Abstract
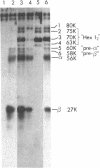
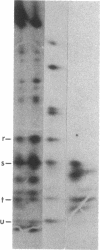
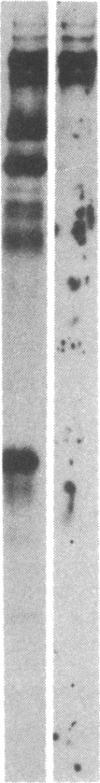
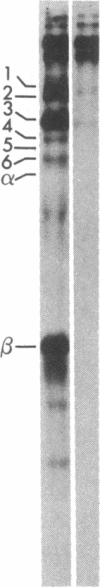
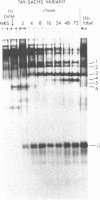
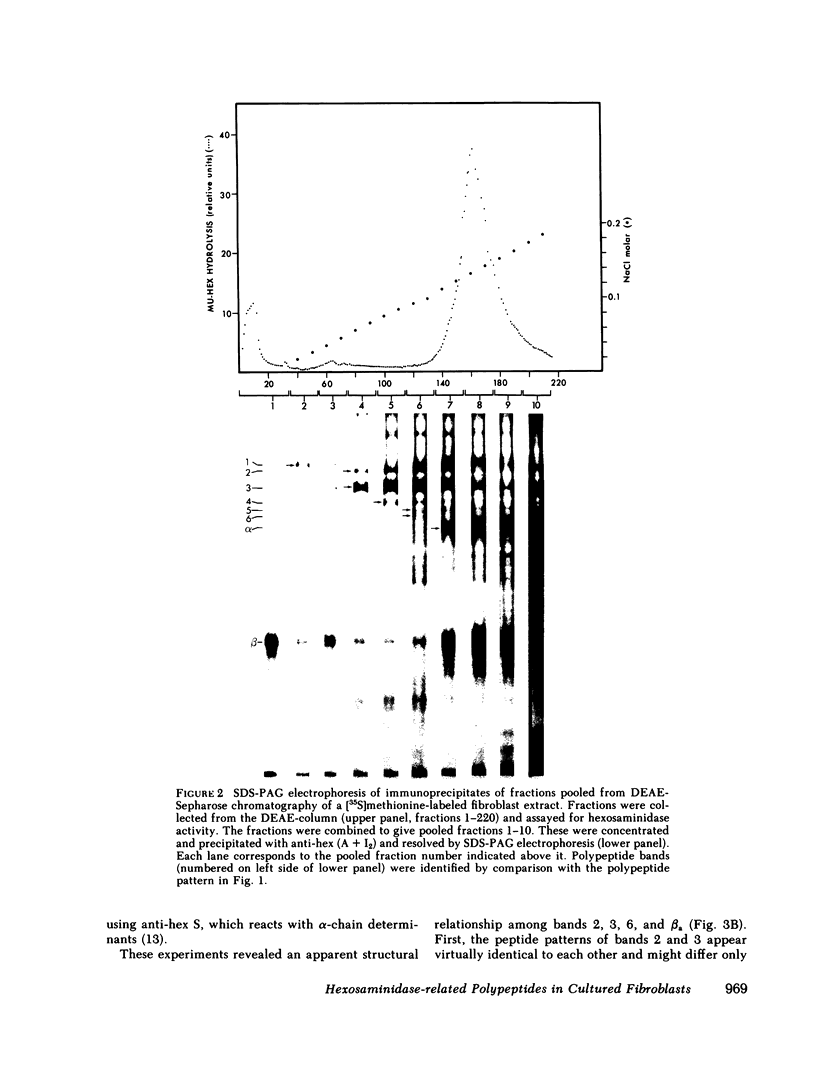
Human fibroblasts synthesize several polypeptides that assort into the various forms of hexosaminidase (hex). We report here the occurrence of three newly identified, hexosaminidase-related polypeptides resolved by sodium dodecyl sulfate-poly-acrylamide gel electrophoresis of immunoprecipitates from [35S]methionine-labeled cell extracts. These polypeptides, called band 2 (75,000), band 3 (70,000), and band 4 (63,000), were immunoprecipitated by an antiserum specific to placental hex I2. They are distinct from pre-alpha- (60,000) and pre-beta- (58,000) precursor polypeptides and the alpha- (56,000), beta a- (27,000), and beta b- (27,000) polypeptides of the mature hex A (alpha beta a beta b) and hex B (2[beta a beta b]). When fibroblast extracts were chromatographed on DEAE-Sepharose, bands 2, 3, and 4 were eluted together in fractions before hex A, in a position characteristic of serum and placental hex I2 and serum hex P. This suggests that bands 2, 3, and 4 might represent the polypeptides of a fibroblast hex I. The analysis of partial proteolytic digests of the radioactively labeled polypeptides revealed that bands 2 and 3, pre-beta, and beta a had several peptides in common, suggesting that they are structurally related to each other. However, bands 2, 3, and 4 were present in extracts of Tay-Sachs (pre-alpha and alpha deficiency) and Sandhoff cells (pre-beta, beta a, and beta b deficiency) and appeared later than pre-beta in pulse-chase experiments. These results suggest that bands 2 and 3 occur independently of pre-beta and beta a and are probably specified by different mRNA, whether from the same gene or distinct but homologous genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., Kuhl W., Comings D. Hexosaminidase isozyme in type O Gm2 gangliosidosis (Sandhoff-Jatzkewitz disease). Am J Hum Genet. 1975 Sep;27(5):628–638. [PMC free article] [PubMed] [Google Scholar]
- Beutler E. The biochemical genetics of the hexosaminidase system in man. Am J Hum Genet. 1979 Mar;31(2):95–105. [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Yoshida A., Kuhl W., Lee J. E. The subunits of human hexosaminidase A. Biochem J. 1976 Dec 1;159(3):541–543. doi: 10.1042/bj1590541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bordier C., Crettol-Järvinen A. Peptide mapping of heterogeneous protein samples. J Biol Chem. 1979 Apr 25;254(8):2565–2567. [PubMed] [Google Scholar]
- Geiger B., Arnon R. Chemical characterization and subunit structure of human N-acetylhexosaminidases A and B. Biochemistry. 1976 Aug 10;15(16):3484–3493. doi: 10.1021/bi00661a014. [DOI] [PubMed] [Google Scholar]
- Geiger B., Calef E., Arnon R. Biochemical and immunochemical characterization of hexosaminidase P. Biochemistry. 1978 May 2;17(9):1713–1717. doi: 10.1021/bi00602a020. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahuran D. J., Tsui F., Gravel R. A., Lowden J. A. Evidence for two dissimilar polypeptide chains in the beta 2 subunit of hexosaminidase. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1602–1605. doi: 10.1073/pnas.79.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahuran D., Lowden J. A. The subunit and polypeptide structure of hexosaminidases from human placenta. Can J Biochem. 1980 Apr;58(4):287–294. doi: 10.1139/o80-038. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Kumin S., Nitowsky H. M. Human hexosaminidase isozymes: chromatographic separation as an aid to heterozygote identification. Clin Chim Acta. 1977 Mar 1;75(2):181–191. doi: 10.1016/0009-8981(77)90189-9. [DOI] [PubMed] [Google Scholar]
- Price R. G., Dance N. The demonstration of multiple heat stable forms of N-acetyl- -glucosaminidase in normal human serum. Biochim Biophys Acta. 1972 Jun 22;271(1):145–153. doi: 10.1016/0005-2795(72)90142-0. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Ansari N. H., Hawkins L. A., Wiktorowicz J. E. Demonstration of cross-reacting material in Tay-Sachs disease. Biochem J. 1979 Jun 1;179(3):657–664. doi: 10.1042/bj1790657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Yoshida A., Awasthi Y. C., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. II. Kinetic and structural properties. J Biol Chem. 1974 Apr 10;249(7):2049–2053. [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Van Elsen A. F., Leroy J. G. Chromatographic components of beta-hexosaminidase in I-cell disease (mucolipidosis II). Hum Genet. 1979 Apr 5;47(3):305–317. doi: 10.1007/BF00321023. [DOI] [PubMed] [Google Scholar]