine (IUSM) and Methodist Hospital. Three-month-old female Ossabaw pigs were obtained from the IUSM and Purdue University breeding colony (West Lafayette, IN). Pigs were anesthetized (induction with ketamine [20 mg/kg] and xylazine [2 mg/ml]; maintenance with 1–3% isoflurane) and the left kidney urinary collecting system visualized using contrast medium (injected into a ureteral catheter) and X-ray fluoroscopy. SWs were targeted to the upper-pole calyx and delivered at 2000 SWs (24 kV, 120 SWs/min) with X-ray verification of SW targeting done every 500 SWs, and with SW treatment paused every 1000 SWs to replace the electrode and check targeting (~1 minute). In the sham-SWL treated group, the lithotripter was not fired.
Magnetic Resonance Imaging (MRI)
Pigs underwent MRI before and after SW treatment. This was done to determine the anatomy of the kidney and pancreas so as to identify targeting of the upper pole calyx of the left kidney that would also target the pancreatic tail, and to assess whether SWL treatment resulted in injury to the kidney and pancreas.
Radiotelemetry instrumentation
Radiotelemetry transmitters were chronically implanted in pigs with arterial blood pressure measurements taken from a catheter introduced into the femoral artery and advanced towards the aorta. A receiver was placed in the pig’s cage with continuous recordings taken weekly (Friday afternoon to Monday morning)—a period of minimal disturbance. Animal rooms had a 12 h light: 12 h dark cycle with pigs housed in individual cages and access to play toys, ad libitum water intake and a fixed daily food intake.
Intravenous Glucose Tolerance Test (IVGTT)
Pigs were prepared for IVGTTs as previously described,21 and performed a few days before SWL or sham-SWL and then at monthly intervals after beginning the hypercaloric atherogenic (HA) diet. Fasted, conscious pigs were restrained in a low-stress body sling and blood samples taken from a jugular vein catheter immediately before and at 5, 10, 20, 30, 40, 50 and 60 min after an intravenous bolus of sterile glucose solution (dextrose, 1 g/kg). Blood samples were assayed for plasma glucose and insulin.
Renal and Pancreas histology
At the time of animal euthanasia, kidneys and pancreas were perfusion fixed in situ with 10% phosphate buffered formalin, harvested, and processed separately for light microscopy.14,21
Calculations
Insulin sensitivity and beta cell function was evaluated from fasting plasma values of glucose and insulin measured on the day of the IVGTT using the equations, QUICKI = 1/(log glucose0 min + log insulin0 min), and HOMA-%BCF = (360 × insulin0 min)/(glucose0 min − 63). From IVGTT we calculated: glucose and insulin AUC; AIRG = insulin5 min − insulin0 min; KG = −slope of ln(glucose)5–20 min × 100); BCF = AIRG/(glucose5 min − glucose0 min); S2 = KG/((AUCinsulin(0 – 20 min)/20 min) × Vd) where Vd = injected glucose dose/(glucose peak × body weight); DI = AIRG × S2.21
Statistical analysis
IVGTT measurements were summarized by means (standard deviations) along time for each time point. For each outcome, a linear mixed effect model was fitted with random intercepts for subjects and time treated as a categorical variable to account for potential nonlinear effects. Based on these models, measurements at month 1–7 were compared to the baseline values. No multiple comparison adjustments were used. For MABP, a linear change point model was fitted for each subject. Both the location of the change point and the necessity of the change point compared to a simple linear regression were selected by Akaike Information Criterion. A two-sided P-value <0.05 was considered significant. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
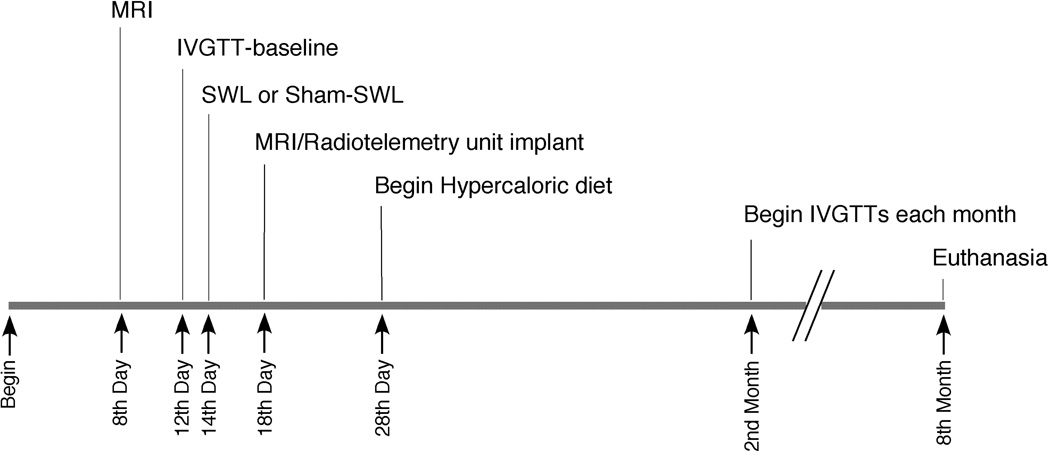
The experimental timeline is shown in Figure 1.
Figure 1.
Experimental timeline.
Abdominal anatomy of the pig
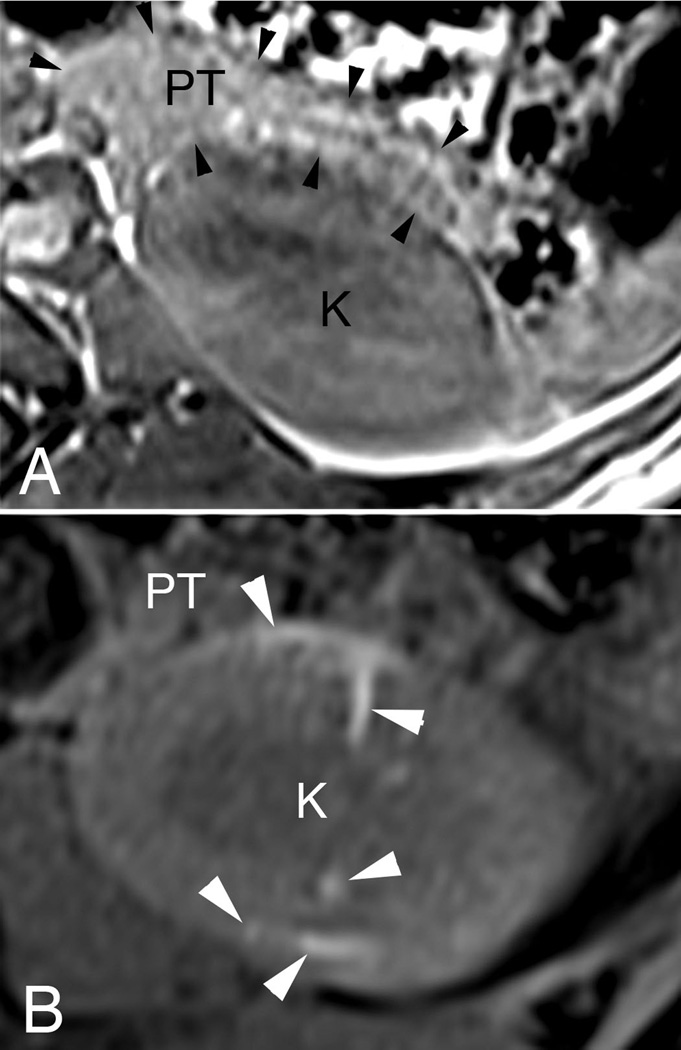
An abdomical MRI (Figure 2A) shows the normal anatomical location of the pancreas to the left kidney in a juvenile Ossabaw pig—the tail of the pancreas lying on the anterior surface of the kidney’s upper pole (panel 2A). SWs were targeted to the left kidney upper pole calyx that was closest to the pancreatic tail. An MRI taken several days after SWL shows evidence of subcapsular and intraparenchymal hemorrhage in the upper pole of the SW-treated kidney with no readily apparent trauma to the pancreas (Figure 2B).
Figure 2.
Shown are MRIs of 3-month-old female Ossabaw pigs on a normal diet before (panel A) and 4 days after (panel B) SWL-treatment. The pancreatic tail (PT, outlined with black arrow heads) lies adjacent to the anterior surface of the upper pole of the left kidney (K)—both regions are within the blast path of the SW generated from the HM3 lithotripter (panel A). Sites of intraparenchymal and subcapsular hemorrhage can be seen as bright areas (see white arrow heads) within the kidney after SWL, with no obvious injury to the pancreatic tail (panel B).
Blood chemistry and caloric intake
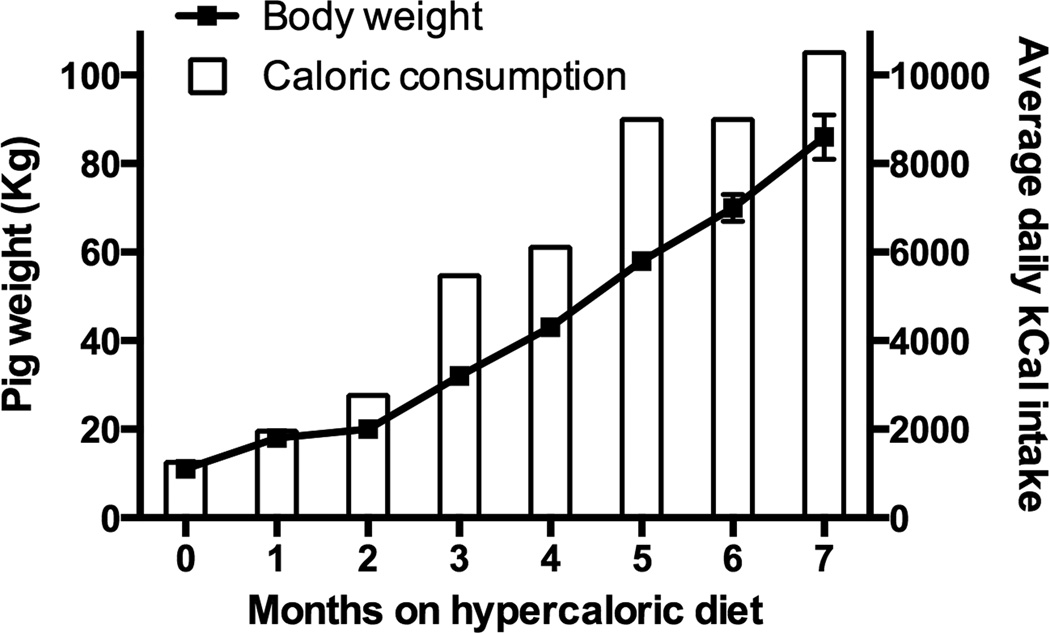
Figure 3 shows body weight and daily caloric intake. Pigs were on a lean diet with a caloric intake of 1250 kCal/day prior to being fed the HA diet19—the daily HA diet intake was increased over the course of the study to drive robust MetS. Body weight gain over the first 2 months on the HA diet was normal and thereafter increased substantially compared to juvenile Ossabaw pigs fed a lean diet (not shown). Body weights and fasting blood chemistries taken at the end of the study established that the pigs were obese (86 ± 5 kg), hyperinsulinemic (18 ± 3 µU/ml) and dyslipidemic (cholesterol = 999 ± 222 mg/dL; triglycerides = 69 ± 34 mg/dL), i.e. they had robust MetS.21
Figure 3.
Body weight and caloric consumption of juvenile Ossabaw pigs on a diet high in fat, cholesterol and fructose. One kg of the diet provides ~6000 kCal and ~2.5 g of sodium.
IVGTT
Initially, most of the juvenile pigs could not tolerate ingesting the pure HA diet for more than one week—resulting in all pigs being returned to the lean diet for ~9 days before placing them on a food intake schedule that consisted of increasing amounts of HA diet mixed with lean diet. This mixed diet was given until pigs could tolerate the complete HA diet by the end of the first month. Therefore, the first data points shown for pigs on the HA diet is at 2-months (Table 1). Metrics of insulin resistance and glucose tolerance during the progression of diet-induced MetS were essentially identical in the sham-treated and SWL-treated pigs. Therefore, we combined the data sets to gain some insight into the progression of MetS in this juvenile swine model. Measurements of fasting plasma glucose and insulin levels indicated that pigs remained normoglycemic with a trend towards hyperinsulinemia at ≥ 5 months on the HA diet (Table 1). Analysis of monthly IVGTTs revealed substantial falls in glucose tolerance (KG), insulin sensitivity index (S2) and disposition index (DI) over the first 2 months on the HA diet—indices that reflect a rise in insulin resistance and impaired glucose tolerance. Thereafter, there was a gradual decrease in S2, whereas KG and DI remained constant until the seventh month on the diet when further decreases were noted (Table 1). Calculation of beta cell function from fasting plasma glucose and insulin and IVGTTs indicated that pancreatic insulin function progressively increased over the course of the study, which likely accounted for the pigs remaining normoglycemic (Table 1).
Table 1.
Glycemic measurements in juvenile Ossabaw pigs fed a HA diet.
| HA DIET DURATION | 0-month | 2-month | 3-month | 4-month | 5-month | 6-month | 7-month |
|---|---|---|---|---|---|---|---|
| Fasting: | |||||||
| Plasma Glucose (mg/dL) | 108 ± 10 | 99 ± 2 | 87* ± 2 | 83** ± 3 | 98 ± 10 | 86* ± 1 | 85* ± 5 |
| Plasma Insulin (µU/ml) | 13 ± 6 | 12 ± 2 | 11 ± 1 | 10 ± 1 | 23* ± 4 | 12 ± 0 | 18 ± 3 |
| QUICKI | 0.334 ± 0.021 | 0.329 ± 0.004 | 0.338 ± 0.007 | 0.345 ± 0.004 | 0.301* ± 0.012 | 0.332 ± 0.000 | 0.317 ± 0.01 |
| HOMA-BCF% | 93 ± 21 | 121 ± 26 | 163 ± 13 | 192* ± 44 | 252*** ± 35 | 190* ± 6 | 305*** ± 53 |
| IVGTT: | |||||||
| AUCglucose/AUCinsulin | 7.23 ± 1.96 | 5.53 ± 0.26 | 3.64** ± 0.51 | 4.19* ± 0.68 | 3.03** ± 0.17 | 2.97** ± 0.83 | 2.91** ± 0.08 |
| BCF (pmol/mmol) | 12.50 ± 3.56 | 11.34 ± 0.37 | 14.05 ± 2.13 | 13.52 ± 0.87 | 16.42 ± 1.50 | 15.59 ± 3.27 | 17.05 ± 2.35 |
| KG (min−1) | 6.37 ± 0.42 | 3.72** ± 0.23 | 4.41* ± 0.53 | 4.17* ± 0.42 | 4.20* ± 0.33 | 4.67* ± 0.84 | 3.01*** ± 0.37 |
| S2 (ml•min−1•pM•kg−1) | 6.78 ± 1.19 | 2.50*** ± 0.21 | 1.93*** ± 0.20 | 1.77*** ± 0.27 | 1.37*** ± 0.05 | 1.14*** ± 0.08 | 0.85*** ± 0.12 |
| DI | 2056 ± 193 | 756*** ± 76 | 773*** ± 57 | 741*** ± 77 | 705*** ± 76 | 642*** ± 62 | 485*** ± 41 |
HA diet = hypercaloric atherogenic diet; QUICKI = quantitative insulin sensitivity check index; HOMA-%BCF = homeostasis model assessment for steady state beta cell function; AUC = area under curve for glucose and insulin; BCF = beta cell function; KG = glucose tolerance; S2 = insulin sensitivity index; DI = disposition index. Equations for each glycemic measure are described in the Calculations section in Methods and Materials. Data shown are mean ± SEM.
= P<0.05,
= P<0.01,
= P<0.001 from baseline values (0-month).
Blood Pressure Radiotelemetry
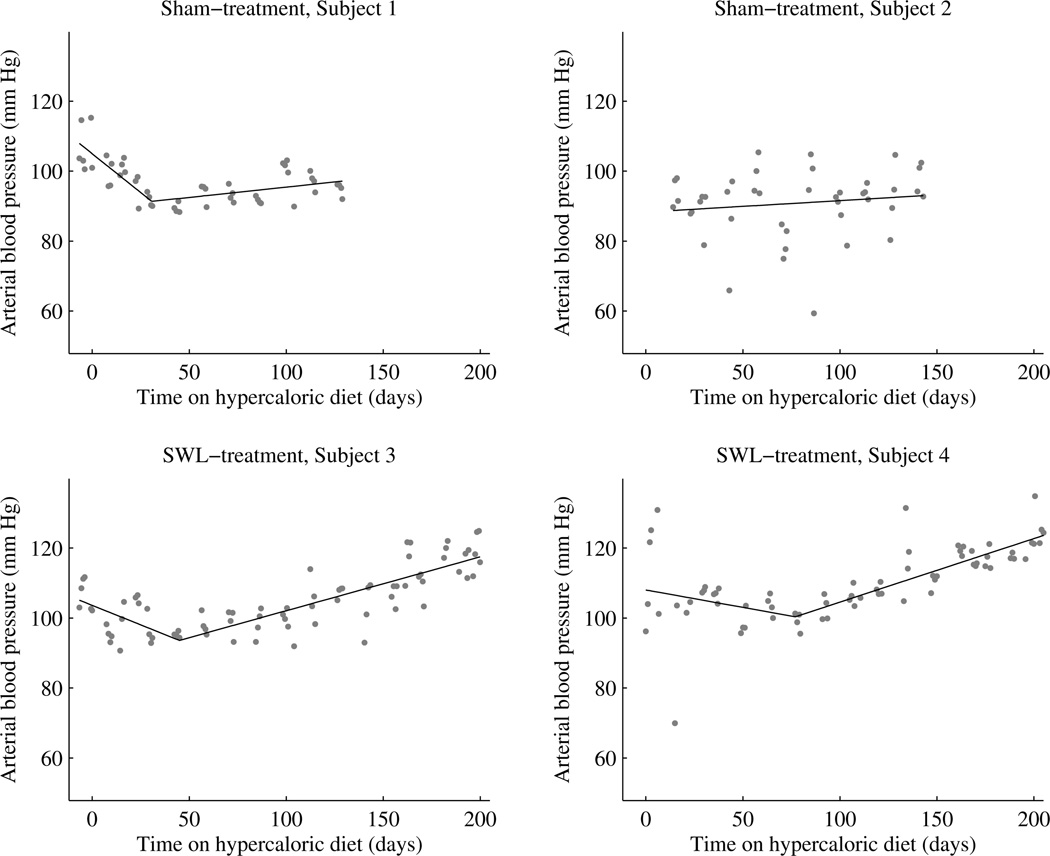
Mean arterial blood pressure (MABP) data for all 4 pig subjects is shown in Figure 4.
Figure 4.
MABP in sham-treated and SWL-treated juvenile Ossabaw pigs fed a HA diet. Each data point represents the average blood pressure during a 12 h daylight cycle—a time when the pig is most active. SWL or sham-treatment was performed ~14 days prior to feeding pigs the HA diet.
Sham-treatment, Subject 1
This pig had consumed the HA diet for a total of 139 days before dying during the initiation of isoflurane anesthesia for a non-surgical procedure. Inspection of the heart revealed substantial atherosclerosis. MABP initially fell during the first 30 days on the diet and then began to rise slowly over the next 100 days. The slope of the MABP rise was shallow with a value of only 0.059 mm Hg/day (P = 0.0062). The calculated rise in MABP would have been 10 mm Hg if the slope of blood pressure rise remained unchanged and the pig had consumed the HA diet for 200 days—ending MABP would have been ~100 mm Hg.
Sham-treatment, Subject 2
MABP was relatively constant during the first 154 days on the HA diet and measured 90–95 mm Hg. Thereafter there was a sudden step-wise fall in MABP of ~30 mm Hg—we suspect a blood clot, but the exact reason is unknown. MABP remained low during the next 17 days (these data points were not used in statistical analysis) at which time telemetry signals were lost.
SWL-treatment, Subject 3
There was a fall in MABP during the first 45 days on the HA diet and then progressively increased with a slope of 0.154 mm Hg/day (P < 0.0001). The calculated rise in MABP after consuming the HA diet for 200 days was 24 mm Hg relative to the lowest pressure at day 45. MABP at the end of the study was ~119 mmHg.
SWL-treatment, Subject 4
MABP decreased during the first 70 days on the HA diet and progressively increased thereafter with a slope of 0.182 mm Hg/day (P < 0.0001). The calculated rise in MABP after consuming the HA diet for 200 days was 24 mm Hg relative to the lowest pressure recorded at day 70. MABP at the end of the study was ~124 mmHg.
Renal and Pancreas histology
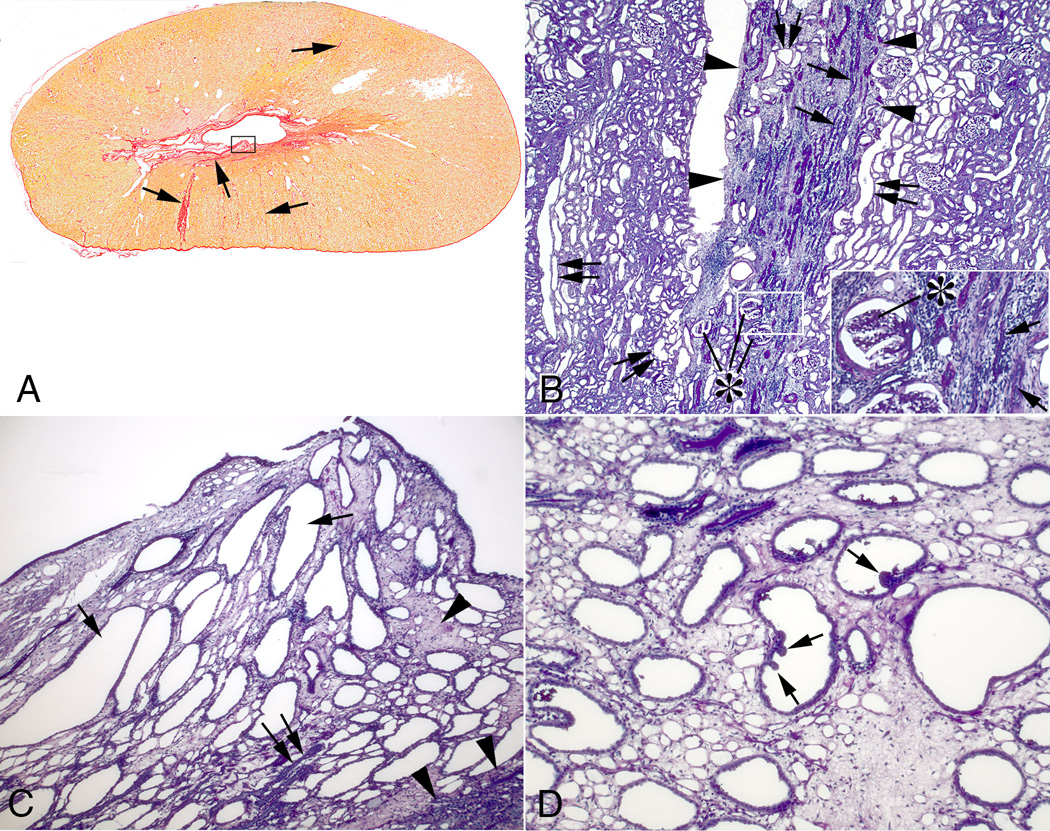
Chronic SWL-induced tissue injury was noted in treated kidneys as fibrotic lesions/bands that extended from the renal capsule to the inner medulla with varying lengths and widths (Figure 5A). Much of the cortex was normal. Sites of cortical damage (arrow, Figure 5A; Figure 5B) possessed sclerotic glomeruli, both atrophic and dilated tubular segments, and interstitial fibrosis. Injured papillae were always associated with regions of cortical damage and the changes varied from sparse regions of interstitial fibrosis with minimal tubular damage to widespread interstitial fibrosis and extensive tubules changes (rectangle, Figure 5A). Tubular changes in the severely damaged papilla included extensively dilated outer and inner medullary collecting ducts while other tubular segments were atrophic (Figure 5C and D). Some dilated collecting ducts showed epithelial in-foldings and polypoid configurations, which is indicative of cell proliferation, i.e. nephron structure remodeling (Figure 5D). There was no evidence of tissue injury and minimal tubule dilation in kidneys from sham-treated MetS pigs (not shown).
Figure 5.
Cortical and medullary changes in SWL-treated kidneys. Panel A show a transverse section of the upper pole of a treated kidney stained with Weigert’s hematoxylin and picro sirius red. Fibrosis extends from the renal capsule to the medulla (arrows) on both sides of the kidney. The largest cortical fibrotic band in panel A is shown in panel B (outlined in arrowheads) and shows sclerotic glomeruli (asterisks), atrophic tubular segments (arrows) and interstitial fibrosis—clearly seen in the panel B insert. Dilated tubule segments were found within fibrotic bands (double arrows) but more commonly in adjacent regions (double arrows). Panel C shows a damaged papilla outlined in a rectangle in panel A. Extensive interstitial fibrosis (arrowheads) surrounds dilated collecting ducts (arrows) and atrophic tubular segments (double arrow). A damaged papilla from a different kidney (panel D) shows a polypoid configuration (arrows) of the lining cells of several dilated inner medullary collecting ducts. Reduced from ×2 (A), ×300 (B), ×600 (B insert), ×1000 (C) and ×1500 (D).
There were no obvious signs of tissue injury in the pancreatic tails from SWL-treated or sham-SWL-treated pigs. That is, pancreas histology appeared normal in both groups (not shown).
Discussion
Herein, we report results from a pilot study on the development of MetS in a juvenile Ossabaw pig model, and the effect of renal SWL on the progression of MetS towards diabetes.
Induction and progression of MetS in a juvenile swine model
Adulthood obesity and associated co-morbidities have reached epidemic proportions in the USA.22 New data from the National Health and Nutrition Examination Survey (NHANES) also shows alarming rates of overweight and obesity in the US pediatric population of 32% and 17%, respectively,18 and annually at least 3700 youth are diagnosed with type 2 diabetes in the USA.16 Alterations in glucose metabolism have been documented in overweight/obese children and adolescents,17 but there is little information on the onset and progression of MetS in such cohorts.
We provide preliminary data on the onset and progression of insulin resistance and glucose tolerance in a juvenile Ossabaw female pig model that could be used to investigate childhood and adolescent obesity and MetS. We found that pigs had profound increases in insulin resistance and decreases in glucose tolerance within a relatively short period (2-month) of consuming the HA diet. Our study did not include control pigs on a lean diet and therefore age cannot be excluded from contributing to these early metabolic abnormalities. There are a small number of reports in the published literature that have used a juvenile swine model to explore the effects of an obesogenic diet in children. One study showed that feeding 2-month-old Göttigen pigs a high-energy diet for only 2-weeks resulted in substantial insulin resistance and impaired glucose tolerance compared to age-matched pigs fed a low-energy diet as measured by oral glucose tolerance tests.23 These early impairments in glucose and insulin metabolism were associated with an increase in the percentage of body fat.23 A second study fed a diet high in fat and sucrose to 3–4 month-old Chinese Guizhou minipigs and compared their findings to age-matched pigs on a normal control diet. Based on fasting blood glucose and insulin levels, only pigs consuming the high fat/sucrose diet for 2-months or longer were hyperglycemic and insulin resistant.24 Therefore, we assume that the dramatic abnormalities in glucose and insulin metabolism observed in the first 2-months of our study were likely a result of consuming the HA diet.
Our study provides new information on the temporal progression of MetS following this early metabolic impairment. There was a shallow decrease in insulin sensitivity over the course of the study—that is, a slow progressive increase in insulin resistance. Despite substantial step-wise increases in daily caloric intake and ever-increasing obesity, there was a plateau in impaired glucose metabolism that was likely due to the adaptive response of the pancreas to continuously increase beta cell insulin function. However, beta cell insulin function at 7 months appeared to be insufficient to compensate for the decrease in insulin sensitivity and led to further impairment in glucose tolerance (lower DI and decreased KG) as pigs advanced towards diabetes. These results, albeit preliminary, suggest that the juvenile Ossabaw pig could be a useful animal model to study childhood obesity, MetS and associated cardiovascular disorders, as well as the mechanisms and factors contributing to these effects.
Effect of renal SWL on the development of MetS
Since the introduction of extracorporeal SWL in the 1980s to treat kidney stones there have been many clinical reports supporting7,9,11 or opposing8,10,12,25 the view that renal SWL can lead to new onset hypertension, and this debate continues to the present day.26,27 Also, there are concerns that renal SWL can lead to diabetes as a result of a 19-year followup retrospective study in adults suggesting that SWL can result in a 3-fold increase in the risk of diabetes.9 However, others have not shown such associations with diabetes in adult patients with long-term follow-ups.10,12,13 The only non-adult study examining the risks of SWL-induced diabetes reported that fasting levels of glucose remained normal over a mean followup period of 5 years in patients (average age of 6.5 years old) treated with single or multiple SWL sessions.28
We examined the issue of whether renal SWL is a risk factor for diabetes and hypertension in the Ossabaw pig, a model that demonstrates similar features of human MetS.19 Juvenile pigs underwent renal SWL to mimic treatment of children, and then MetS was induced by daily feeding of a diet high in fat, cholesterol and fructose.19 Although we had only 2 pigs in the SWL-treated and sham-treated groups, we found a similar onset and progression of insulin resistance and glucose intolerance. Furthermore, we found no evidence of SWL-induced acute injury to the pancreas based on MRIs or plasma pancreatic amylase levels (not shown), and no signs of chronic injury to the pancreatic tail based on tissue histology. These preliminary results support the notion that renal SWL is not a risk factor for diabetes, and is in agreement with our most recent findings that renal SWL does not worsen insulin resistance and glucose tolerance in adult pigs with robust MetS.21
The conflicting reports of whether renal SWL can induce hypertension were largely based on studies of the general nephrolithiasis population and then stratified for correlates, as well as using a variety of lithotripters and SW delivery protocols. Perhaps not surprisingly, a meta-analysis of eleven clinical studies found no association between SWL and the development of hypertension.26 Some investigators have suggested that older patients—a cohort that is at greater risk of developing high blood pressure—may be more prone to hypertension after SWL, which correlated well with elevations in renal resistive indexes.29 Animal models with a genetic disposition towards the development of hypertension have also shown that SWL-treatment can evoke a rise in blood pressure.30 Although arterial blood pressure remained relatively constant in our sham-treated pigs over the course of the study, such obese pigs consuming a HA diet will undoubtedly become hypertensive at some point.19 On the other hand, there was a substantial elevation in arterial blood pressure in each of the two SWL-treated juvenile pigs that began at 30–70 days on the HA diet and continued to rise throughout the 200 day study. This implies that renal SWL accelerated the onset of hypertension in MetS, which was likely due to the detrimental effects of SWs on the kidney.7,29,present study Indeed, renal histology revealed permanent loss of parenchyma as well as structural abnormalities seven months after SWL treatment. Therefore, our preliminary findings are in agreement with those clinical and animal studies that implicate renal SWL as a risk factor for elevating blood pressure—the onset being accelerated in those predisposed towards hypertension.
Limitations of this pilot study include: the small number of pigs in each group; the early death of a control (sham-SWL) pig; and the study was not powered to detect significance between groups. Therefore, our results should be viewed as exploratory in nature.
Acknowledgments
This work was supported by a Showalter grant (RKH) and PHS grant P01-DK43881.
Abbreviations and Acronyms
- SW
shock wave
- SWL
SW lithotripsy
- MetS
metabolic syndrome
- MRI
magnetic resonance imaging
- IVGTT
intravenous glucose tolerance test
- MABP
mean arterial blood pressure
- HA diet
hypercaloric atherogenic diet
- AIRG
acute insulin response to glucose
- KG
glucose tolerance
- S2
insulin sensitivity index
- AUC
area under curve
- BCF
beta cell function
- HOMA-%BCF
homeostasis model assessment of steady state BCF
- QUICKI
quantitative insulin sensitivity check index
- DI
disposition index
References
- 1.Gerber GS. Trends in Endourological practice: Management of lower-pole caliceal stones. J Endourol. 2003;17:501. doi: 10.1089/089277903769013676. [DOI] [PubMed] [Google Scholar]
- 2.Nelson CP. Extracorporeal shock wave lithotripsy in the pediatric population. Urol Res. 2010;38:327. doi: 10.1007/s00240-010-0291-4. [DOI] [PubMed] [Google Scholar]
- 3.Kaude JV, Williams CM, Millner MR, et al. Renal morphology and function immediately after extracorporeal shock wave lithotripsy. AJR. 1985;145:305. doi: 10.2214/ajr.145.2.305. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner BR, Dickey KW, Ambrose SS, et al. Kidney changes after extracorporeal shock wave lithotripsy: appearance on MR imaging. Radiology. 1987;163:531. doi: 10.1148/radiology.163.2.3562837. [DOI] [PubMed] [Google Scholar]
- 5.Dhar NB, Thornton J, Karafa MT, et al. A multivariate analysis of risk factors associated with subcapsular hematoma formation following electromagnetic shock wave lithotripsy. J Urology. 2004;172:2271. doi: 10.1097/01.ju.0000143459.03836.2d. [DOI] [PubMed] [Google Scholar]
- 6.Razvi H, Fuller A, Nott L, et al. Risk factors for perinephric hematoma formation after shockwave lithotripsy: A matched case-control analysis. J Endourology. 2012;26:1478. doi: 10.1089/end.2012.0261. [DOI] [PubMed] [Google Scholar]
- 7.Williams CM, Thomas WC. Permanently decreased renal blood flow and hypertension after lithotripsy. NEJM. 1989;321:1269. doi: 10.1056/NEJM198911023211814. [DOI] [PubMed] [Google Scholar]
- 8.Eassa WA, Sheir KZ, Gad HM, et al. Prospective study of the long-term effects of shock wave lithotripsy on renal function and blood pressure. J Urol. 2008;179:964. doi: 10.1016/j.juro.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Krambeck AE, Gettman MT, Rohlinger AL, et al. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175:1742. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Tanda H, Kato S, et al. Shock wave lithotripsy for renal stones is not associated with hypertension and diabetes mellitus. Urol. 2008;71:586. doi: 10.1016/j.urology.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 11.Barbosa PV, Makhlouf AA, Thorner D, et al. Shockwave lithotripsy associated with greater prevalence of hypertension. Urology. 2011;78:22. doi: 10.1016/j.urology.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Chew BH, Zavaglia B, Sutton C, et al. Twenty-year prevalence of diabetes mellitus and hypertension in patients receiving shock-wave lithotripsy for urolithiasis. BJU Int. 2012;109:444. doi: 10.1111/j.1464-410X.2011.10291.x. [DOI] [PubMed] [Google Scholar]
- 13.de Cógáin M, Krambeck AE, Rule AD, et al. Shock wave lithotripsy and diabetes mellitus: a population-based cohort study. Urol. 2012;79:298. doi: 10.1016/j.urology.2011.07.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willis LR, Evan AP, Connors BA, et al. Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. JASN. 1999;10:1753. doi: 10.1681/ASN.V1081753. [DOI] [PubMed] [Google Scholar]
- 15.Sas DJ. An update on the changing epidemiology and metabolic risk factors in pediatric kidney stone disease. Clin J Am Soc Nephrol. 2011;6:2062. doi: 10.2215/CJN.11191210. [DOI] [PubMed] [Google Scholar]
- 16.Writing Group for the SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among US youth: Prevalence estimates from the SEARCH for diabetes in Youth Study. Pediatrics. 2006;118:1511. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 17.Brufani C, Ciampalini P, Grossi A, et al. Glucose tolerance status in 510 children and adolescents attending an obesity clinic in Central Italy. Pediatric Diabetes. 2010;11:47. doi: 10.1111/j.1399-5448.2009.00527.x. [DOI] [PubMed] [Google Scholar]
- 18.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee L, Alloosh M, Saxena R, et al. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology. 2009;50:56. doi: 10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome. Diabetes Care. 2005;28:1769. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 21.Handa RK, Evan AP, Connors BA, et al. Shock wave lithotripsy targeting of the kidney and pancreas does not increase the severity of metabolic syndrome in a porcine model. J Urol. 2014 doi: 10.1016/j.juro.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 23.Christoffersen B, Golozoubova V, Pacini G, et al. The young Gottingen minipig as a model of childhood and adolescent obesity: Influence of diet and gender. Obesity. 2013;21:149. doi: 10.1002/oby.20249. [DOI] [PubMed] [Google Scholar]
- 24.Xi S, Yin W, Wang Z, et al. A minipig model of high-fat/high-sucrose diet-induced diabetes and atherosclerosis. Int J Exp Path. 2004;85:223. doi: 10.1111/j.0959-9673.2004.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krambeck AE, Rule AD, Li X, et al. Shock wave lithotripsy is not predictive of hypertension among community stone formers at long-term followup. J Urol. 2011;185:164. doi: 10.1016/j.juro.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu C, Longfei L, Long W, et al. A systemic review and meta-analysis of new onset hypertension after extracorporeal shock wave lithotripsy. Int Urol Nephrol. 2014;46:719. doi: 10.1007/s11255-013-0588-7. [DOI] [PubMed] [Google Scholar]
- 27.Eterovic D, Situm M, Markovic V, et al. Are we estimating the adverse effects of shock-wave lithotripsy on a faulty scale? Med Hypotheses. 2014 doi: 10.1016/j.mehy.2014.03.005. http://dx.doi.org/10.1016/j.mehy.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 28.El-Nahas RA, Awad BA, El-Assmy AM, et al. Are there long-term effects of extracorporeal shockwave lithotripsy in paediatric patients? BJUI. 2013;111:666. doi: 10.1111/j.1464-410X.2012.11420.x. [DOI] [PubMed] [Google Scholar]
- 29.Janetschek G, Frauscher F, Knapp R, et al. New onset hypertension after extracorporeal shock wave lithotripsy: Age related incidence and prediction by intrarenal resistive index. J Urol. 1997;158:346. doi: 10.1016/s0022-5347(01)64475-6. [DOI] [PubMed] [Google Scholar]
- 30.Weber C, Gluck U, Staehler G, et al. Extracorporeal shock wave treatment raises blood pressure in borderline hypertensive rats. J Urol. 1995;154:232. [PubMed] [Google Scholar]