Abstract
Objective
To test the hypothesis that knee cartilage changes over five years are associated with baseline peak knee adduction moment (KAM) and peak knee flexion moment (KFM) during early stance.
Design
Baseline KAM and KFM were measured in sixteen subjects with medial knee OA. Regional changes in cartilage thickness and changes in medial-to-lateral thickness ratio were quantified using magnetic resonance imaging at baseline and again after five years. Multiple regression was used to determine whether baseline measures of KAM and KFM were associated with cartilage changes over five years. Associations with baseline pain score, Kellgren-Lawrence grade, walking speed, age, gender, and body mass index were tested one-by-one in the presence of KAM and KFM.
Results
Changes over five years in femoral medial-to-lateral thickness ratio were associated with baseline KAM, KFM, and pain score (R2=0.60, p=0.010), and most significantly with KAM (R2=0.33, p=0.019). Changes in tibial medial-to-lateral thickness ratio were associated with baseline KAM, KFM, and walking speed (R2=0.49, p=0.039), with KFM driving this association (R2=0.40, p=0.009). Changes in medial tibial thickness were associated with baseline KAM, KFM, and walking speed (R2=0.49, p=0.041); KFM also drove this association (R2=0.42, p=0.006).
Conclusions
The findings that the KAM has a greater influence on femoral cartilage change and the KFM has a greater influence on tibial cartilage change provide new insight into the tibiofemoral variations in cartilage changes associated with walking kinetics. These results suggest that both KAM and KFM should be considered when designing disease interventions as well as when assessing the risk for OA progression.
Keywords: Gait, ambulatory mechanics, kinetics, disease progression, cartilage thickness, MRI
INTRODUCTION
Osteoarthritis (OA) represents a growing yet unsolved problem1, with knee OA being the most prevalent symptomatic form of this disease2,3. While there are biological, mechanical, and structural factors associated with the progression of knee OA, mechanical loading during walking has been identified as a critical factor4–7 that represents an opportunity for low cost and low risk intervention. Most commonly, the external knee adduction moment has been the target of various interventions that aim to reduce joint loading and slow disease progression8. However, recent studies showing that the external knee flexion moment is also significantly associated with total medial compartment load suggest that analyzing the flexion and adduction moments together could provide a better assessment of joint loading9–11. The need to improve our understanding of the relationship between these knee moments and structural disease progression is particularly important because, in the absence of a cure, interventions are aimed at reducing joint loads by focusing only on the adduction moment12–14. Currently, there is a lack of knowledge that supports the reduction of the adduction moment without any consideration for the flexion moment.
The external knee adduction moment has received much attention in the past because it complements the static mechanical axis alignment measure by characterizing the ambulatory distribution of load between the medial and lateral compartments15–17. The adduction moment has been shown to be associated with cartilage changes in medial knee OA18–20 as well as being prospectively sensitive to disease progression as measured by radiographs21 and cartilage volume22. On the other hand, the knee flexion moment has also been shown to be important in knee loading in patients with OA9,23,24 as it provides a surrogate for net muscle contraction15, which corresponds to forces that are multiples of body weight in early stance of walking. Furthermore, the flexion moment is substantially influenced by OA-related knee joint pain25–27 and can change in response to an intervention9.
Literature is particularly deficient in longitudinal studies of ambulatory loads and structural markers for OA, with no such study analyzing the flexion moment. Consequently, there is a need for prospective studies that analyze the relative influence of the adduction and flexion moments on cartilage changes. These types of studies would elucidate the effect of knee loading during gait in the broader context of evaluating future directions for treating knee OA. Given the complexity of knee mechanics during gait, the adduction and the flexion moments can influence different regions of tibiofemoral cartilage. Testing for regional differences associated with these components of joint load would provide new insight into the pathomechanics of knee OA. Thus the purpose of this study was to assess which of these knee moments are prospectively associated with changes in cartilage structure. This study tested the hypotheses that the knee adduction moment and the knee flexion moment are significantly correlated with changes in cartilage thickness and medial-to-lateral cartilage thickness ratio over five years in specific load-bearing regions of the femoral and tibial cartilage.
METHOD
Patients
As part of this study, 16 subjects were recruited from a cohort of 42 patients with medial knee OA who had participated in a previous study approximately five years prior28. With Institutional Review Board (IRB) approval, the subjects were initially recruited from printed advertisements through the Palo Alto Veterans Affairs and the local community. The inclusion criteria at baseline and follow-up testing were: age greater than 40 years; radiographically-diagnosed medial compartment knee OA (Kellgren-Lawrence grade≥1) in at least one knee. Exclusion criteria at baseline and follow-up testing were: serious lower extremity injury or surgery including ACL reconstruction, meniscus surgery, and knee arthroscopy; diagnosed or symptomatic OA in the ipsilateral hip or ankle; radiographic evidence of lateral compartment OA; gout or recurrent pseudo gout; age greater than 85 years; body mass index (BMI) greater than 35 kg/m2; total knee or hip replacement in either leg; and inability to have a magnetic resonance imaging (MRI) scan. The index knee was selected based on baseline pain and Kellgren-Lawrence (KL) grade that was determined from radiographs by an experienced orthopaedic surgeon. With a second IRB approval, subjects were recalled and eligibility determined by mail and phone. Of the 26 subjects who were not retested, 10 did not respond, 4 had total knee replacements, 5 had other knee surgeries, 3 had moved to other states, 3 were unwilling to participate, and 1 was deceased.
Gait
At baseline, each subject’s kinematic data during self-selected normal walking speed was recorded using a multi-camera system (Qualisys AB, Gothenburg, Sweden) and the point cluster technique29. Ground reaction forces were collected using a floor-embedded force plate (Bertec Corporation, Columbus, OH). Both systems were synchronized and data sampled at 120 Hz. A standing reference pose was captured before the walking trials with markers placed on palpable bony landmarks throughout the lower limb to define an anatomical reference frame for each segment according to a previously-described protocol30. The knee adduction moment and knee flexion moment, expressed as external moments in the tibial anatomical frame, were calculated with the software BioMove (Stanford University, Stanford, CA) as detailed in prior work31. The first peak knee adduction moment (KAM) and first peak knee flexion moment (KFM) were defined as the maximum value during the first half of stance phase on the force plate. These maximum values for the index knee were averaged over three walking trials for every subject, and expressed as a percentage of body weight and height (%BW*Ht). For each subject, the three trials were controlled to a self-selected normal walking speed that was found to be repeatable and comfortable.
MRI
The index knee of each patient was imaged at baseline and follow-up on the same General Electric Signa 1.5 Tesla MRI machine (GE Medical Systems, Milwaukee, WI) with a standard transmit-receive extremity coil. Sagittal plane images of the knee were obtained using a fat-suppressed three-dimensional spoiled gradient recalled echo (SPGR) sequence with TR=60ms, TE=5ms, flip angle=40°, field of view=140×140 mm, slice thickness=1.5mm, number of slices=60, and matrix=256×256.
Cartilage Thickness
The boundaries of the femoral and tibial cartilages were defined on the two-dimensional sagittal slices of the MRI scans using a semi-automatic segmentation method by a single experienced operator32; intra-observer variability for this segmentation method has been shown to be less than 3%32. Three-dimensional models of the femoral and tibial cartilages were then constructed using custom software32. Mean cartilage thicknesses were calculated over classical load-bearing regions at the femoral condyles and the tibial plateau; these cartilage regions were described as medial femoral, medial tibial, lateral femoral, and lateral tibial33. The posterior limit for the load-bearing region of the femur was defined at 60% of the distance between the notch and the posterior end of the condyles34. Change in cartilage thickness was defined as the mean cartilage thickness at follow-up minus the mean cartilage thickness at baseline. Femoral and tibial medial-to-lateral cartilage thickness ratios were defined as the mean medial region thickness divided by mean lateral region thickness; change in medial-to-lateral ratio was defined as the medial-to-lateral ratio at follow-up minus the medial-to-lateral ratio at baseline.
Pain Score
Pain was quantified at baseline using a modified version of the Rush Hospital for Special Surgery functional knee evaluation35. In this survey, a higher score indicates less knee pain; a score of 50 indicates ‘no pain or ignores pain’ and score of 0 indicates ‘continuous pain regardless of activity’4.
Data Analysis
Normal distribution of the data was tested and determined using the Shapiro-Wilk test. To identify the baseline measures that were significantly associated with changes in cartilage, multiple linear regression was used to correlate baseline KAM and KFM with mean thickness changes in the medial femoral, medial tibial, lateral femoral, and lateral tibial regions, as well as with changes in femoral medial-to-lateral thickness ratio and tibial medial-to-lateral thickness ratio. The effect of age, gender, BMI, KL grade, pain score, and walking speed were each tested by entering one of these measures into the statistical model with KAM and KFM; this method is preferred in order to avoid an over-fitted model with all eight independent measures. The multivariate regression models were considered statistically significant when the model’s p<0.05; multivariate results are shown in terms of standardized coefficients and p-values to show each measure’s relative contribution to the final statistical model. Correlations between the independent measures in the regression model and cartilage change were considered to be statistically significant when p<0.05. Statistics were performed with SPSS version 20 (IBM Corp., Armonk, NY) and MATLAB version 2010b (The MathWorks, Natick, MA).
RESULTS
This study’s participant cohort consisted of 16 subjects (10 females, 6 males) with medial compartment knee OA (KL1: 4, KL2: 5, KL3: 6, KL4: 1). At baseline, the mean ± standard deviation age was 60.1 ± 9.4 years, BMI was 28.3 ± 4.5 kg/m2, pain score was 36 ± 19, and walking speed was 1.31 ± 0.14 m/s. The time between baseline and follow-up tests was 4.7 ± 0.6 years. There was no significant relationship between the baseline KAM and baseline KFM (R2=0.00, p=0.96).
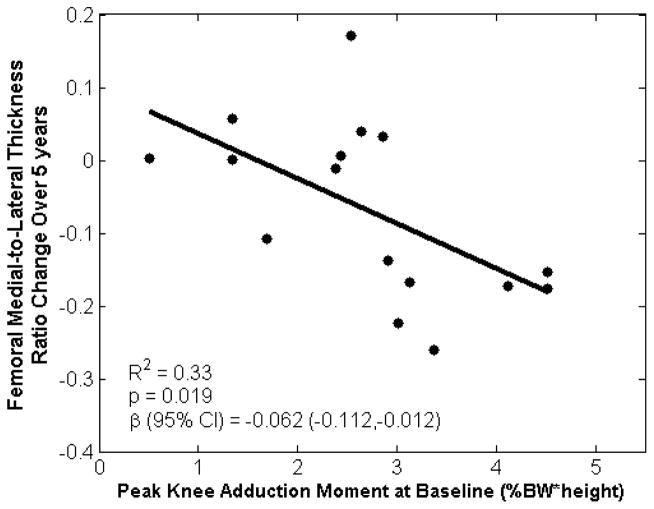
For the femoral cartilage, changes in the medial-to-lateral thickness ratio were associated with baseline KAM and KFM (R2=0.40, p=0.038; Table 1), as well as baseline KAM, KFM, and pain score (R2=0.60, p=0.010; Table 1). The baseline KAM was the measure that was most strongly correlated femoral thickness ratio change, such that the unstandardized univariate regression showed that a 1% BW*Ht increase in the baseline KAM indicated an average reduction of 0.06 unit in the femoral medial-to-lateral cartilage thickness ratio over five years (R2=0.33, p=0.019; Figure 1). There were no statistically significant correlations between baseline measures and changes in mean thickness in the medial femoral or lateral femoral regions.
Table 1.
Baseline knee moments are associated with five-year cartilage changes in femoral medial-to-lateral thickness ratio, tibial medial-to-lateral thicknes ratio, and medial tibial thickness. This was assessed using a multiple linear regression with KAM and KFM as independent measures. The addition of baseline pain score to femoral medial-to-lateral thickness ratio produced a statistically significant model, as well as the addition of walking speed to tibial medial-to-lateral thickness ratio and medial tibial thickness. Models were considered significant when the overall model p<0.05; baseline measures are considered significant and are bolded when p<0.05.
| Cartilage Measure | Baseline Measures | Overall Model | |||
|---|---|---|---|---|---|
| Name | Standardized Coefficient | p-value | R2 | p-value | |
| Femoral Medial-to-Lateral Thickness Ratio | KAM | −0.580 | 0.019 | 0.40 | 0.038 |
| KFM | −0.251 | 0.265 | |||
| Femoral Medial-to-Lateral Thickness Ratio | KAM | −0.507 | 0.018 | 0.60 | 0.010 |
| KFM | −0.292 | 0.137 | |||
| Pain score | −0.459 | 0.029 | |||
| Tibial Medial-to-Lateral Thickness Ratio | KAM | 0.124 | 0.570 | 0.41 | 0.032 |
| KFM | −0.627 | 0.011 | |||
| Tibial Medial-to-Lateral Thickness Ratio | KAM | 0.070 | 0.746 | 0.49 | 0.039 |
| KFM | −0.776 | 0.006 | |||
| Walking Speed | 0.323 | 0.197 | |||
| Medial Tibial Thickness | KAM | 0.032 | 0.881 | 0.43 | 0.027 |
| KFM | −0.651 | 0.008 | |||
| Medial Tibial Thickness | KAM | −0.015 | 0.943 | 0.49 | 0.041 |
| KFM | −0.780 | 0.006 | |||
| Walking Speed | 0.281 | 0.259 | |||
Figure 1.
Baseline peak knee adduction moment during early stance was associated with five-year changes in femoral medial-to-lateral cartilage thickness ratio. Plot represents a univariate regression with unstandardized coefficient β.
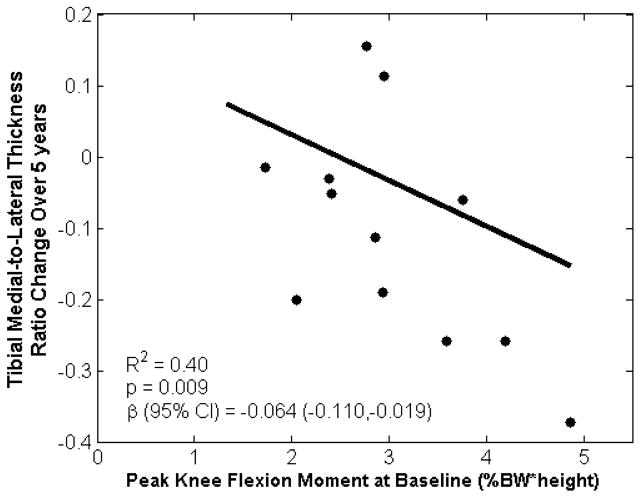
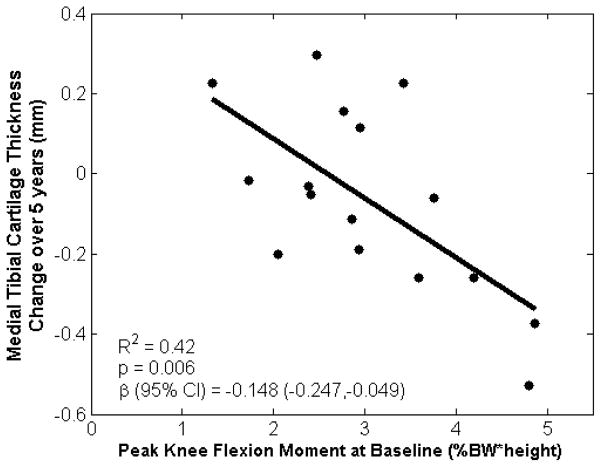
The tibial cartilage changes in the medial-to-lateral thickness ratio were associated with baseline KAM and KFM (R2=0.41, p=0.032; Table 1), as well as baseline KAM, KFM, and walking speed (R2=0.49, p=0.039; Table 1). These associations were driven by KFM, such that the unstandardized univariate regression showed that a 1% BW*Ht increase in the baseline KFM indicated an average reduction of 0.06 unit in the tibial medial-to-lateral cartilage thickness ratio over five years (R2=0.40, p=0.009; Figure 2). In addition, cartilage thickness changes in the medial tibial cartilage region were associated with baseline KAM and KFM (R2=0.43, p=0.027; Table 1), as well as baseline KAM, KFM, and walking speed (R2=0.49, p=0.041; Table 1). Again, these associations were primarily driven by KFM, such that unstandardized univariate regression showed that a 1% BW*Ht increase in the baseline KFM indicated an average loss of 0.15 mm of medial tibial cartilage over five years (R2=0.42, p=0.006; Figure 3). There were no statistically significant correlations between baseline measures and changes in mean thickness in the lateral tibial region.
Figure 2.
Baseline peak knee flexion moment during early stance was associated with five-year changes in tibial medial-to-lateral cartilage thickness ratio. Plot represents a univariate regression with unstandardized coefficient β.
Figure 3.
Baseline peak knee flexion moment during early stance was associated with five-year changes in medial tibial cartilage thickness. Plot represents a univariate regression with unstandardized coefficient β.
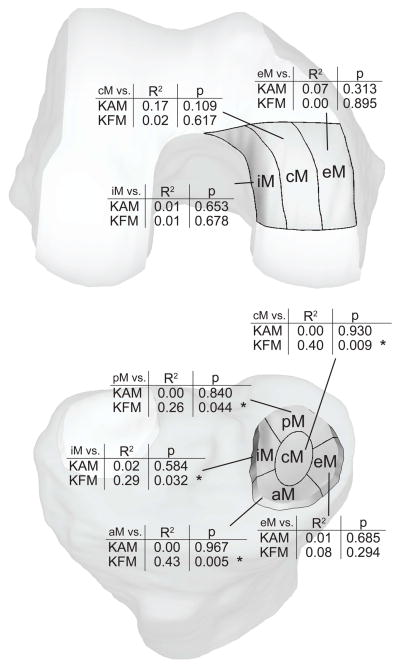
The KAM or KFM had the lowest individual p-value in each of the previously-described multivariate models (Table 1), indicating that baseline kinetics were the factors that were most strongly associated with cartilage changes in the medial region. In order to gain further insight into the role of the KAM and KFM in medial knee OA, post-hoc univariate correlations between baseline KAM and baseline KFM with sub-regions of the load-bearing cartilage region were tested (Figure 4). Three femoral sub-regions (external, central, and internal) and five tibial subregions (anterior, posterior, external, central, and internal) commonly used in literature were defined33. The KAM was most highly (but non-significantly) correlated with femoral medial central cartilage loss over five years (R2=0.17, p=0.109). The KFM was significantly correlated with cartilage changes in the tibial medial central (R2=0.40, p=0.009), tibial medial posterior (R2=0.26, p=0.044), tibial medial internal (R2=0.29, p=0.032), and tibial medial anterior (R2=0.43, p=0.005) regions over five years.
Figure 4.
Post-hoc analysis revealed that baseline knee flexion moment (KFM) was significantly associated with medial tibial cartilage thickness changes in the central (cM), internal (iM), posterior (pM), and anterior (aM) subregions. eM correspond to the external medial subregion. * p<0.05
DISCUSSION
The results of this study showing that cartilage thinning and reduction of the medial-to-lateral thickness ratio during the five year follow-up period were associated with higher KAM and KFM at baseline provide new insights into the pathomechanics of the disease and possible interventions to treat medial compartment knee OA. Specifically, the finding that the KFM was also shown to be related to changes in the medial-to-lateral thickness distribution is important since this load can change when interventions are introduced. Thus these observations have important implications for gait interventions for knee OA because they suggest that it is the overall loading environment, and not only the KAM, that should be considered. More specifically, these results suggest that reducing the KAM while increasing the KFM might be detrimental for cartilage health in specific regions and they invite further studies about how the combination of KAM and KFM should be modified to slow disease progression.
This study and others found that the medial-to-lateral thickness ratio in the weight-bearing regions was associated with the KAM18,36,37, suggesting that this measure influences the medial-to-lateral balance of cartilage thickness and is most sensitive to load during walking. The association between changes in medial tibial thicknesses with the KFM suggests that the KFM has a different influence on joint load than the KAM alone. This is supported by the fact that the two moments are defined in orthogonal planes. Specifically, an increase in the KAM may reflect increased loading in the medial compartment while an increase in the KFM may reflect increased loading in both the medial and lateral compartments. Furthermore, a significant increase in the magnitude of one moment may not necessarily have a significant effect on the magnitude of the other, and previous studies show that the combination of the KAM and KFM yields a better association with medial compartment loading9,11 than the KAM alone11. Changes in either the KAM or KFM could effectively disrupt the cartilage matrix’s ability to withstand and adapt to cyclic loading as changes in loading conditions may damage cartilage, which in turn alters the loading paradigm and further increases cartilage damage36.
This study found peak knee moments to be sensitive to changes in cartilage when thicknesses are averaged over the entire medial or lateral regions. Other studies have also shown that peak moments at baseline are associated with clinical outcomes in patients with medial knee OA21,38. This suggests that although peak measures represent only one point in the gait cycle, they act as viable surrogate measures for the moment waveform and the actual loading environment at the knee. Therefore, studies that test associations between peak moments and cartilage structure may benefit from selecting weight-bearing cartilage regions, which are subject to OA-related cartilage changes39. This allows for an increase in sensitivity that could explain this study’s significant association between baseline KAM and changes in femoral medial-to-lateral thickness ratio, while a previous study found no association between baseline KAM and changes in cartilage volume that included regions that were not weight-bearing22.
After the KAM and KFM, pain was the independent variable that was most strongly associated with cartilage changes, with greater levels of baseline pain (lower score) associated with less change in the femoral medial-to-lateral thickness ratio (Table 1). This relationship may be explained by the fact that pain level is negatively correlated with the knee joint moments during walking20,25,40; patients with more painful knees tend to walk with lower knee moments. This could suggest that individuals who had greater levels of baseline pain altered their mechanics to reduce tibiofemoral load and potentially slow disease progression. This finding is particularly important for disease treatment because it suggests that clinical interventions that only target reductions in knee pain might be counter-effective if they are associated with an increase in loads at the knee41. In addition, the KFM has been associated with knee pain and alterations in OA25,26, further indicating that knee moments should be considered in clinical interventions and in a more detailed description of the relationship between pain and joint loads in OA.
Baseline self-selected normal walking speed also appeared to trend towards significant associations with cartilage changes, as assessed by the statistical models for changes in tibial medial-to-lateral thickness ratio and medial tibial thickness that included baseline walking speed (Table 1). Subjects who walked faster when asked to walk at ‘normal’ speed experienced less cartilage change, perhaps indicating that their relative joint health and mechanics were less severe than those who walked slower at ‘normal’ speed. It is possible that walking speed is not actually a measure that directly influences cartilage change and the rate of OA progression, but is rather a representation of general lower limb musculoskeletal health and relative severity of the mechanical environment associated with knee OA.
The post-hoc subregion analysis revealed that there is a regional component in the relationship between cartilage thickness and knee loading (Figure 4). The KAM was most related to changes in the femoral medial central region, which has been previously shown to be the subregion that is subject to greatest cartilage loss in OA progression39,42. The KFM was principally associated to changes in the tibial medial anterior and central regions, the latter of which has been reported to be a subregion that is subject to significant changes in OA progression39. The differences in correlations between the baseline moments and changes in cartilage subregions support the notion that the KAM and KFM do indeed offer distinct insights into the pathomechanics of cartilage degradation in OA.
This study is limited by the absence of truly sensitive outcome measures that are able to reliably detect progression of this complex disease. With degradation of articular cartilage being the main structural outcome of disease progression, the preferred tool for cartilage analysis in patients with OA remains MRI43. Specifically, cartilage thickness in the load-bearing regions have been shown to be among the most sensitive measures of cartilage morphology44 to gain a sense of the cartilage’s relative health. In addition, the medial-to-lateral cartilage thickness ratio has been shown to be a measure that is sensitive to OA16,18, with a reduced medial-to-lateral thickness ratio being indicative of subjects at a more advanced stage of medial knee OA18. The thickness ratio is complementary to crude thickness measures because it provides a quantitative sense of the medial and lateral distribution, while allowing for an inter-subject normalization of cartilage thickness. Further complicating estimates of cartilage thickness are the facts that structural changes are neither temporally nor spatially constant; femoral changes are particularly associated with early OA45 and cartilage may both thicken and thin in a given region46. As a result, the lack of associations with femoral thickness changes may be attributed to varied rates of OA progression between subjects as well as the selection of predefined cartilage boundaries. Testing for changes in thickness distribution as well as absolute regional thickness was recently recommended in literature to gain a better understanding of OA-related structural differences47,48.
While these results suggest associations between biomechanical markers and change in cartilage, this study has several other limitations. First, the population sample size is small and the stage of OA at baseline (as assessed by KL grade) is heterogeneous. This could potentially confound associations shown here since the moments change with disease severity19. In addition, these results did not indicate statistically significant associations with baseline BMI, even though increased BMI is associated with the progression of knee OA49; this is likely due to the study’s limited statistical power. While there is a risk of model over-fitting with a limited sample size and up to three variables in the multivariate regressions, this study was able to detect strong relationships related to OA progression with only 16 subjects using both multivariate and univariate models. This suggests that baseline KAM, KFM, and pain score are measures that can be particularly sensitive to longitudinal cartilage change. In addition, the outcome measures of this study were based on predefined cartilage regions that are agnostic to individual variability in anatomical structure and potentially load-bearing regions. Therefore, averaging mean thicknesses in such regions might not detect time-based changes in thickness that occur between or across multiple regions. However, these limitations only restrict this study’s sensitivity and still support the findings that the KAM and KFM can be associated with markers for OA progression. There is also evidence that the KAM and KFM differ between men and women6,50, so a future study with a larger cohort could focus on gender-specific indicators of OA progression.
In conclusion, this study shows that multiple biomechanical measures can provide distinct insights into the pathomechanics of OA progression. Specifically, these results further support the prospective association of the KAM with OA progression by way of change in the medial-to-lateral thickness ratio. In addition, this study identifies the KFM as a biomechanical measure that is prospectively associated with change in the medial-to-lateral thickness ratio and absolute change in cartilage thickness. While there may be some level of mechanical interaction between these two moments, they appear to impact different anatomical regions of cartilage change in OA. These results offer important implications into the design of OA interventions, where focusing solely on the KAM when treating OA may not be sufficient for reducing the rate of OA progression. The KFM plays an important role in knee loading, and should not be ignored when intervening to alter a patient’s medial tibiofemoral contact force through interventions such as valgus bracing of the knee12, foot orthoses13, and gait retraining14. Future studies of gait kinetics need to consider both the KAM and KFM to gain a more complete description of the mechanical pathway to knee OA.
Acknowledgments
The authors would like to acknowledge Anne Mündermann and Jessica L Asay for their contributions to the data collection as well as administrative and logistic support.
ROLE OF THE FUNDING SOURCE
This study was supported by the National Institute of Health (grant AR039421), the Veterans Administration (grants VA A02-2577R and VA A04-3583R), and the Arthritis Foundation (grant 6289).
Footnotes
CONFLICT OF INTEREST
None of the authors had any conflict of interest with this study.
AUTHOR CONTRIBUTIONS
EFC: conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article. Eric Chehab takes responsibility for the integrity of the work as a whole, from inception to finished article.
JF: conception and design, analysis and interpretation of the data, critical revision of the article for important intellection content, final approval of the article.
JCEH: collection and assembly of data, obtaining of funding, critical revision of the article for important intellection content, final approval of the article.
TPA: conception and design, obtaining of funding, analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eric F. Chehab, Email: echehab@stanford.edu.
Julien Favre, Email: jfavre@stanford.edu.
Jennifer C. Erhart-Hledik, Email: jennifer.erhart@gmail.com.
Thomas P. Andriacchi, Email: tandriac@stanford.edu.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and Rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. Journal of Rheumatology. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 3.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. Journal of Rheumatology. 2007;34:172–180. [PubMed] [Google Scholar]
- 4.Baliunas A, Hurwitz D, Ryals A, Karrar A, Case J, Block J, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis and Cartilage. 2002;10:573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 5.Astephen JL, Deluzio KJ. Changes in frontal plane dynamics and the loading response phase of the gait cycle are characteristic of severe knee osteoarthritis application of a multidimensional analysis technique. Clinical Biomechanics. 2005;20:209–217. doi: 10.1016/j.clinbiomech.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman KR, Hughes C, Morrey BF, Morrey M, An KN. Gait characteristics of patients with knee osteoarthritis. Journal of Biomechanics. 2001;34:907–15. doi: 10.1016/s0021-9290(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 7.Hunt MA, Birmingham TB, Giffin JR, Jenkyn TR. Associations among knee adduction moment, frontal plane ground reaction force, and lever arm during walking in patients with knee osteoarthritis. Journal of Biomechanics. 2006;39:2213–2220. doi: 10.1016/j.jbiomech.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Reeves ND, Bowling FL. Conservative biomechanical strategies for knee osteoarthritis. Nature Reviews Rheumatology. 2011;7:113–22. doi: 10.1038/nrrheum.2010.212. [DOI] [PubMed] [Google Scholar]
- 9.Walter JP, D’Lima DD, Colwell CW, Fregly BJ. Decreased knee adduction moment does not guarantee decreased medial contact force during gait. Journal of Orthopaedic Research. 2010;28:1348–54. doi: 10.1002/jor.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manal K, Buchanan TS. An electromyogram-driven musculoskeletal model of the knee to predict in vivo joint contact forces during normal and novel gait patterns. Journal of Biomechanical Engineering. 2013;135:021014. doi: 10.1115/1.4023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manal K, Gardinier E, Snyder-Mackler L, Buchanan T. American Society of Biomechanics. 2013. An alternate predictor of peak medial compartment loading: the product of the peak knee extensor and abductor moments. [Google Scholar]
- 12.Fantini Pagani CH, Potthast W, Brüggemann G-P. The effect of valgus bracing on the knee adduction moment during gait and running in male subjects with varus alignment. Clinical Biomechanics. 2010;25:70–76. doi: 10.1016/j.clinbiomech.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Erhart JC, Mündermann A, Elspas B, Giori NJ, Andriacchi TP. Changes in knee adduction moment, pain, and functionality with a variable-stiffness walking shoe after 6 months. Journal of Orthopaedic Research. 2010;28:873–9. doi: 10.1002/jor.21077. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler JW, Shull PB, Besier TF. Real-time knee adduction moment feedback for gait retraining through visual and tactile displays. Journal of Biomechanical Engineering. 2011;133:041007. doi: 10.1115/1.4003621. [DOI] [PubMed] [Google Scholar]
- 15.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. Journal of Orthopaedic Research. 1991;9:113–9. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 16.Koo S, Andriacchi TP. A comparison of the influence of global functional loads vs. local contact anatomy on articular cartilage thickness at the knee. Journal of Biomechanics. 2007;40:2961–6. doi: 10.1016/j.jbiomech.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. Journal of Orthopaedic Research. 2002;20:101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 18.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. Journal of Bone and Joint Surgery. 2009;91 (Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mündermann A, Dyrby CO, Hurwitz DE, Sharma L, Andriacchi TP. Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: reduced walking speed. Arthritis and Rheumatism. 2004;50:1172–8. doi: 10.1002/art.20132. [DOI] [PubMed] [Google Scholar]
- 20.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis and Rheumatism. 1998;41:1233–40. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Annals of the Rheumatic Diseases. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennell KL, Bowles K-A, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Annals of the Rheumatic Diseases. 2011;70:1770–4. doi: 10.1136/ard.2010.147082. [DOI] [PubMed] [Google Scholar]
- 23.Chen CPC, Chen MJL, Pei Y-C, Lew HL, Wong P-Y, Tang SFT. Sagittal plane loading response during gait in different age groups and in people with knee osteoarthritis. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2003;82:307–12. doi: 10.1097/01.PHM.0000056987.33630.56. [DOI] [PubMed] [Google Scholar]
- 24.Creaby MW, Hunt MA, Hinman RS, Bennell KL. Sagittal plane joint loading is related to knee flexion in osteoarthritic gait. Clinical Biomechanics. 2013;28:916–920. doi: 10.1016/j.clinbiomech.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Schnitzer TJ, Popovich JM, Andersson GBJ, Andriacchi TP. Effect of piroxicam on gait in patients with osteoarthritis of the knee. Arthritis and Rheumatism. 1993;36:1207–1213. doi: 10.1002/art.1780360905. [DOI] [PubMed] [Google Scholar]
- 26.Boyer KA, Angst MS, Asay J, Giori NJ, Andriacchi TP. Sensitivity of gait parameters to the effects of anti-inflammatory and opioid treatments in knee osteoarthritis patients. Journal of Orthopaedic Research. 2012;30:1118–24. doi: 10.1002/jor.22037. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz DE, Ryals AR, Block JA, Sharma L, Schnitzer TJ, Andriacchi TP. Knee pain and joint loading in subjects with osteoarthritis of the knee. Journal of Orthopaedic Research. 2000;18:572–9. doi: 10.1002/jor.1100180409. [DOI] [PubMed] [Google Scholar]
- 28.Mündermann A, King KB, Smith RL, Andriacchi TP. Change in serum COMP concentration due to ambulatory load is not related to knee OA status. Journal of Orthopaedic Research. 2009;27:1408–13. doi: 10.1002/jor.20908. [DOI] [PubMed] [Google Scholar]
- 29.Andriacchi TP, Alexander EJ, Toney MK, Dyrby C, Sum J. A point cluster method for in vivo motion analysis: applied to a study of knee kinematics. Journal of Biomechanical Engineering. 1998;120:743–9. doi: 10.1115/1.2834888. [DOI] [PubMed] [Google Scholar]
- 30.Dyrby CO, Andriacchi TP. Secondary motions of the knee during weight bearing and non-weight bearing activities. Journal of Orthopaedic Research. 2004;22:794–800. doi: 10.1016/j.orthres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Zabala ME, Favre J, Scanlan SF, Donahue J, Andriacchi TP. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. Journal of biomechanics. 2013;46:515–20. doi: 10.1016/j.jbiomech.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthritis and Cartilage. 2005;13:782–9. doi: 10.1016/j.joca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2008;27:737–44. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 34.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Annals of the Rheumatic Diseases. 2006;65:433–41. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Insall JN, Ranawat CS, Paolo A, JS A comparison of four models of total knee-replacement prostheses. The Journal of Bone & Joint Surgery. 1976;58:754–765. [PubMed] [Google Scholar]
- 36.Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Annals of Biomedical Engineering. 2004;32:447–57. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 37.Blazek K, Favre J, Asay J, Erhart-Hledik J, Andriacchi T. Age and obesity alter the relationship between femoral articular cartilage thickness and ambulatory loads in individuals without osteoarthritis. Journal of orthopaedic research3: official publication of the Orthopaedic Research Society. 2014;32:394–402. doi: 10.1002/jor.22530. [DOI] [PubMed] [Google Scholar]
- 38.Prodromos CC, Andriacchi TP, Galante JO. A relationship between gait and clinical changes following high tibial osteotomy. The Journal of bone and joint surgery American volume. 1985;67:1188–94. [PubMed] [Google Scholar]
- 39.Wirth W, Hellio Le Graverand M-P, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis and Cartilage. 2009;17:291–7. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henriksen M, Graven-Nielsen T, Aaboe J, Andriacchi TP, Bliddal H. Gait changes in patients with knee osteoarthritis are replicated by experimental knee pain. Arthritis Care & Research. 2010;62:501–9. doi: 10.1002/acr.20033. [DOI] [PubMed] [Google Scholar]
- 41.Henriksen M, Simonsen EB, Alkjær T, Lund H, Graven-Nielsen T, Danneskiold-Samsøe B, et al. Increased joint loads during walking – A consequence of pain relief in knee osteoarthritis. The Knee. 2006;13:445–450. doi: 10.1016/j.knee.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Hellio Le Graverand M-P, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Subregional femorotibial cartilage morphology in women – comparison between healthy controls and participants with different grades of radiographic knee osteoarthritis. Osteoarthritis and Cartilage. 2009;17:1177–1185. doi: 10.1016/j.joca.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Eckstein F, Cicuttini F, Raynauld J-P, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis and Cartilage. 2006;14:46–75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR in Biomedicine. 2006;19:822–54. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 45.Cicuttini FM. Tibial and femoral cartilage changes in knee osteoarthritis. Annals of the Rheumatic Diseases. 2001;60:977–980. doi: 10.1136/ard.60.10.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frobell RB, Nevitt MC, Hudelmaier M, Wirth W, Wyman BT, Benichou O, et al. Femorotibial subchondral bone area and regional cartilage thickness: a cross-sectional description in healthy reference cases and various radiographic stages of osteoarthritis in 1,003 knees from the Osteoarthritis Initiative. Arthritis Care & Research. 2010;62:1612–23. doi: 10.1002/acr.20262. [DOI] [PubMed] [Google Scholar]
- 47.Andriacchi TP. Valgus alignment and lateral compartment knee osteoarthritis: a biomechanical paradox or new insight into knee osteoarthritis? Arthritis and rheumatism. 2013;65:310–3. doi: 10.1002/art.37724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Favre J, Scanlan SF, Erhart-Hledik JC, Blazek K, Andriacchi TP. Patterns of Femoral Cartilage Thickness are Different in Asymptomatic and Osteoarthritic Knees and Can be Used to Detect Disease-Related Differences Between Samples. Journal of Biomechanical Engineering. 2013;135:101002–10. doi: 10.1115/1.4024629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reijman M, Pols HAP, Bergink AP, Hazes JMW, Belo JN, Lievense AM, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Annals of the rheumatic diseases. 2007;66:158–62. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKean KA, Landry SC, Hubley-Kozey CL, Dunbar MJ, Stanish WD, Deluzio KJ. Gender differences exist in osteoarthritic gait. Clinical Biomechanics. 2007;22:400–409. doi: 10.1016/j.clinbiomech.2006.11.006. [DOI] [PubMed] [Google Scholar]