Abstract
Inflammatory diseases are highly associated with affective disorders including depression and anxiety. While the role of the innate immune system on emotionality has been extensively studied, the role of adaptive immunity is less understood. Considering that chronic inflammatory conditions are mediated largely by maladaptive lymphocyte function, the role of these cells on brain function and behavior during inflammation warrants investigation. In the present study we employed mice deficient in lymphocyte function and studied behavioral and inflammatory responses during challenge with bacterial lipopolysaccharides (LPS). Rag2−/− mice lacking mature lymphocytes were susceptible to death under sub-septic (5 mg/kg) doses of LPS and survived only to moderate (1 mg/kg) doses of LPS. Under these conditions, they displayed attenuated TNF-alpha responses and behavioral symptoms of sickness when compared with immunocompetent mice. Nevertheless, Rag2−/− mice had protracted motivational impairments after recovery from sickness suggesting a specific function for lymphocytes on the reestablishment of motivational states after activation of the innate immune system. The behavioral impairments in Rag2−/− mice were paralleled by an elevation in plasma corticosterone after behavioral tests. These results provide evidence that the absence of adaptive immunity may be associated with emotional deficits during inflammation and suggest that depressive states associated with medical illness may be mediated in part by impaired lymphocyte responses.
Keywords: Open Field Test, Forced Swim Test, Cytokines, Corticosterone, T cells, microglia
1. Introduction
Activation of the innate immune system in peripheral compartments of the body by means of bacterial products such as lipopolysaccharides (LPS) is known to induce a group of symptoms collectively referred to as sickness behavior [1–5]. These include, among others, lethargy and/or immobility, sleepiness, ptosis, piloerection, inhibition of sexual behavior and reduced food and water intake. These behavioral symptoms are often accompanied by physiological changes such as hyper- or hypothermia, changes in blood pressure, activation of the hypothalamic-pituitary-adrenal axis and increased monoamine turnover in the brain [1, 4]. These symptoms are mainly mediated by the actions of macrophages and cytokines in the periphery and mechanisms of transduction of inflammation from the periphery to the brain. During this process, the cytokines interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL6) play a major inflammatory role in the brain by acting initially in the circumventricular organs, choroid plexus, menigeal spaces and median eminence [3]. The inflammatory signal is then transduced into the brain parenchyma inducing de-novo synthesis of cytokines by perivascular cells of the brain and microglia [6–10]. As this inflammatory cascade resolves, behavioral symptoms of sickness also recede. Nevertheless certain motivational deficits persist for some time, supporting the notion that immunological processes are capable of causing emotional disturbances by mechanisms triggered by inflammatory processes and cytokines [3].
The ability to separate sickness behavior from emotional disturbances in experimental animals has provided supportive evidence that activation of the innate immune system involves specific pathways that lead to symptoms of depressive-like behaviors including anhedonia and behavioral despair as well as symptoms of anxiety-like behaviors [3, 11–14]. This knowledge has contributed to the understanding of the relationship between inflammatory conditions and the development of depression and anxiety in humans. Indeed, chronic inflammatory conditions including autoimmune and allergic diseases, are highly associated with these mental disorders [15–21]. An important aspect of these diseases is that they are largely mediated by a defective adaptive immune response. Furthermore, studies in rodents have shown that activation of adaptive immune processes are capable of inducing emotional disturbances that are different from those triggered by the innate immune response [22–25]. Nevertheless, the role of the adaptive arm of the immune system on brain function and behavior is poorly understood.
Thus far, the effects of activation of the innate or the adaptive immune system on behavior have been studied separately; nevertheless, it is known that innate and adaptive immune processes act synergistically to produce an integral immune response to maximize survival and recovery. In the present study we evaluated the role of adaptive immunity during activation of the innate immune system by means of LPS challenge. Mice deficient in lymphocyte function by deletion of the recombination-activating gene II (RAG2) were compared with immunocompetent mice during LPS challenge. Rag2−/− mice have an intact innate immune system and normal hematopoiesis, but lack mature T and B cells due to the inability to perform recombination, processes necessary for T cell and immunoglobulin receptor function [26, 27]. Following LPS administration, immune deficient and immunocompetent mice on a BALB/c background were assessed for sickness behavior and motivational deficits. Furthermore, body temperature and plasma cytokine and corticosterone levels were determined at multiple time points. Finally, hypothalamic cytokine mRNA expression, as well as microglial activation, was also evaluated. This region of the brain was selected due to its critical role in regulating body temperature and neuroendocrine responses during an LPS challenge. Furthermore, the ability of this region to produce cytokines and, in turn, be modulated by peripherally derived cytokines indicates that the hypothalamus is an important site of neuro-immune interaction [8, 9, 28–30].
2. Methods and Materials
2.1 Animals
Male BALB/c (n = 44) and Rag2−/− mice on a BALB/c background (n=43) (Taconic Farms, model 601), 8–10 weeks old were housed under normal conditions (12 hrs light/dark cycle, 2–4 mice per cage) with ad libitum access to food and water. Prior to beginning any experiments all animals were handled daily for several days to monitor overall health and habituate the animals to the experimenter. All procedures were carried out under approved IACUC protocols and institutional guidelines at the University of Maryland, School of Medicine.
2.2 LPS injections and tissue processing
Experiment 1
BALB/c and Rag2−/− mice (n = 6 BALB/c and 5 Rag2−/−) were administered subseptic doses of LPS (5mg/kg, i.p.; Sigma-Aldrich, Serotype 055:B5) or saline via intraperitoneal (i.p) injections between 9:00 and 11:00 AM. They were monitored for vital signs every hour for the first 6 hours and every 3 hours for a total of 12 hours. They were then left undisturbed overnight and monitored again at 24, 36 and 48 hours after LPS injection. Body temperature was recorded at baseline, 3, 6, 9 and 12 hours after LPS administration (Figure 1A).
Figure 1.
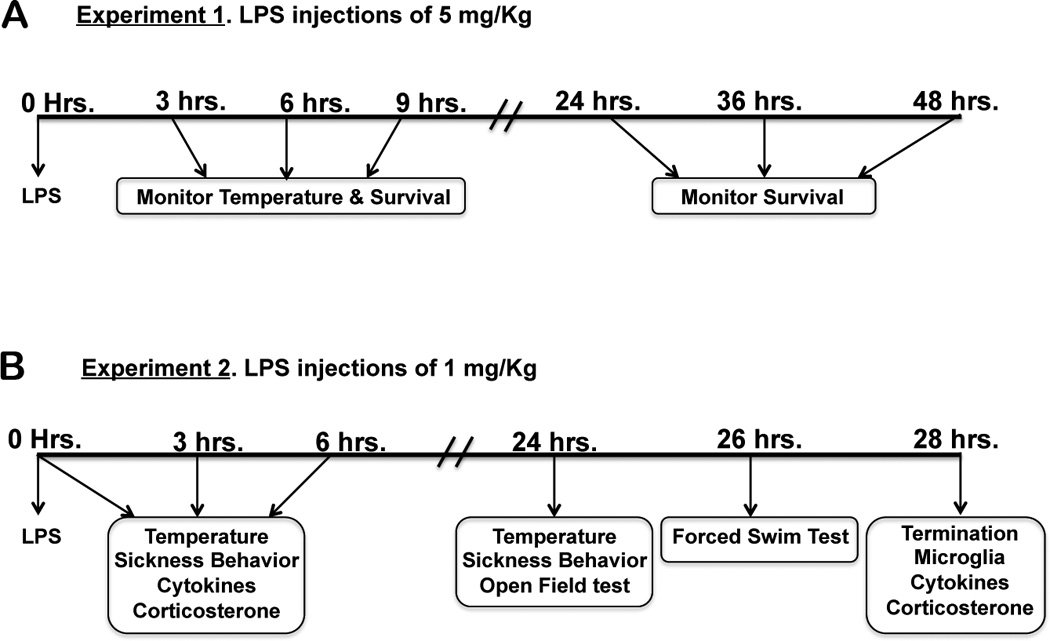
Flow chart of the experimental design for mice receiving intraperitoneal LPS injections of 5 mg/kg or 1 mg/kg of body weight. The parameters evaluated at each time point are indicated inside the boxes.
Experiment 2
BALB/c and Rag2−/− mice (n = 38 BALB/c and 38 Rag2−/−) were administered a sub-septic dose of LPS (1mg/kg, i.p.) or saline between 9:00 and 11:00 AM. Body temperature and sickness behavior were recorded at baseline prior to injection and at 3, 6, 12 and 24 hours after LPS administration. Groups of mice were terminated at 3 and 6 hours after LPS injections and tissue collected for biochemical determinations. Additional groups of mice were left undisturbed overnight and tested for temperature and sickness behavior 24 hours after LPS administration. They were then tested in the open field test (OFT) approximately 15 minutes after recording sickness behavior, followed by testing in the forced swim test (FST) two hours later, 26 hours after LPS administration. Two hours after the completion of the FST, the experiments were terminated and tissue collected for biochemical determinations (Figure 1B). At each end point of analysis, mice were anesthetized with 5% isofluorane and blood was collected via cardiac puncture. Mice were euthanized either by cervical dislocation and the brain immediately removed and frozen in isopentane or they underwent transcardial perfusion with 0.01% heparin in PBS followed by 4% paraformaldehyde (PFA).
2.3 Evaluation of sickness behavior and temperature
Sickness behavior was scored on a four-point scale as described by Gandhi, et al. [31]. Briefly, mice were checked for lethargy (demonstrated by diminished locomotion and curled body posture), ptosis (drooping eyelids), and piloerection (ruffled, greasy fur) with each symptom equal to 1 point resulting in a scale of 0 to 3 with 0 = no symptom and 3 = all symptoms present. For lethargy, delayed movement after prompting was scored as 0.5 points. Similarly, piloerection of fur only at the nape of the neck was also given 0.5 points. Scoring was performed by three independent observers. After confirming that independent scoring produced similar results, the values were averaged for statistical analyses. Core body temperature was determined using a digital rectal probe.
2.4 Open field test
Horizontal exploratory locomotor activity and anxiety behavior were assessed in the OFT. Mice were placed in the corner of one of four square arenas (50 × 50 cm) and allowed to freely explore while being recorded with an overhead camera. Illumination of the room was set to 5 lux as measured in the center of the arena. Constant background noise of 70 dB was provided by a white noise machine. Total distance traveled and the time spent in the center (interior 50%) of the arena was determined during the 10 min session using TopScan software (CleverSys, Inc; Reston, VA).
2.5 Forced swim test
Two hours after completion of the OFT mice were assessed for behavioral despair in the FST. Briefly, four individual mice (2 control and 2 LPS treated mice) were tested simultaneously by being placed into clear glass cylinders, 19 cm in diameter, 29 cm high and filled to a depth of 14 cm with tap water at 24° C. Behavior during the forced swim test was digitally recorded for 6 minutes and analyzed for floating/immobility for the last 4 minutes of the test. The videos were manually scored by two independent observers blind to treatment, with immobility defined as the lack of directional movement using either front or hind legs.
2.6 Plasma Corticosterone
Plasma corticosterone levels were determined by radioimmunoassay using the ImmuChem Corticosterone Double Antibody RIA kit (ImmuChem, MP Biomedicals, Orangeburg, NY) according to manufacturer’s instruction. Intra assay variation was less than 5%.
2.7 Plasma levels of cytokines
Plasma levels of IL-1β, IL6 and TNF-α were determined using a Luminex Multianalyte System at the University of Maryland cytokine laboratory core facility. In brief, after processing blood plasma with assay buffers, 25 µl of each sample, along with standards and controls were transferred to appropriate wells in a 96 well filter plate (Millipore) and vacuum filtered. A mixture containing IL-1β, IL6 and TNF-α that have been conjugated to beads was added to each well and incubated overnight at 4° C under shaking. The plate was then vacuumed and washed twice with 200 µl of buffer. After the last vacuum step, the detection antibody was added and incubated under shaking for 1 hour at room temp. Finally, 25 µl of phycoerythrin (1:25 dilution) was added to each well and incubated for 30 minutes. After thorough washing, 150 µl of Sheath Fluid was added to each well and the plate was read and the data collected using a Luminex 100 reader.
2.8 Real-time RT-PCR determination for brain cytokines
Dissected tissue from the whole hypothalamic region was processed for mRNA extraction using the Trizol reagent (Invitrogen, USA) as described previously [25]. Total RNA was treated with DNAse (Invitrogen) for 15 minutes at room temperature according to manufacturer’s instruction. Five hundred ng of total RNA per sample were reverse transcribed into cDNA in a 20 µl reaction using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to manufacturer's instructions. Real-time RT-PCR was conducted using the iQ SYBR Green Supermix (Bio-Rad) in a 25 µl reaction using the following set of primers: IL1-β forward 5'CCCATTAGACAACTGCACTAC3', IL1-β reverse 5'GATTCTTTCCTTTGAGGCCC3'; IL6 forward 5'CAGAAACCGCTATGAAGTTCC3', IL6 reverse 5'CTCATTTCCACGATTTCCCAG3'; TNF-α forward 5'AAGAGGCACTCCCCCAAAAG3', TNF-α reverse 5'CTGGGCCATAGAACTGATGAG3'. The PCR products derived from all sets of primers were run on 0.9% agarose gel to confirm a single amplification product. All primer pairs were designed using the Accelrys Gene 2.0v software. The real-time PCR reaction was run on a MyiQ instrument (Bio-Rad) with a three step cycling program as follows: an initial hot start for 5 min at 95° C followed by 40 cycles with a denaturation step of 15 sec at 95° C, an annealing step of 30 sec at 55° C, an extension step of 30 sec at 72° C with the optics on at this last step. In preparation of a melt curve, the samples were heated for 1 minute at 95° C then cooled for 1 minute at 55° C, and the melt curve was executed in 10 second increments of 0.5° C with the temperature increasing from 55° C to 95° C with the optics on. All the primers used were selected and tested for the described amplification conditions. Efficiency and consistency of the cDNA synthesis was determined by amplification of the 18S gene as a control. For each round of amplifications, only those samples that were within 1 cycle of difference respect to the mean for 18S were considered for further analysis. For each sample of a specific target gene, each cycle threshold was normalized with respect to the average of 3 control genes including 18S, GAPDH and Actin-beta using previously published primer sets [25]. Relative expression was determined using the 2−ΔΔCt method [32].
2.9 Immunohistochemistry
Serial consecutive coronal sections (30 µm) were cut in a cryostat and mounted directly onto gelatinized slides. To perform antigen retrieval slides were washed in 0.5% Tx-100 in PBS (PBST) and then incubated in 1% sodium borohydride at room temperature for 20 min. Slides were then washed thoroughly and endogenous peroxidase activity blocked via incubation in 0.5% hydrogen peroxide for 15 min. After washing, slides were incubated in anti-Iba1 (1:1000 in PBST, Wako BioProducts; # 019-19741) for one hour at room temp, then overnight at 4° C. The following day slides were washed and incubated in biotinylated goat anti-rabbit IgG (1:500 in PBST, Vector Labs) for 1 hour at room temp. Following secondary antibody application slides were washed and then incubated in A/B solution (Vectastain ABC kit, Vector Labs; # PK-6100) for 1 hour. Antibody complexes were then precipitated using nickel enhanced 3, 3’-diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich; #D5905). Slides were then washed, air dried and dehydrated in a graded ethanol series followed by xylene and coverslipped with DPX mounting media.
2.10 Imaging and Microglia Quantification
A total of 15 to 20 coronal sections per animal (n = 3/group) of the antero-medial hypothalamus (Bregma −1.06 to −2.54) were processed and used for semiquantitative analysis of microglia. One to two images per section, with the second image directly superior to the first, were taken to ensure that the entire hypothalamic region was included. Each image constituted a field for quantification. Each image was taken at 100× magnification with a Zeiss Axioscop and the ZEN 2011 microimaging software (Carl Zeiss AG, Oberkochen, Germany) and covered a field of 2.7 mm × 3.5 mm vertically centered on the third ventricle (3V). Microglia were identified by positive Iba-1 labeling of the cell body and processes, and were quantified using the ImageJ software cell counter plugin (National Institutes of Health, Bethesda, MD). Data was processed and presented as the average number of microglia per field as determined by two independent analyzers.
2.11 Statistical Analysis
All statistical analyses were conducted using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). Differences in survival curves were determined using the Log-Rank test. A two-way ANOVA followed by the Fisher’s LSD post hoc test was used to determine differences in temperature, sickness behavior, cytokines, plasma corticosterone and microglial cells. Exploratory and motivational behavior was analyzed with a two-way ANOVA for main effects of treatment and genotype. Newman-Keuls post hoc test was used for multiple comparisons. The effect of treatment and genotype on microglia activation was determined by two-way ANOVA. Planned comparisons were conducted within genotypes using Student’s t tests to evaluate the a priori hypothesis that LPS would induce changes in Rag2−/− and BALB/c mice due to the presence of an intact innate immune system in both genotypes. A p ≤ 0.05 was considered significant.
3. Results
3.1 Experiment 1. Survival to 5 mg/kg LPS challenge
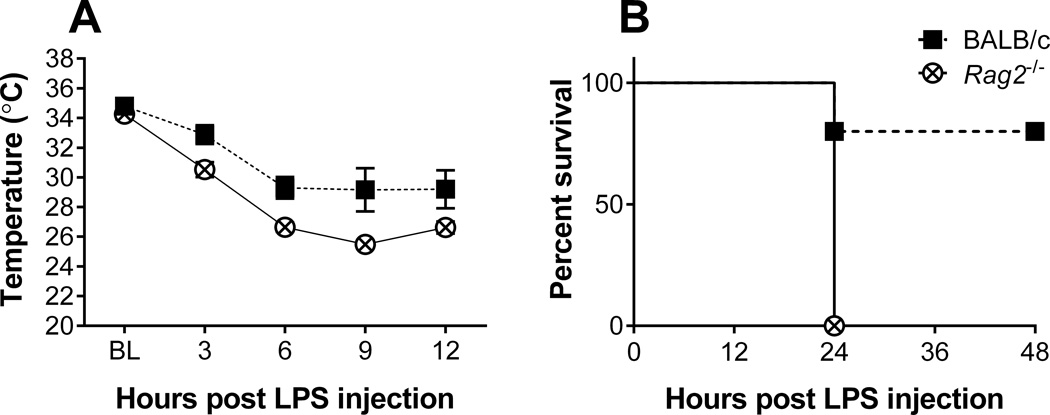
Mice administered an LPS dose of 5 mg/kg displayed marked and evident signs of sickness in both genotypes as early as 1 hour after injections. Analysis of body temperature revealed a significant drop over time [F (4, 32) = 41.78, p < 0.0001], with Rag2−/− mice becoming significantly more hypothermic than BALB/c mice [F (1, 8) = 15.58, p = 0.004] (Figure 2A). While mean baseline temperatures did not differ between genotypes (BALB/c: 34.8° C ± 1.1 vs. Rag2−/−: 34.3° C ± 1.1), there was a maximum mean difference in temperature of 3.7° C ± 1.1 by 9 hours post injection with Rag2−/− mice becoming severely hypothermic. All mice of the Rag2−/− genotype died between 12 and 24 hours post LPS injection while BALB/c mice had a survival of 80% at 48 hours post injection (p = 0.014, Log-rank test) (Figure 2B). These results show the critical role of lymphocytes and recombination processes for survival against septic shock.
Figure 2.
Core body temperature (A) and survival rate (B) of BALB/c and Rag2−/− mice receiving an LPS dose of 5 mg/kg of body weight. Statistical analysis by two-way ANOVA for temperature revealed main effects for time (p < 0.0001) and genotype (p = 0.0043). Statistical comparisons for survival by Log Rank (Mantel Cox) test showed a significant effect (p = 0.0143), with a median survival time of 24 hr for Rag2−/− mice.
3.2 Experiment 2. Sickness behavior and temperature after 1 mg/kg LPS challenge
All mice administered with an LPS dose of 1 mg/kg survived the challenge. Significant changes in temperature over time [F (3, 162) = 35.20, p < 0.0001] accompanied by a significant effect of genotype [F (1, 162) = 4.12, p = 0.044] were observed, with a significant interaction [(3, 162) = 3.20, p = 0.025 (Figure 3A). After initially dropping, the body temperature of Rag2−/− mice began to recover by 6 hours after the injection with a significant increase over BALB/c mice (p = 0.002, Fisher’s LSD post hoc), whose temperature had continued to drop (Figure 3A). Mean body temperature had returned to normal by 24 hrs after LPS administration in both groups.
Figure 3.
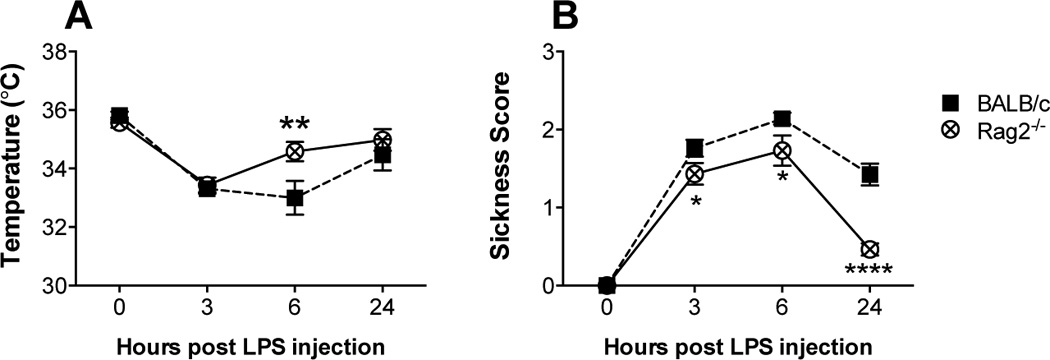
Core body temperature (A) and sickness behavior (B) of BALB/c and Rag2−/− mice after receiving an LPS dose of 1 mg/kg of body weight. Analysis by two-way ANOVA revealed significant differences. (A) Temperature: main effects of time (p < 0.0001) and genotype (p = 0.0454) with a significant interaction (p = 0.025). (B) Sickness behavior: main effects of time (p < 0.0001) and genotype (p < 0.0001), interaction (p = 0.0005). Fisher’s LSD post hoc test: * p < 0.05, ** p < 0.01, **** p < 0.0001
A significant difference in the manifestation of sickness behavior between Rag2−/− and BALB/c mice was observed [genotype × time point: F (3, 150) = 6.33, p = 0.0005] (Figure 3B). While both groups of mice showed a significant change in sickness behavior over time [F (3, 150) = 116.0, p < 0.0001], Rag2−/− mice displayed significantly fewer symptoms of sickness compared to BALB/c mice [genotype: F (1, 150) = 28.77, p < 0.0001]. Post hoc analysis showed that Rag2−/− mice display a lower degree of sickness behavior 3 and 6 hours after LPS injection and fully recovered by 24 hours after LPS administration (Figure 3B).
3.3 Experiment 2. Open field and forced swim tests
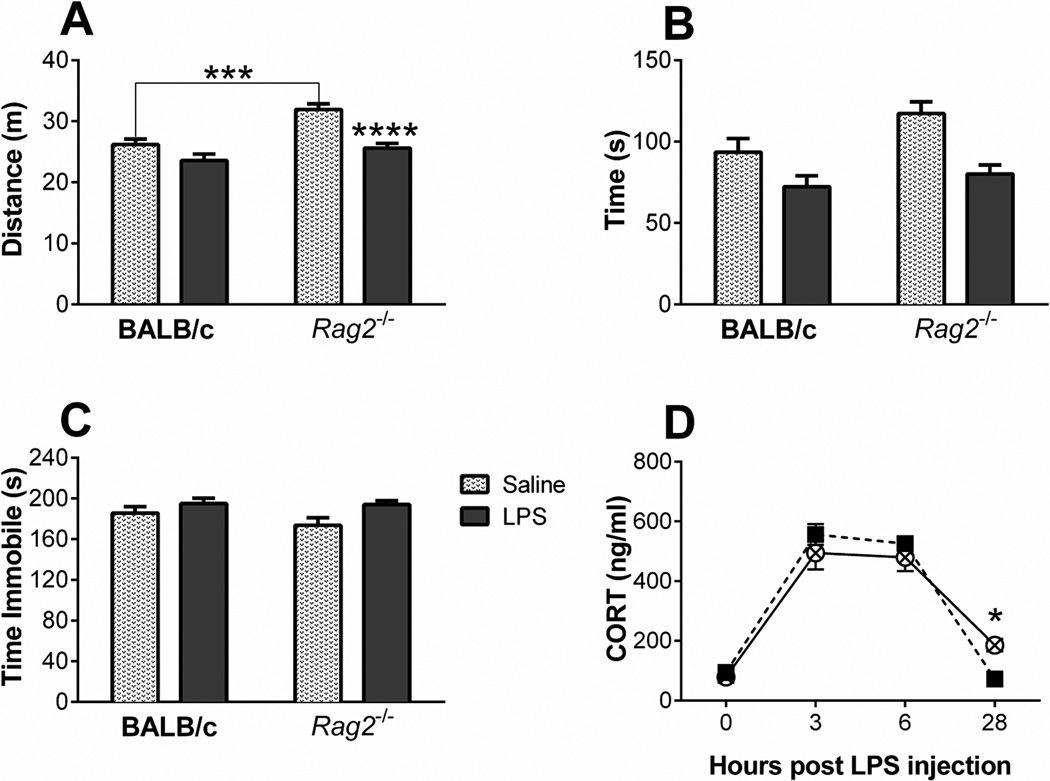
Exploratory behavior in the OFT was recorded 24 hours after LPS administration, about 15 minutes after recording sickness behavior. LPS treatment significantly decreased locomotion [F (1, 44) = 23.58, p < 0.0001] (Figure 4A) and center time [F (1, 43) = 18.27, p = 0.0001] (Figure 4B). However, the degree to which LPS affected these parameters of exploration and anxiety-like behavior was dependent upon genotype. Two-way ANOVA analysis indicated a significant interaction between genotype and LPS treatment for distance traveled [F (1, 44) = 4.07, p = 0.049]. Newman-Keuls post hoc analysis revealed that Rag2−/− saline treated mice travel significantly more that BALB/c controls and although both genotypes displayed decreased locomotion, the effect of LPS was only significant between Rag2−/− saline treated and LPS treated mice. Evaluation of time spent in the center of the arena produced similar findings (Figure 4B); although there was not a significant interaction, there was a main effect of genotype [F (1, 43) = 5.36, p = 0.026). Nevertheless, planned pair comparisons within genotype showed that Rag2−/− mice spent significantly less time in the LPS condition when compared to saline control [t (20) = 2.01, p = 0.003] while BALB/c mice showed no significant differences between treatments.
Figure 4.
Total distance traveled (A) and total time spent in the center field (B) of a square enclosed arena for BALB/c and Rag2−/− mice 24 hours after receiving an LPS dose of 1 mg/kg of body weight. Immobility time (C) in the forced swim test 26 hours after LPS administration. (D) Time-course of plasma corticosterone concentrations. Analysis by two-way ANOVA revealed significant differences: (A) Distance traveled, main effects of LPS (p < 0.0001) and genotype (p = 0.0001), interaction (p = 0.0499). (B) Time in center, main effects of LPS (p = 0.0001) and genotype (p = 0.0255). (C) Immobility time: main effect of LPS (p = 0.0147). (D) Plasma corticosterone: main effects of time (p < 0.0001) with an interaction between time and genotype (p = 0.034). Newman-Keuls or Fisher’s LSD post hoc test: * p < 0.05, *** p < 0.001, **** p < 0.0001. See text for planned comparisons differences.
Assessment for behavioral despair in the FST two hours later indicated that LPS treatment induced a significant increase in immobility time [F (1, 40) = 6.50, p = 0.015] with no main effect of genotype and no significant interaction (Figure 4C). Nevertheless, planned pair comparisons within group revealed a significant increase in immobility by Rag2−/− LPS treated mice over Rag2−/− saline controls [t (20) = 2.54, p = 0.02], but no difference within the BALB/c mice.
3.4 Experiment 2. Plasma levels of corticosterone, cytokines and hypothalamic mRNA expression of cytokines
Analysis of plasma corticosterone levels revealed a main effect of time [F (3, 36) = 125.0, p < 0.0001) with no effect of genotype. However, there was a significant interaction between both factors [F (3, 36) = 3.218, p = 0.034) and Fisher’s LSD post hoc analysis indicates that corticosterone is significantly elevated in Rag2−/− mice compared to BALB/c mice at the conclusion of the experiment (Figure 4D).
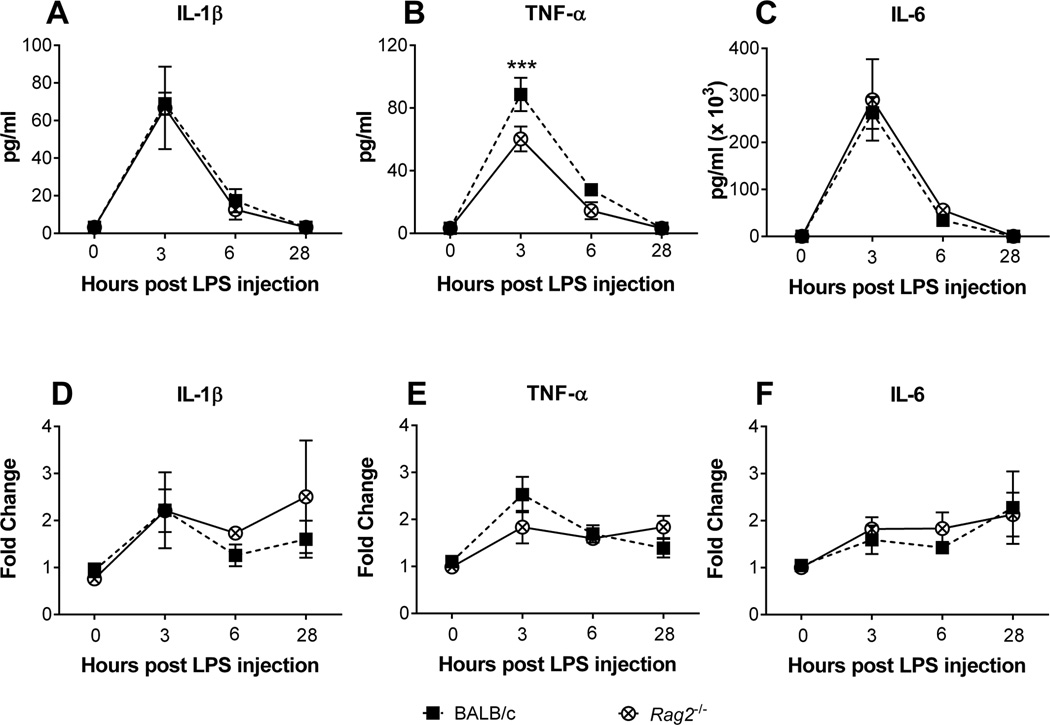
Plasma cytokine levels showed a time dependent change in response to LPS administration in both BALB/c and Rag2−/− mice (Figure 5A, B, C). Of those cytokines evaluated (IL-1β, IL6 and TNF-α) only TNF-α was dependent upon genotype, with Rag2−/− mice expressing significantly lower levels than BALB/c mice [F (1, 36) = 9.658; p = 0.004; genotype × time: F (3, 36) = 3.980, p = 0.015] (Figure 5 B). Post hoc analysis revealed that Rag2−/− mice had significantly lower levels of TNF-α 3 hrs after LPS administration. Although hypothalamic mRNA expression of IL6 and TNF-α also showed a time-dependent change in both genotypes [IL6: F (3, 34) = 3.797, p = 0.0189; TNF-α: F (3, 34) = 9.482, p = 0.0001], there were no significant differences detected between genotypes, nor any interaction between factors, for any of the cytokines measured in the hypothalamus (Figure 5 D, E, F).
Figure 5.
Plasma circulating levels of interleukin-1 beta (A) tumor necrosis factor alpha (B) and interleukin-6 (C) in BALB/c and Rag2−/− mice receiving a LPS dose of 1 mg/kg of body weight. Hypothalamic mRNA expression of interleukin-1 beta (D) tumor necrosis factor alpha (E) and interleukin-6 (F) in the same groups of mice. Two-way ANOVA analysis indicates significant effects of time for all plasma cytokine levels, as well as mRNA levels for interleukin-6 and tumor necrosis factor alpha (A–C, E, F: p ≤ 0.001), with a significant effect of genotype (p = 0.004) and an interaction (p = 0.015) for levels of tumor necrosis factor alpha. Fisher's LSD post hoc. *** p < 0.001.
3.5 Experiment 2. Semiquantitative analysis of microglia in the hypothalamus
Analysis of Iba-1 positive microglia in the hypothalamus revealed main effects of LPS [F (1, 331) = 293.8, p < 0.0001] and genotype [F (1, 331) = 18.29, p < 0.0001; interaction = n.s.] on the average number of microglia in BALB/c and Rag2−/− mice (Figure 6A). Subsequent quantification of activated microglia, as specified by a hypertrophied morphology, showed significant effects of LPS [F (1, 331) = 511.0, p < 0.0001] and genotype [F (1, 331) = 16.25, p < 0.0001] in the hypothalamus of BALB/c and Rag2−/− mice. However, the effect of LPS was dependent upon genotype [interaction: F (1, 331) = 15.47, p = 0.0001] and post hoc analysis indicated that, following LPS administration, BALB/c mice had significantly more activated microglia than Rag2−/− mice (Figure 6B).
Figure 6.
Average of number per field of Iba1 positive microglia (A) and activated microglia (B) in the anteromedial hypothalamic region of BALB/c and Rag2−/− mice receiving a LPS dose of 1 mg/kg of body weight, 28 hours after LPS administration. Statistical comparisons by two-way ANOVA revealed significant differences. (A) Microglia per field: main effects of LPS (p < 0.0001) and genotype (p < 0.0001). (B) Activated microglia: main effects of LPS (p < 0.0001) and genotype (p < 0.0001) with a significant interaction (p = 0.0001). Representative digital microphotographs of the hypothalamus at the level of the median eminence (ME) in saline control (C, D) or LPS treated (E, F) mice. Scale bar in F applicable to all panels: 100 µm. 3V: third ventricle.
4. Discussion
The present study provides evidence that adaptive immune processes involving T and B cell receptor recombination play an important role in the manifestation of behavioral symptoms of sickness and on appropriate behavioral and hormonal recovery following LPS challenge. It also shows the critical survival role of lymphocytes during activation of the innate immune system and their modulatory function on TNF-α production. Since Rag2−/− mice have an intact innate immune response, these studies also confirm that activation of the innate immune system by LPS is the main process by which behavioral responses to LPS challenge are driven. Together, these results suggest that behavioral adaptation to LPS challenge is the resultant of a coordinated response of the innate as well as the adaptive arm of the immune system.
The endotoxin LPS is a component of bacterial cell walls that activate the innate immune response by activating the toll-like receptor 4 (TLR4). Detection of LPS initially occurs via binding to LPS-binding protein (LBP); the LPS-LBP complex then associates with the CD14 receptor and activates TLR4 resulting in induction of nuclear factor kappa-b (NFκ-b) mediated transcription and production of pro-inflammatory cytokines [33]. This process initiates a cellular signaling cascade that culminates in the recruitment and expansion of macrophages and neutrophils aimed at eliminating the invading pathogen. This mechanism primarily involves the innate immune response, and because purified LPS is non-infectious, the physiological and neurobehavioral effects are known to be mediated by the host immune response. Under these conditions, the reported dissociation between sickness behavior and the effects on motivational states induced by peripheral administration of LPS provides the experimental basis for understanding the effects of inflammation and cytokines on adaptive behaviors and emotional states [3, 4]. It has been shown that the behavioral effects of LPS challenge follow a bi-phasic temporal sequence in which the symptoms of sickness develop at early time points while the effects on motivation and emotion appear at later time points [3, 4]. In different strains of mice and rats, and at the approximate doses used in the present study [34–37], the symptoms of sickness are maximal between 6 and 12 hours while the effects on motivation are greatest at 24 hours, when the symptoms of sickness are believed to have minimal influence on behavioral performance. The present study shows that the adaptive arm of the immune system plays a role in the manifestation of sickness behavior and recovery to motivational states. Immunocompetent BALB/c mice displayed a higher degree of sickness behavior than immune deficient Rag2−/− mice, where activation of the innate immune system alone also resulted in a faster resolution of the symptoms of sickness. Core body temperature was also significantly different after 6 hours of LPS injections at the time that symptoms of sickness were maximal. While LPS can induce both hyper or hypothermia depending on the species, strain and dosage of LPS, hypothermic responses to LPS challenge are very common [38, 39] and usually parallel symptoms of sickness behavior. These effects in Rag2−/− mice were accompanied by an attenuated peripheral TNF-α response in the hypothalamus when compared to BALB/c mice. Notably, BALB/c and Rag2−/− mice had a similar peripheral IL1-β and IL-6 response as well as hypothalamic mRNA expression. Thus, attenuated behavioral symptoms of sickness were correlated with reduced peripheral TNF-α responses suggesting that lymphocytes may play a role in TNF-α production during LPS challenge, and that differences in symptoms of sickness may be mediated by the action of this cytokine. Moreover, the manifestation of sickness behavior in Rag2−/− mice in parallel with comparable expression of IL1-β and IL6 confirms that these cytokines likely participate in the manifestation of sickness behavior.
In the Rag2−/− model of immune deficiency, the absence of functional lymphocytes resulted in protracted motivational abnormalities at the time when sickness behavior had completely resolved. Our study explored specific aspects of emotionality related to motivational states and anxiety-like responses as evaluated in the OFT and behavioral despair as measured by the FST. Nevertheless, these two tests employed may be also sensitive to other behavioral and motivational components induced by inflammation such as fatigue, a component that should be studied further in immune deficient mice. Similar to our previous reports on Rag2−/− mice on the BALB/c background [27], control Rag2−/− mice exhibit enhanced locomotor exploratory behavior and reduced anxiety-like behavior in the OFT compared to wild type controls. Thus, an enhanced effect of LPS in Rag2−/− versus wild type mice in the OFT may be attributed to significant differences in basal behavior. Nevertheless, under basal conditions Rag2−/− mice display similar habituation to the arena compared to wild type mice [27] and further examination of the behavior showed the same effect on habituation suggesting that LPS affected components of motivation and/or fatigue in Rag2−/− mice. This is also supported by the results in the FST where swimming behavior was comparable between wild type and Rag2−/− in control conditions while LPS affected immobility time in Rag2−/− mice as revealed by planned comparisons. Considering that Rag2−/− mice showed no symptoms of sickness behavior 24 hours after LPS administration, the present results support the notion that adaptive immunity may modulate recovery of motivational states during resolution of inflammation and also may also influence fatigue states. This may have implications for many diseases in which the adaptive arm of the immune system is dysfunctional, including autoimmune and allergic diseases, as well as immune deficiency conditions such as HIV infections.
The potential mechanisms by which lymphocytes may modulate motivational states remain poorly understood. The present study showed that peripheral pro-inflammatory cytokine levels as well as hypothalamic mRNA of the same cytokines were similar between wild type and Rag2−/− mice shortly after behavioral testing. In contrast, plasma corticosterone levels were elevated in Rag2−/− with respect to wild type mice indicating this as a potential factor that may modulate behavior. It is possible that elevated corticosterone levels may result from compensatory anti-inflammatory responses to LPS challenge due to the absence of modulatory functions of lymphocytes over other immune cells. Nevertheless, wild type mice showed increased total numbers of microglial cells in the hypothalamus. Thus, is possible that lymphocytes may have a function in promoting certain stimulatory mechanisms such as microglial proliferation, independent of pro-inflammatory cytokine expression, and that this mechanisms favor adequate return to homeostasis. It must be noted that lymphocytes, specifically CD4+ T cells, have been shown to provide protective functions on several CNS processes, including regeneration after nerve injury [40, 41] and promoting cognitive functions [42, 43], as well as homeostatic neuronal cell proliferation [44]. Several mechanisms have been proposed for how T cells participate on these processes. Perhaps the most consistent hypothesis across several studies is via production of anti-inflammatory cytokines, that upon acting on other cells types including microglia and astrocytes, control inflammation and stimulate the production of trophic factors [41, 43, 45–47]. Another mechanism by which lymphocytes may contribute to the recovery process from inflammation is through modulation of the cholinergic anti-inflammatory reflex [48–51]. It has been shown that upon challenge with LPS, a neural reflex mechanisms, involving either the vagus nerve and/or sympathetic splenic nerves [49, 52], stimulates a subset of CD4+ T cells to produce acetylcholine. T cell-derived acetylcholine in turn, modulates inflammation through a mechanism involving the alpha-7 subunit nicotinic acetylcholine receptor [48, 49]. The potential mechanisms of CD4+ T cells in modulating sickness behavior and recovery deserves further investigation.
In summary, the present study showed that the adaptive immune systems contribute to the manifestation of sickness behavior during activation of the innate immune response probably by modulating TNF-α production. It also showed that effective lymphocyte responses are required for restoring appropriate motivational and hormonal states during recovery from an inflammatory challenge.
Research Highlights.
Lymphocyte deficient mice are susceptible to death by septic shock
Adaptive immunity contributes to the manifestation of sickness behavior after LPS
Adaptive immunity contributes to the production of TNF-alpha in plasma
Lymphocytes modulates motivational states during resolution of inflammation
Lymphocytes affect corticosterone responses after behavioral testing
Acknowledgements
Supported by the National Institute of Mental Health Research Grant R01MH097676 (OppNet) and R21 MH091493 to LHT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dantzer R, Bluthe RM, Gheusi G, Cremona S, Laye S, Parnet P, et al. Molecular basis of sickness behavior. Annals of the New York Academy of Sciences. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 2.Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, Kelley KW. Cytokines and sickness behavior. Annals of the New York Academy of Sciences. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibb J, Audet MC, Hayley S, Anisman H. Neurochemical and behavioral responses to inflammatory immune stressors. Frontiers in bioscience (Scholar edition) 2009;1:275–295. doi: 10.2741/S26. [DOI] [PubMed] [Google Scholar]
- 5.Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, et al. Illness, cytokines, and depression. Annals of the New York Academy of Sciences. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 6.Tonelli LH, Maeda S, Rapp KL, Sternberg EM. Differential induction of interleukin-I beta mRNA in the brain parenchyma of Lewis and Fischer rats after peripheral injection of lipopolysaccharides. Journal of neuroimmunology. 2003;140:126–136. doi: 10.1016/s0165-5728(03)00171-1. [DOI] [PubMed] [Google Scholar]
- 7.Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurological research. 2005;27:679–684. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- 8.Quan N, Stern EL, Whiteside MB, Herkenham M. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. Journal of neuroimmunology. 1999;93:72–80. doi: 10.1016/s0165-5728(98)00193-3. [DOI] [PubMed] [Google Scholar]
- 9.Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–293. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 10.Quan N, Whiteside M, Kim L, Herkenham M. Induction of inhibitory factor kappaBalpha mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10985–10990. doi: 10.1073/pnas.94.20.10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andre C, O'Connor JC, Kelley KW, Lestage J, Dantzer R, Castanon N. Spatio-temporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. Journal of neuroimmunology. 2008;200:90–99. doi: 10.1016/j.jneuroim.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Paiva VN, Lima SN, Fernandes MM, Soncini R, Andrade CA, Giusti-Paiva A. Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behavioural brain research. 2010;215:146–151. doi: 10.1016/j.bbr.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Gibb J, Al-Yawer F, Anisman H. Synergistic and antagonistic actions of acute or chronic social stressors and an endotoxin challenge vary over time following the challenge. Brain, behavior, and immunity. 2013;28:149–158. doi: 10.1016/j.bbi.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Hayley S, Lacosta S, Merali Z, van Rooijen N, Anisman H. Central monoamine and plasma corticosterone changes induced by a bacterial endotoxin: sensitization and cross-sensitization effects. The European journal of neuroscience. 2001;13:1155–1165. doi: 10.1046/j.0953-816x.2001.01496.x. [DOI] [PubMed] [Google Scholar]
- 15.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, behavior, and immunity. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neuroscience and biobehavioral reviews. 2012;36:764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC medicine. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes M, Song C, Yirmiya R. Targeting IL-1 in depression. Expert opinion on therapeutic targets. 2012;16:1097–1112. doi: 10.1517/14728222.2012.718331. [DOI] [PubMed] [Google Scholar]
- 19.Palagini L, Mosca M, Tani C, Gemignani A, Mauri M, Bombardieri S. Depression and systemic lupus erythematosus: a systematic review. Lupus. 2013;22:409–416. doi: 10.1177/0961203313477227. [DOI] [PubMed] [Google Scholar]
- 20.Di Marco F, Santus P, Centanni S. Anxiety and depression in asthma. Current opinion in pulmonary medicine. 2011;17:39–44. doi: 10.1097/MCP.0b013e328341005f. [DOI] [PubMed] [Google Scholar]
- 21.Sansone RA, Sansone LA. Allergic rhinitis: relationships with anxiety and mood syndromes. Innovations in clinical neuroscience. 2011;8:12–17. [PMC free article] [PubMed] [Google Scholar]
- 22.Costa-Pinto FA, Basso AS. Neural and behavioral correlates of food allergy. Chemical immunology and allergy. 2012;98:222–239. doi: 10.1159/000336525. [DOI] [PubMed] [Google Scholar]
- 23.Costa-Pinto FA, Basso AS, De Sa-Rocha LC, Britto LR, Russo M, Palermo-Neto J. Neural correlates of IgE-mediated allergy. Annals of the New York Academy of Sciences. 2006;1088:116–131. doi: 10.1196/annals.1366.028. [DOI] [PubMed] [Google Scholar]
- 24.Mirotti L, Castro J, Costa-Pinto FA, Russo M. Neural pathways in allergic inflammation. Journal of allergy. 2010;2010:491928. doi: 10.1155/2010/491928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonelli LH, Katz M, Kovacsics CE, Gould TD, Joppy B, Hoshino A, et al. Allergic rhinitis induces anxiety-like behavior and altered social interaction in rodents. Brain, behavior, and immunity. 2009;23:784–793. doi: 10.1016/j.bbi.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 27.Clark SM, Sand J, Francis TC, Nagaraju A, Michael KC, Keegan AD, et al. Immune status influences fear and anxiety responses in mice after acute stress exposure. Brain, behavior, and immunity. 2014;38:192–201. doi: 10.1016/j.bbi.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Frontiers in bioscience : a journal and virtual library. 2004;9:1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- 29.McCann SM, Kimura M, Karanth S, Yu WH, Mastronardi CA, Rettori V. The mechanism of action of cytokines to control the release of hypothalamic and pituitary hormones in infection. Annals of the New York Academy of Sciences. 2000;917:4–18. doi: 10.1111/j.1749-6632.2000.tb05368.x. [DOI] [PubMed] [Google Scholar]
- 30.Turnbull AV, Lee S, Rivier C. Mechanisms of hypothalamic-pituitary-adrenal axis stimulation by immune signals in the adult rat. Annals of the New York Academy of Sciences. 1998;840:434–443. doi: 10.1111/j.1749-6632.1998.tb09582.x. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi R, Hayley S, Gibb J, Merali Z, Anisman H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain, behavior, and immunity. 2007;21:477–489. doi: 10.1016/j.bbi.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Tan Y, Kagan JC. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Molecular cell. 2014;54:212–223. doi: 10.1016/j.molcel.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressivelike behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson MA, McCusker RH, Kelley KW. Interleukin-1 beta converting enzyme is necessary for development of depression-like behavior following intracerebroventricular administration of lipopolysaccharide to mice. Journal of neuroinflammation. 2013;10:54. doi: 10.1186/1742-2094-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O'Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Hormones and behavior. 2012;62:202–209. doi: 10.1016/j.yhbeh.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1038–1048. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman MN, Mukhopadhyay P, Belyavskaya E, Tonelli LH, Revenis BD, Doran JH, et al. Glucocorticoid receptor dimerization is required for proper recovery of LPS-induced inflammation, sickness behavior and metabolism in mice. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.131. [DOI] [PubMed] [Google Scholar]
- 39.Skelly DT, Hennessy E, Dansereau MA, Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1Beta, TNF-alpha and IL-6 challenges in C57BL/6 mice. PloS one. 2013;8:e69123. doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raposo C, Graubardt N, Cohen M, Eitan C, London A, Berkutzki T, et al. CNS repair requires both effector and regulatory T cells with distinct temporal and spatial profiles. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:10141–10155. doi: 10.1523/JNEUROSCI.0076-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin J, Mesnard NA, Beahrs T, Wainwright DA, Serpe CJ, Alexander TD, et al. CD4+ T cell-mediated neuroprotection is independent of T cell-derived BDNF in a mouse facial nerve axotomy model. Brain, behavior, and immunity. 2012;26:886–890. doi: 10.1016/j.bbi.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. The Journal of experimental medicine. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. Journal of immunology. 2012;189:4213–4219. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. Journal of immunology. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- 45.Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain, behavior, and immunity. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, et al. Vaccination as a novel approach for treating depressive behavior. Biological psychiatry. 2009;65:283–288. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D'Acquisto F. CD4(+) but not CD8(+) T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Translational psychiatry. 2013;3:e280. doi: 10.1038/tp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawashima K, Fujii T, Moriwaki Y, Misawa H, Horiguchi K. Reconciling neuronally and nonneuronally derived acetylcholine in the regulation of immune function. Annals of the New York Academy of Sciences. 2012;1261:7–17. doi: 10.1111/j.1749-6632.2012.06516.x. [DOI] [PubMed] [Google Scholar]
- 49.Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Autonomic neuroscience : basic & clinical. 2014;182:65–69. doi: 10.1016/j.autneu.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Reardon C, Duncan GS, Brustle A, Brenner D, Tusche MW, Olofsson PS, et al. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1410–1415. doi: 10.1073/pnas.1221655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martelli D, Yao ST, McKinley MJ, McAllen RM. Reflex control of inflammation by sympathetic nerves, not the vagus. The Journal of physiology. 2014;592:1677–1686. doi: 10.1113/jphysiol.2013.268573. [DOI] [PMC free article] [PubMed] [Google Scholar]