Abstract
Purpose
To compare the surgical results of trabeculectomy and Ahmed glaucoma valve implantation after a previous failed trabeculectomy.
Methods
A retrospective comparative case series review was performed on 31 eye surgeries in 20 patients with primary congenital glaucoma who underwent trabeculectomy or Ahmed glaucoma valve implantation after a previous failed trabeculectomy with mitomycin C.
Results
The preoperative mean intraocular pressure was 25.5 mmHg in the trabeculectomy group and 26.9 mmHg in the Ahmed glaucoma valve implantation group (p = 0.73). The 48-month postoperative mean intraocular pressure was 19.6 mmHg in the trabeculectomy group and 20.2 mmHg in the Ahmed glaucoma valve implantation group (p = 0.95). The 12-month trabeculectomy success rate was 69%, compared with 64% for Ahmed glaucoma valve implantation, and the 48-month success rates were 42% and 36% for trabeculectomy and valve implantation, respectively. The success rates following the entire follow-up period were not significantly different between the two groups (p > 0.05 by log rank test). Postoperative complications occurred in 25% of the trabeculectomy-operated eyes and 9% of the Ahmed-implanted eyes (p = 0.38).
Conclusions
There was no significant difference in surgical outcome between the trabeculectomy and Ahmed glaucoma valve implantation groups, neither of which had favorable results. However, the trabeculectomy group demonstrated a higher prevalence of adverse complications such as post-operative endophthalmitis.
Keywords: Ahmed valve implantation, Congenital glaucoma, Surgical outcomes, Trabeculectomy
The incidence of congenital glaucoma is low, but it is the most common form of pediatric glaucoma and an important cause of blindness [1]. The treatment of choice for congenital glaucoma is surgery, with angle surgery usually being performed first, followed by trabeculectomy or Ahmed glaucoma valve implantation, if the angle surgery fails [1,2]. However, even these interventions are not always effective for controlling intraocular pressure (IOP), and, when they fail, it can be difficult to decide on a next step. The aim of this study was to investigate and compare the surgical results of trabeculectomy with those of Ahmed glaucoma valve implantation following a previous trabeculectomy failure in patients with primary congenital glaucoma.
Materials and Methods
With institutional review board approval, we completed a retrospective chart review of 167 congenital glaucoma patients who had undergone glaucoma surgery. Thirty-one eyes of 20 patients with primary congenital glaucoma who underwent trabeculectomy with mitomycin C (n = 20) or Ahmed glaucoma valve implantation (n = 11) after a previous failed trabeculectomy between June 1990 and April 2007 were included in this study. This study followed the tenets of the Declaration of Helsinki.
Diagnoses were confirmed with clinical features and signs, such as gonioscopic angle abnormality, enlargement of the corneal diameter, increase in axial length, optic nerve change, and persistently elevated IOP. Patients with other ocular or systemic abnormalities that could result in glaucoma, such as Axenfeld-Rieger syndrome, were excluded, as were patients with less than six months follow-up time. All patients had a history of previous failed trabeculectomy. The following data were analyzed between the two groups: 1) preoperative and postoperative IOP, 2) number of glaucoma medications used during the preoperative and postoperative periods, 3) surgical complications, and 4) achievement of surgical success.
IOP was measured with a tonopen or, when possible, with a Goldmann applanation tonometer (GAT). Visual acuity was determined with a Teller acuity card or a Snellen acuity chart, when possible. A successful operation was defined as 1) intraocular ocular pressure between 5 and 21 mmHg, 2) no decrease in vision due to complications or changes in IOP, and 3) no need for an additional glaucoma operation. Postoperative use of antiglaucoma medication was not a criterion of success or failure.
All surgeries were performed by a single surgeon (YJH). For trabeculectomy, a limbus-based conjunctival flap was prepared, followed by a rectangular half-thickness scleral flap measuring 4 × 4 mm. A sponge soaked in mitomycin C at a concentration of 0.25 mg/mL was applied intraoperatively on the sclera and underneath the conjunctiva for 3.9 ± 1.6 minutes, according to the patient's condition. When the time since previous glaucoma surgery was less than three months, mitomycin C was not applied. After careful rinsing with saline, trabeculectomy and peripheral iridectomy were performed. The scleral flap was closed with two to four 10-0 nylon sutures, and the Tenon capsule and conjunctiva were sutured ensuring a watertight closure. Ahmed glaucoma valve implantation was performed following the conventional method. Briefly, a fornix-based conjunctival incision was made, and the Tenon capsule was dissected with spring scissors. A rectangular-shaped half-thickness scleral flap was made in the supratemporal position of the limbus. The Ahmed glaucoma valve (S2 or S3 model; New World Medical Inc., Rancho Cucamonga, CA, USA) was secured to the sclera in the superotemporal quadrant 8-mm posterior to the limbus. An entrance site into the anterior chamber was made under the scleral flap with a 23-G needle. After temporary ligation using an absorbable suture, the tube of the valve was then passed through this 23-G opening into the anterior chamber, and a donor sclera was used to cover the flap and tube.
Kaplan-Meier survival curves and log rank test were used to investigate and compare the success rates between the two procedures. To compare between the two groups, we used the Mann Whitney U-test or χ2 test. Statistical analyses were carried out using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Results
The mean follow-up period between the two groups was not significantly different; 85.0 months (range, 7 to 140 months) for the trabeculectomy group and 94.6 months (range, 55 to 120 months) for the Ahmed glaucoma valve implantation group ( p > 0.05 by Mann-Whitney U-test). The mean age of the patients at the time of surgery was 86.3 months (range, 1 to 162) in the trabeculectomy group and 85.1 months (range, 5 to 227) in the valve implantation group. This difference was not statistically significant (p > 0.05 by Mann-Whitney U-test). Both the preoperative IOP (trabeculectomy, 25.5 mmHg; valve implantation, 26.9 mmHg) and the 48-month postoperative IOP (20.2 vs. 19.6 mmHg in the trabeculectomy group and the Ahmed glaucoma valve group, respectively) were similar in the two groups (p > 0.05, Mann-Whitney U-test). There was no significant difference between the two groups in the number of glaucoma medications used either preoperatively or postoperatively (1.7 ±1.1 vs. 2.3 ±1.4, p=0.25; 1.7 ±1.1 vs. 1.8 ±1.5, p = 0.97, respectively). Preoperative IOP, 48-month postoperative IOP, visual acuity, and glaucoma medications are summarized in Tables 1 and 2.
Table 1. Preoperative and postoperative characteristics of the trabeculectomy group.
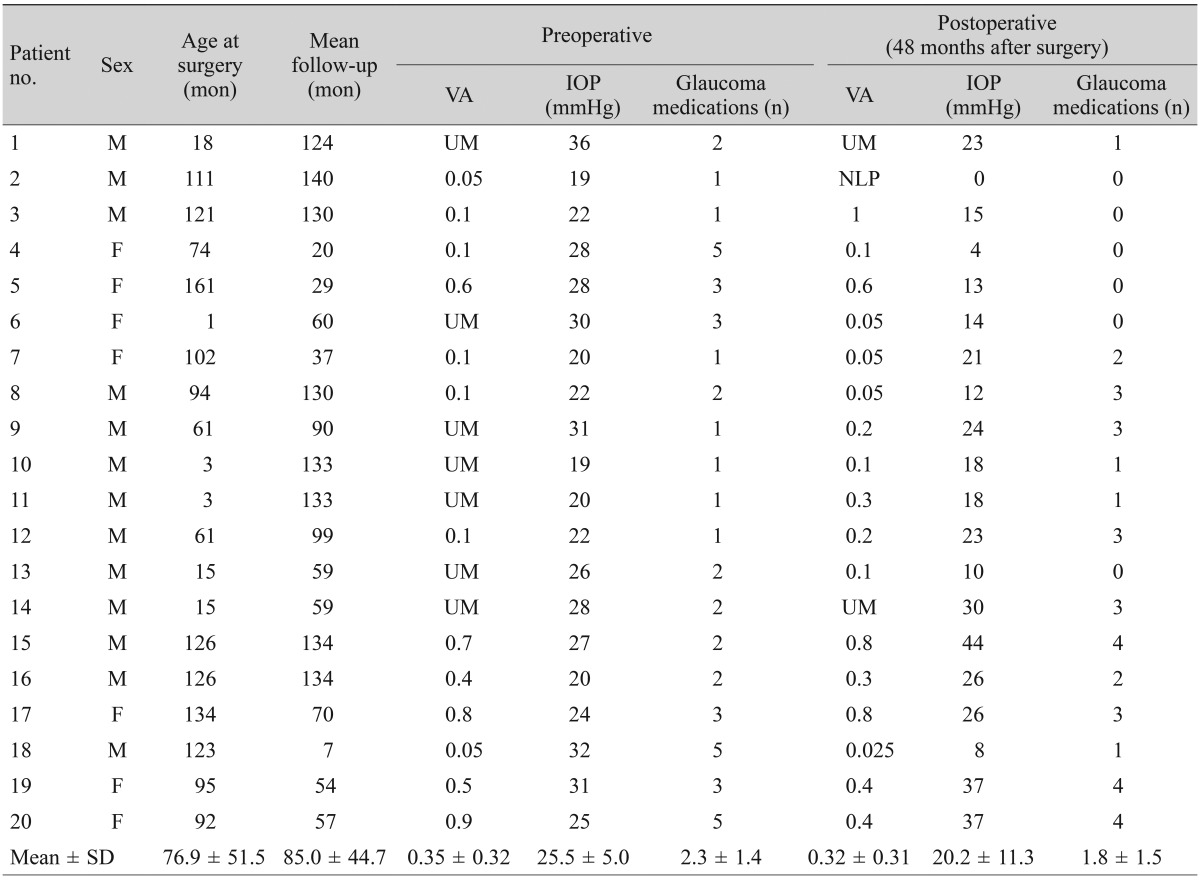
VA = visual acuity; IOP = intraocular pressure; UM = unmeasurable; NLP = no light perception.
Table 2. Preoperative and postoperative characteristics of the Ahmed glaucoma valve group.
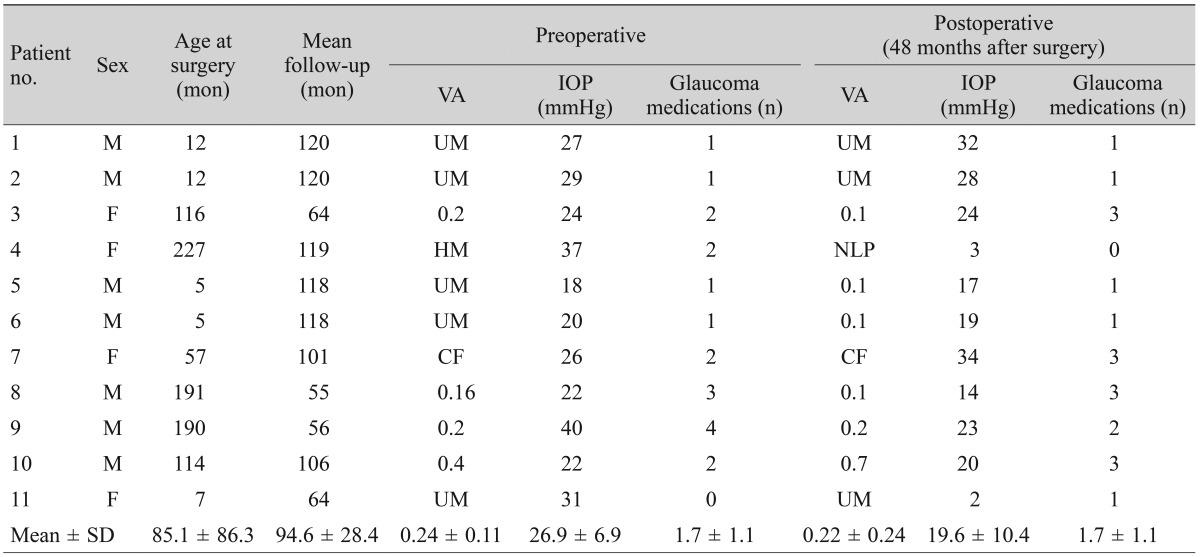
VA = visual acuity; IOP = intraocular pressure; UM = unmeasurable; HM = hand motion; NLP = no light perception; CF = counting finger.
Table 3 presents the observed postoperative complications, which occurred in five (25%) of the trabeculectomy-operated eyes and one (9%) of the Ahmed-implanted eyes (p = 0.38). We observed one case of bleb-related endophthalmitis in a trabeculectomy with mitomycin C patient (5%), which improved with injection of intravitreal antibiotics. In the Ahmed glaucoma valve group, we observed one case of tube-cornea contact due to tube migration (9%), which required surgical repositioning. Additionally, we saw two cases of shallow anterior chamber in the trabeculectomy group (10%), one of which required surgical intervention.
Table 3. Postoperative complications.
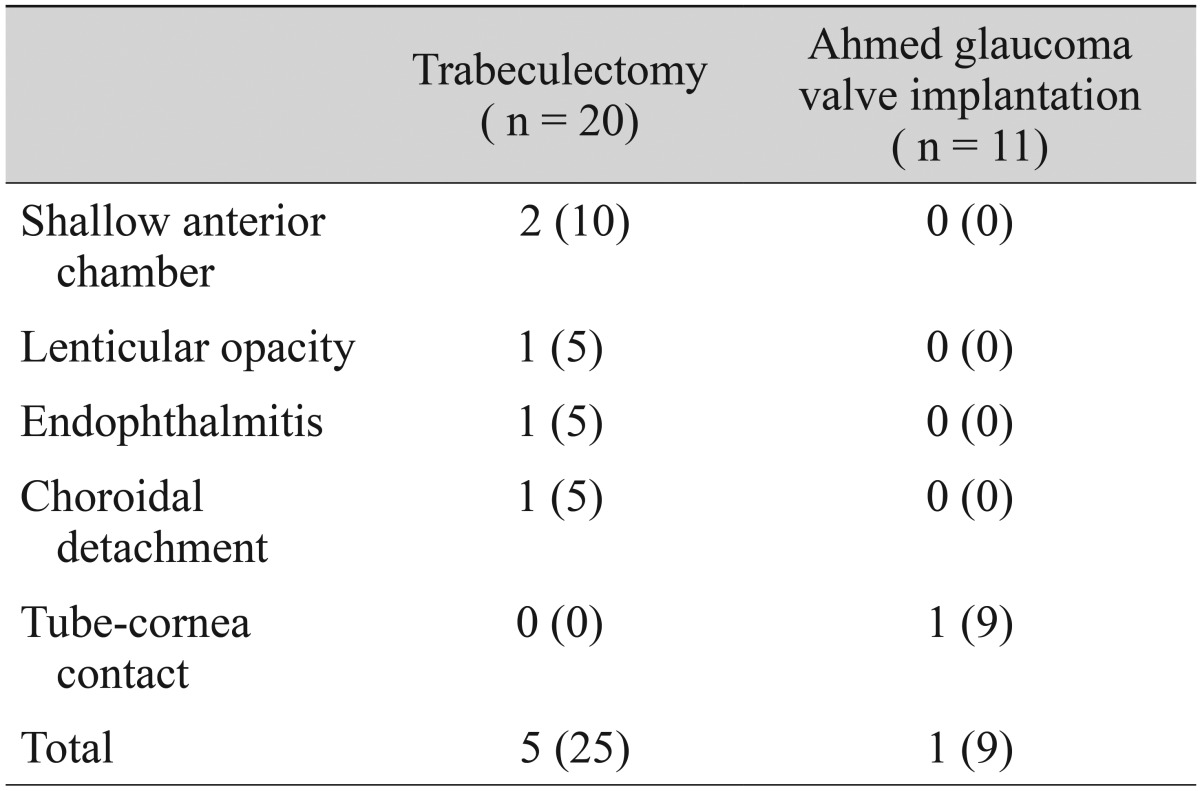
Data are shown as number (%).
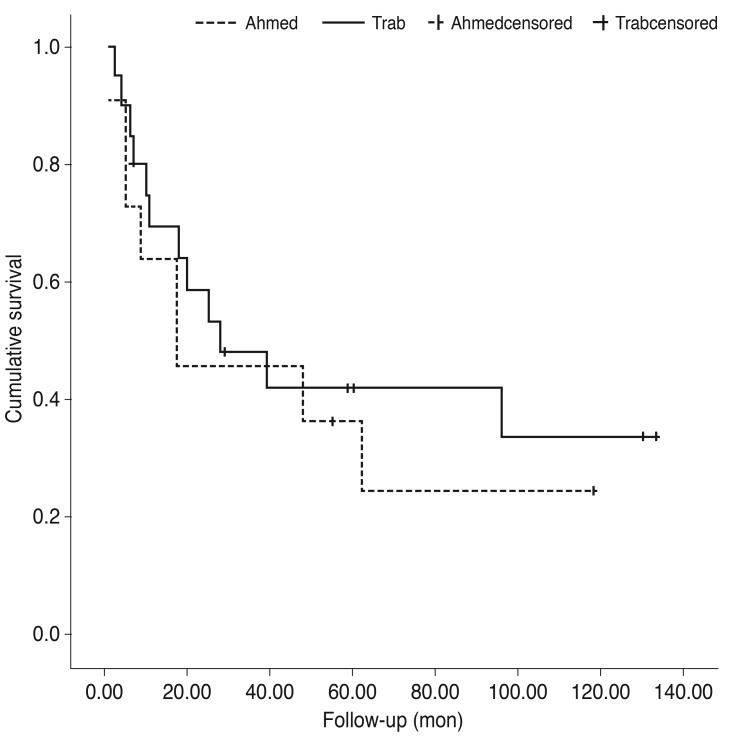
The success rates at one year intervals following the procedures were as follows (reported as trabeculectomy vs. Ahmed valve implantation): 69% vs. 64% (12 months), 59% vs. 46% (24 months), and 42% vs. 36% (48 months). Additionally, the trabeculectomy group showed a 34% success rate after 96 months of follow-up, while the valve implantation group showed a 24% success rate after 62 months. However, no significant difference in the time to failure was observed between the groups (p = 0.529 by log rank test) (Fig. 1).
Fig. 1. Kaplan-Meier survival curve showing the proportion of eyes in which the operation was successful over time after trabeculectomy or Ahmed glaucoma valve implantation.
Discussion
While angle surgery, with a success rate of 70% to 90% [3,4,5], is the primary surgical procedure utilized for congenital glaucoma, congenital glaucoma is not always successfully controlled using this technique. Therefore, when an unacceptable increase in IOP occurs after angle surgery, trabeculectomy with or without mitomycin C is usually the next treatment choice. The reported success rates of treatments for refractory congenital glaucoma show wide variation (39% to 94%) according to the study group, surgeon, success criteria, and follow-up period [6,7,8,9,10,11,12,13]. However, when trabeculectomy p erformed as a second operation also fails, it is unclear whether an additional trabeculectomy or implantation of a glaucoma drainage device is a better choice as the next intervention. Re-trabeculectomy might be more effective than the first surgery, but use of a glaucoma drainage device may also be an option when a previous trabeculectomy has failed. The efficacy of Ahmed glaucoma valve implantation, which is one of the most widely used glaucoma drainage devices, as the surgical choice for treatment of refractory congenital glaucoma has recently been reported and showed similar success rates to trabeculectomy (53% to 88%) [1,2]. However, studies comparing the results of those two surgeries in refractory congenital glaucoma after failure of the first trabeculectomy surgery have not yet been performed because of the rarity of the occurrence. Therefore, we evaluated the success rates and complications of a second trabeculectomy versus those of an Ahmed glaucoma valve implantation following a failed first trabeculectomy.
Using the log rank test, we found that the two had comparable success rates. Although the trabeculectomy group showed slightly higher success rates than the Ahmed glaucoma valve group, there was no significant difference between the two groups (p = 0.529). However, with respect to postoperative complications, trabeculectomy led to more complications; five cases (25%) in the trabeculectomy group showed complications, while tube migration (9%) was the only observed complication in the Ahmed glaucoma valve implantation group.
The success rate we observed was lower than that found by others for this type of primary surgery. This may be due to the fact that all subjects included in this study had already experienced failures of the angle surgery and trabeculectomy, and previous failure of surgery is known to be a poor prognostic factor for subsequent surgery. To the best of our knowledge, this is the first report of success rates of additional surgical intervention (trabeculectomy or Ahmed glaucoma valve implantation) for refractory congenital glaucoma in patients who underwent a previous failed trabeculectomy. Therefore, the findings of this study will inform the choice of surgery for repeatedly uncontrolled congenital glaucoma.
The main limitations of our study were the small number of participants and the fact that it was a retrospective study. Because congenital glaucoma has such a low prevalence, it was difficult to find patients who had undergone primary operations followed by re-operation, and this limited our sample size. On the other hand, the long follow-up period was one of the major strengths of our study.
In conclusion, this study revealed that both trabeculectomy and Ahmed glaucoma valve implantation resulted in unsatisfactory IOP control in the majority of cases of primary congenital glaucoma in patients who had previously undergone trabeculectomy with mitomycin C. There was no significant difference in surgical outcome between the two groups. Both groups experienced complications following their respective procedures, although the complications arising from trabeculectomy were more frequent and serious than those arising from Ahmed glaucoma valve implantation. Additional research is needed to find a more successful method for treating primary congenital glaucoma patients who have previously undergone glaucoma surgery.
Acknowledgements
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A101727).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ho CL, Walton DS. Primary congenital glaucoma: 2004 update. J Pediatr Ophthalmol Strabismus. 2004;41:271–288. doi: 10.3928/01913913-20040901-11. [DOI] [PubMed] [Google Scholar]
- 2.Tanimoto SA, Brandt JD. Options in pediatric glaucoma after angle surgery has failed. Curr Opin Ophthalmol. 2006;17:132–137. doi: 10.1097/01.icu.0000193091.60185.27. [DOI] [PubMed] [Google Scholar]
- 3.Meyer G, Schwenn O, Grehn F. Trabeculotomy in congenital glaucoma: comparison to goniotomy. Ophthalmologe. 2000;97:623–628. doi: 10.1007/s003470070050. [DOI] [PubMed] [Google Scholar]
- 4.McPherson SD, Jr, Berry DP. Goniotomy vs external trabeculotomy for developmental glaucoma. Am J Ophthalmol. 1983;95:427–431. doi: 10.1016/0002-9394(83)90260-x. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DR. Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmology. 1983;90:805–806. doi: 10.1016/s0161-6420(83)34484-5. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher T, Chan J, Lanigan B, et al. Long-term follow up of primary trabeculectomy for infantile glaucoma. Br J Ophthalmol. 1996;80:499–502. doi: 10.1136/bjo.80.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozkiris A, Tamcelik N. Long-term results of trabeculectomy with different concentrations of mitomycin C in refractory developmental glaucoma. J Pediatr Ophthalmol Strabismus. 2005;42:97–102. doi: 10.3928/01913913-20050301-04. [DOI] [PubMed] [Google Scholar]
- 8.Mandal AK, Prasad K, Naduvilath TJ. Surgical results and complications of mitomycin C-augmented trabeculectomy in refractory developmental glaucoma. Ophthalmic Surg Lasers. 1999;30:473–480. [PubMed] [Google Scholar]
- 9.Mandal AK, Walton DS, John T, Jayagandan A. Mitomycin C-augmented trabeculectomy in refractory congenital glaucoma. Ophthalmology. 1997;104:996–1001. doi: 10.1016/s0161-6420(97)30195-x. [DOI] [PubMed] [Google Scholar]
- 10.Beck AD, Wilson WR, Lynch MG, et al. Trabeculectomy with adjunctive mitomycin C in pediatric glaucoma. Am J Ophthalmol. 1998;126:648–657. doi: 10.1016/s0002-9394(98)00227-x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hazmi A, Zwaan J, Awad A, et al. Effectiveness and complications of mitomycin C use during pediatric glaucoma surgery. Ophthalmology. 1998;105:1915–1920. doi: 10.1016/S0161-6420(98)91041-7. [DOI] [PubMed] [Google Scholar]
- 12.Sidoti PA, Belmonte SJ, Liebmann JM, Ritch R. Trabeculectomy with mitomycin-C in the treatment of pediatric glaucomas. Ophthalmology. 2000;107:422–429. doi: 10.1016/s0161-6420(99)00130-x. [DOI] [PubMed] [Google Scholar]
- 13.Azuara-Blanco A, Wilson RP, Spaeth GL, et al. Filtration procedures supplemented with mitomycin C in the management of childhood glaucoma. Br J Ophthalmol. 1999;83:151–156. doi: 10.1136/bjo.83.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]