Abstract
Antibiotic resistance is a global crisis driven by appropriate and inappropriate antibiotic use to treat human illness and promote animal growth. The antimicrobial resistance epidemic continues to spread due to the triple threat of unfettered access, minimal product regulation and oversight of antibiotic prescription, and lack of clinical diagnostic tools to support antibiotic de-escalation in low-resource settings. In high-resource settings, evidence-based strategies have improved appropriateness of antibiotic use, limiting the spread of drug-resistant organisms and reducing hospital-associated infections, which may also be effective to stop the spread of resistance in resource-poor countries. Current research and surveillance efforts on antimicrobial resistance and hospital-associated infections in low-resource settings are extremely limited, largely focused intensive care units. Many challenges exist to improving antibiotic use and infection control in resource-limited settings, and turning the tide requires intensifying research and surveillance, antimicrobial stewardship, and developing new bedside diagnostic tools for bacterial infections and antimicrobial susceptibility.
Keywords: antimicrobial, resistance, antibiotic, low-resource, healthcare-associated infection, stewardship
The rise of antimicrobial resistance
Since the discovery of Penicillin in 1928 by Alexander Fleming, societies have relied on antibiotics in everyday clinical practice. Health care providers prescribe these ‘miracle drugs’ to our patients more than any other class of medications, with impressive clinical results and improved patient outcomes[1]. Clinicians and patients rely on antibiotics, accustomed to having effective antibiotics to cure nearly any bacterial infection.
Though antibiotics are prescribed for an individual patient’s condition, unlike other medications, antibiotics have effects reaching far beyond the individual[2]. Even when used appropriately and as prescribed, antibiotics and bacteria resistant to antibiotics seep into our local drinking water sources[3–5] after human and agricultural and animal use[6] and wastewater treatment[7]. They are also common contaminants of locally-produced and imported meat and poultry for human consumption[8–13], and direct conduits for causing human illness or colonization. Resistant bacterial have the potential to affect the natural bacterial flora of any person, regardless of who first swallowed the pill or received the injection. Indeed, substantial evidence demonstrates a causal link between wide spread appropriate and inappropriate antimicrobial use and the emergence of antimicrobial resistance[14–19].
Antibiotic resistance is defined as the ability of a specific bacterium to survive in the presence of an antibiotic that was originally effective to treat infections caused by the bacterium, or acquisition of a specific antibiotic resistance mechanism[20, 21]. There are four major mechanisms of bacterial antibiotic resistance: production of enzymes that inactivate the drug; production of modified targets against which the antibiotic has a reduced effect; reduction of permeability to the drug; and active export antibiotics using various pumps[22]. Bacteria may be intrinsically resistant to antimicrobial agents, or may acquire resistance to one or more classes of antibiotics by de novo mutation or exchange of resistance genes from other organisms. Acquired resistance genes may enable a bacterium to produce enzymes that cleave and destroy the antibiotic, to express efflux pumps preventing the drug from reaching a bacterial intracellular target, modify the drug's target site and thwart binding of drug to target, or to produce alternative metabolic pathways bypassing the drug’s target pathway (Table 1)[22]. Antibiotic-susceptible bacteria may acquire new genetic material from antibiotic-resistant strains through conjugation, transformation, or transduction, with simple transposons often facilitating the incorporation of the multiple resistance genes into the genome or plasmids[22].
Table 1.
Common antibacterial drug targets and selected mechanisms of resistance, by antibiotic class.
| Antibiotic class | Antibiotic mechanism of action |
Mechanism(s) of antibiotic resistance |
|---|---|---|
β-lactams
|
Interference with bacterial cell wall synthesis |
|
Glycopeptides
|
Interference with bacterial cell wall synthesis |
|
| Macrolides, Chloramphenicol, Clindamycin, Quinupristin-dalfopristin, Linezolid | Inhibition of protein synthesis- bind to 50S ribosomal subunit |
|
| Aminoglycosides, Tetracyclines | Inhibition of protein synthesis- bind to 30S ribosomal subunit |
|
| Fluoroquinolones | Interference with bacterial DNA synthesis |
|
| Rifampin | Interference with bacterial RNA synthesis | Mutation or duplication of drug target, modification cell permeability |
| Trimethoprim-sulfamethoxazole | Inhibition of metabolism (bacterial folate synthesis) | Single amino acid substitutions in the chromosomal dihydrofolate reductase (as in S. pneumoniae) leading to decreased binding of drug |
| Polymixins, Daptomycin | Disruption of bacterial membrane structure | Mutations altering cell surface charge |
Though dozens of ‘superbugs’ resistant to antibiotics have made headlines over the last quarter century, many clinical microbiologists increasingly agree multidrug-resistant gram-negative bacteria pose the greatest risk to public health[23]. Resistance in gram-positive bacteria, especially Staphylococcus aureus and Enterococcus also continues to rise, with broad implications for loss of effective treatments for skin and soft tissue infections, urinary tract infections and pneumonias[24, 25], all common healthcare-associated infections. Antibiotic resistance is common in healthcare-associated infections (HCAIs), which are localized or systemic infections that not present at admission to a healthcare facility but occur while patients are receiving treatment for another condition in the facility[26]. Common HCAIs include central line associated blood stream infections, catheter associated urinary tract infections, and surgical site infections[26]. Preventing and treating HCAIs should be considered as part of the infection control package when considering ways to stem the tide of antimicrobial resistance worldwide.
As antibiotic resistance becomes increasingly prevalent and recognized, health providers are in danger of losing effective antibiotics to treat both routine infections, and infections caused by antibiotic-resistant organisms. To most effectively address this public health crisis, it is necessary to understand the history and magnitude of the problem as well as plausible solutions. In this review, we will detail the current understanding of global antimicrobial resistance, its detection, how resistance to antibiotic affects treatment choice, and the major factors contributing to the rise of antimicrobial resistance, all with a focus on resource limited settings. We will then review how lower-income countries can turn the tide on global antimicrobial resistance by emphasizing need for additional data collection, diagnostics development, antimicrobial stewardship, and discuss which existing strategies may be effective in these settings.
The state of the art—healthcare-associated infections and antimicrobial resistance in low-resource settings
The global infectious disease burden is disproportionately distributed across countries. The majority of the infectious burden is found in lower and middle-income countries (LMICs)[27] as defined by the World Bank (Table 2). The burden of both antimicrobial resistance and healthcare associated infections (HCAI) is high in all LMICs, where pooled infection data suggest HCAIs rates at least three times as high as rates in resource-rich countries[28]. In fact, HCAIs, whether or not they are associated with high-level antibiotic resistance, are on the rise in LMICs. A recent review[28] highlighted the rise of HCAIs in developing countries, while other sources have deemed HCAIs to be the most frequent hospital-associated adverse event worldwide[29–31].
Table 2.
World Bank definitions of countries by resource distribution.
| Gross National Income (GNI) per capita 2013 U.S. dollars |
World Bank Classification |
|---|---|
| ≤ $1,045 | Low-income |
| $1,045 to $12,746 | Middle-income |
| ≥ $12,746 | High-income |
Source: The World Bank Country and Lending Groups. The World Bank Group; 2014
Though antibiotic resistance is widespread and affects the entire world’s population, the effects of antimicrobial resistance are even more significant in LMICs[7]. Patients in resource-limited countries may suffer the most from the increasing prevalence of antimicrobial resistance due to challenges in identifying and diagnosing these infections and lack of second- and third-line antibiotics to treat resistant bacteria. When antimicrobial resistance becomes prevalent in resource-rich clinical practice settings, providers are generally able to select second- and thirdline treatments. These therapies are often difficult to obtain in LMICs secondary to high cost and low availability.
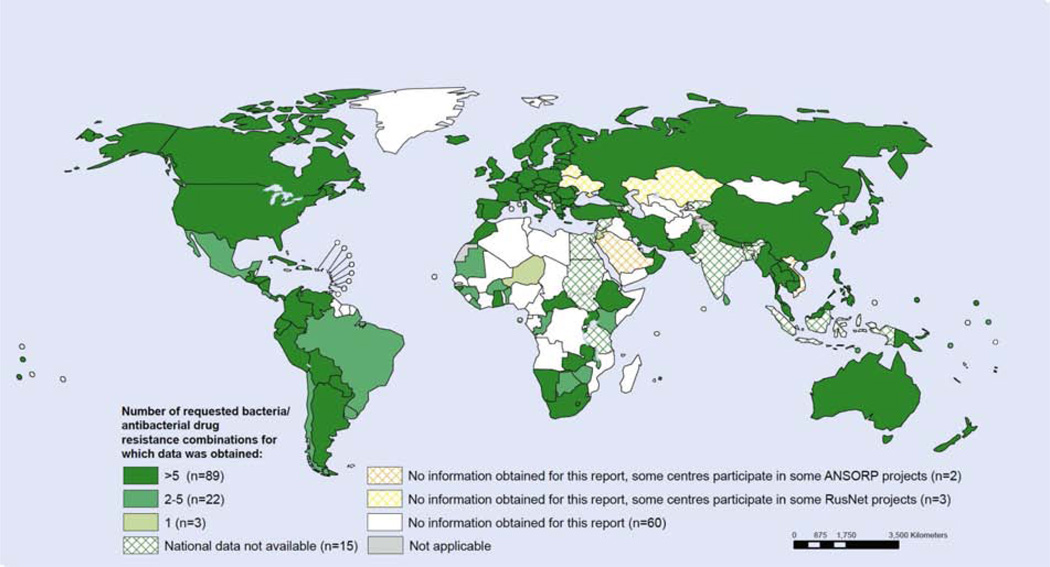
Overall, few data are available on antimicrobial resistance in most LMIC settings (Figure 1)[25, 32]. The most comprehensive description of patterns antimicrobial resistance in low resource countries is a 2011 review in The Lancet by Allegranzi et al., summarizing data from only 28 individual articles representing data on approximately 5,000 organisms[28]. The few scattered studies of reasonable size reporting antimicrobial susceptibility have largely focused on adult intensive care units (ICU)s. These reports are suggestive that ICUs globally are hotbeds of emerging, high-level resistance[33–39]. Such alarming reports merit further study in other countries and healthcare settings.
Figure 1.
WHO report on availability of data on resistance for selected bacteria-antibacterial drug combinations, 2013, in Antimicrobial resistance: global report on surveillance, 2014[25].
Number of reported bacteria is based on the information obtained based on request to national official sources on antibacterial susceptibility testing of at least one of the requested combinations, regardless of denominator data. Data from United Arab Emirates originate from Abu Dhabi only.
Outside of the adult ICU, the bulk of antimicrobial resistance research to date in LMICs has focused on infections in neonates, one of the world’s most vulnerable populations. To treat infections diagnosed within the first 28 days of life, the World Health Organization (WHO) recommends empiric combination antibiotic therapy with gentamycin and ampicillin, but hospital data from developing countries suggest that up to 71% of Klebsiella and 50% of E. coli isolates are resistant to gentamycin[40], often limiting effective therapy to the carbapenem class of antibiotics, which are not widely available in sub-Saharan Africa and many other low-resource settings. These early-onset neonatal infections are likely maternally acquired, and parallel studies in LMIC mothers report similar levels of ampicillin resistance, including gentamycin resistance amongst 60–70% of E. coli and nearly 100% of Klebsiella isolates, in addition to 40–60% of other Enterobacteriaceae[41]. These levels of gram-negative rod (GNR) resistance, including extended-spectrum β-lactamase (ESBL) production, have led countries with access to carbapenems, such as India, to use them as first-line treatment for neonatal sepsis. However, even countries with access to these advanced antibiotics are not immune to encroaching antibiotic resistance; the emergence of carbapenem-resistant neonatal infections amongst Enterobactereiacae and Acinetobacter in these settings is particularly problematic—such infections are essentially untreatable and associated with high mortality[42]. Compounding the issue, aside from research and surveillance activities, clinicians in most LMICs have limited access to useful diagnostics for bacterial infections. Without diagnostic support, LMIC clinicians often lack the ability to diagnose infections caused by resistant bacteria with certainty, leading to uninformed prescribing and complicated treatment decisions.
Antimicrobial resistance—a threat to LMICs, but also a global public health emergency?
Resistance to Penicillin has been detected in low levels in historical samples of bacteria even prior to its widespread use[43]. This finding illustrates that some mechanisms of resistance occur naturally in the environment and may be enhanced and selected for by drug use, even if the use is appropriate. Other types of antibiotic resistance develop only under direct selection pressure through inappropriate use of antibiotics. Inappropriate antibiotic use can take many forms, including courses of therapy which are either too long or too short, incorrect dosing, or use of antibiotics when not clinically indicated. Antibiotics are misused in all regions of the world[44].
While mutations conferring resistance are common to all regions of the world, and thousands of individual mutations have been isolated and described, NDM-1 mutations found in gram negative bacteria (see boxed text) and similar ‘superbugs’ are among the most disturbing because of the very limited spectrum of available antibiotics to treat them. While people living in densely populated areas of India and Bangladesh as well as other countries are known to be at risk of infection and colonization with resistant bacteria, how does mobile, high-level antibiotic resistance affect other populations not living in slum communities, neonates and mothers in LMICs, and urban centers of resource-rich countries? Recent case reports have demonstrated that antimicrobial resistance does not respect borders. For example, one study described European leisure travelers to India who had no contact with the Indian healthcare system, remained healthy throughout their trip and after return home, and then tested positive for carbapenemase-producing Enterobacteriaceae in their stool after coming home[60]. Such demonstrations prove that it is possible to acquire multi-drug-resistant colonizers in the absence of direct selection pressure or healthcare contact. Many examples exist of cross-border resistance; the average person living in resource-rich countries cannot ignore the rising prevalence of antibiotic resistance and the interplay between resource-rich and LMIC nations in promoting the spread of highly-drug resistant organisms. This rapid shift of resistant bacteria as well as the genes conveying resistance may herald the dawn of the post-antibiotic area[47]. Clear evidence is mounting that antibiotic resistance is not a local, but rather a global and highly mobile public health challenge. Our dependence on these medications to treat infections—and expectation that we will always need for effective antibiotics—the rapid rise of high-level antimicrobial resistance constitutes a global public health emergency.
Global factors contributing to antimicrobial resistance and inappropriate antibiotic use
Access to antimicrobials and product regulation
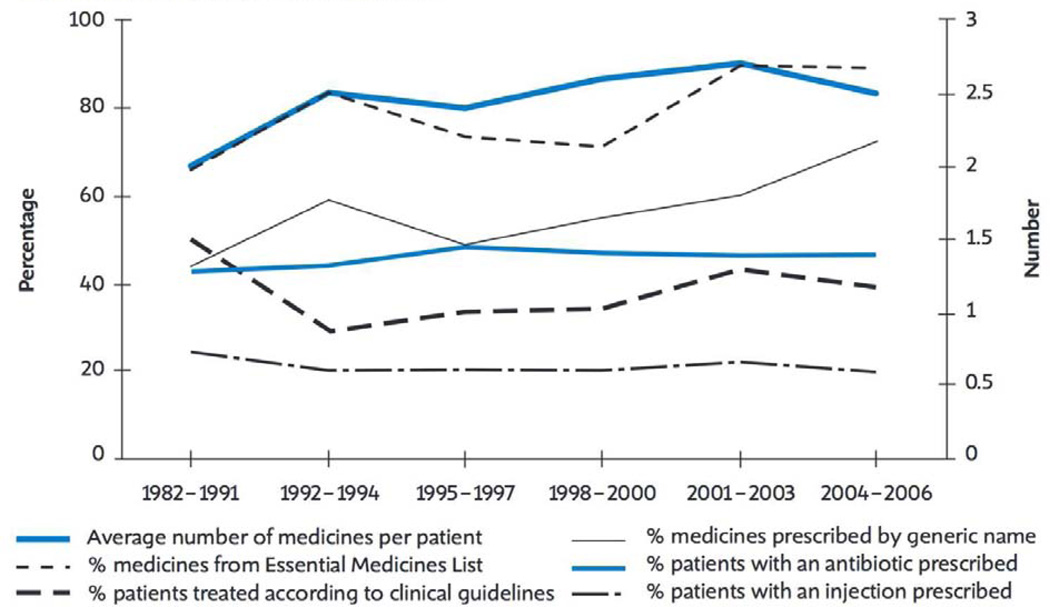
Antibiotic use varies widely across the globe within and between low-income to high-income countries. The health systems of most LMICs are challenged by low spending on population-based health programs. In many LMICs, less than 5% of GDP is spent on healthcare and many countries additionally suffer from a low healthcare worker to population ratio of less than 1 in 1000 people[61]. As a result of low healthcare spending and inadequate staffing, funding priorities have traditionally been focused on the most common and devastating diseases. Monitoring and preventing antimicrobial resistance has not featured among those[62]. However, in many LMICs, rates of hospitalization and antibiotic use are increasing, leading to an overall increase in the amount of antibiotic prescribing (Figure 2)[44]. Institutional and government policies on antibiotic use in LMICs, though variable, are in general less restrictive than in higher-income countries, leaving antibiotic prescribing practices unfettered, at the discretion of the prescriber[25, 44, 63]. In LMICs, two thirds of all antibiotics are sold without a prescription, through unregulated private sectors[44], and data from the WHO database show that approximately 80% of all prescribed medicines in LMICs are dispensed by unqualified personnel[44]. Many countries allow over-the-counter sales of antibiotics and few have a national strategy to contain antimicrobial resistance, as is recommended by the WHO[64]. In India, though prohibited by law, over-the-counter sales and use of antibiotics are extremely common[65]. The situation is similar in Vietnam and many other resource-limited countries, where policies often exist to regulate antibiotic use but enforcement is insufficient or lacking[66]. Such unregulated antibiotic use contributes to development of antibiotic resistance [25, 44], made worse through crowded hospital and clinic conditions and low rates of hand hygiene[67–69].
Figure 2.
WHO report on medicines use in primary care in developing and transitional countries over time, as reported in the World Medicines Report, 2011[44].
Data on the relationship between antibiotic use and antibiotic resistance are scarce in LMICs, and few high quality studies have been published[32]. What is known is that low-resource settings have a higher proportion of antibiotic use[70] and a higher proportion of inappropriate antibiotic prescriptions than high-resource settings[71]. Though the data on antibiotic use and development of bacterial resistance in LMICs are sparse, data in other settings support the correlation between antibiotic use and resistant bacteria highlighting reason for concern from high, unmonitored antibiotic use in these settings, emphasizing the importance of restrictive antibiotic prescribing policies[72]. A prudent global strategy to reduce the spread of antimicrobial resistance would include increasing restrictions on antibiotic prescribing worldwide.
Restrictions on antibiotic use are difficult to institute. Regulatory policies on antibiotic prescriptions tend to be more common in high-income compared to low-income countries[73]. In resource-rich countries, regulatory agencies such as the United States (US) Food and Drug Administration and European Medicines Agency restrict entry of antibiotics into the market and ensure high quality products are distributed in areas under their jurisdiction. In most LMIC countries, there is little to no oversight of prescribing as described, no standardized antibiogram showing local antibiotic resistance patterns, lack of quality control over production resulting in fraudulent or less than fully potent antibiotics, and limited pharmacy stocks resulting in few readily available choices[44, 65]. As of 2007, less than 40% of all countries worldwide had national policies in place limiting availability of antibiotics to prescription-only[44], with enforcement of these policies occurring almost exclusively in resource-rich settings. There is some evidence that restricting antibiotics to prescription-only does work to improve rational antibiotic use in LMICs. One study from Chile, where a new regulation in 2000 prohibited the dispensing by private retail outlets of antibiotics without prescription, was associated with a significant reduction in overall sales of antibiotics in the private sector[74]. Unfortunately, competing agendas and conflict of interest may make passing and enforcing such regulations difficult. According to the WHO, in both 2003 and 2007, approximately 27% of ministries of health reported that revenue from the sale of medicines was used to pay for or supplement health worker salaries, representing a significant incentive for over-prescribing[44]. Additionally, added pressure comes from the pharmaceutical industry promoting increased use of its products. Globally, prescribers receive most of their prescribing information from the pharmaceutical industry directly, and in many countries this is the only information they receive[44].
Lack of support for clinical decision-making
While inappropriate antibiotic use is high in LMICs, this is exacerbated by a paucity of appropriate diagnostic and clinical tools to assist clinicians in safely de-escalating antibiotics or avoiding their use when unnecessary. The lack of diagnostic assays and equipment is so profound, it has been termed the ‘Achilles Heel’ of antibiotic resistance containment[75, 76]. Basic diagnostic assays such as routine blood counts to assess for leukocytosis, urinalysis and urinary culture, blood cultures, and plain radiographs are considered essential tools for the practice of modern medicine. Each of these diagnostic tests play a cornerstone role in medical decision-making, increasing or decreasing the probability of infection in a patient based on the result. Many resource-poor settings do no offer these tests or when offered, the tests are too expensive for the vast majority of patients to afford. Furthermore, testing for antimicrobial resistance in bacterial isolates is out of reach in most LMIC clinical practice settings. Where available, microbiologic assays such as disk diffusion method for antimicrobial susceptibility enable providers to assess for resistance patterns and guide therapy. Without this information, clinicians do not have sufficient information to prescribe the narrowest-spectrum antibiotic needed to treat the patient’s disease, or to decide that no antibiotic is needed. In addition to the expense of diagnostic technologies and supplies, personnel trained to run the assays are often lacking, and understaffing of LMIC laboratories and microbiology departments is unfortunately too common.
Worldwide, there is general consensus among experts that 50% or more of current antibiotic use could be avoided as unnecessary or inappropriate for the illnesses being treated with antibiotics, without negative consequence to the patient[77]. However, when diagnostic support is not available, clinicians tend to prescribe antimicrobials as a safeguard against severe infection, implicitly calculating that the benefits outweigh the risks for an individual patient. While few studies have examined the impact of basic laboratory and molecular diagnostic tests on detection and therapy for infections, one study performed in sub-Saharan Africa showed that one third of neonatal meningitis cases could be misdiagnosed without lumbar puncture studies[78]. Such reports underscore why antibiotics may be over-prescribed in settings without diagnostic testing; they are a theoretical protection for individual patients. This effect is amplified on a population level, leading to gross antibiotic overuse in settings lacking adequate diagnostic support to de-escalate or stop antibiotic therapy.
Though diagnostic interventions have not been well-studied as strategies to reduce antibiotic use in LMICs, it is rational to conclude that the availability of diagnostic testing would lead to more appropriate antibiotic use by providing decision support for clinicians to safely start, deescalate, change, or stop antibiotics. In LMICs, implementing the same basic laboratory and microbiologic diagnostic assays used in resource-rich settings may be challenging or impossible due to lack of reliable cold chain transport and storage, instability of equipment and reagents in hot and dusty climates, impractical service and replacement contracts, and understaffing or inadequate training of lab personnel. This challenge is only exacerbated by the increasing complexity of improved diagnostic equipment, making repairs and upkeep difficult. Equipment service contracts are a necessity, but these may be unaffordable or unavailable in these settings.
Another challenge is that diagnostic technology must be able to keep pace with evolving antimicrobial resistance, a constantly moving target with new resistance mutations and patterns reported regularly. Historically, there has been little incentive for for-profit companies to create rapid diagnostic solutions for low-resource settings, since sales in LMICs may not be lucrative enough to generate adequate return on investment[79]. One possible advantage of the global spread of antimicrobial resistance is that a common bedside testing platform for detection of bacterial infection and antimicrobial resistance profiling could be used in all country settings, leading to higher return on investment through demand from richer countries. Alternatively, affordable devices developed in the LMIC setting could be reverse innovated to be useful in resource-rich settings. The Infectious Diseases Society of America has recently called for the increased development and approval of rapid, accurate microbiologic testing for specific diagnosis of infection, while acknowledging that globalization of diagnostics could be challenging due to varying disease prevalence globally, affecting the pre-test probability of a diagnostic, and limiting its usefulness outside of its intended target area[80]. The ideal characteristics for LMIC diagnostics include low cost, minimal required sample preparation, quick return of results relevant to patient care[80]. The creation of rapid, heat-stable, accurate and simple bedside diagnostics for common bacterial infections is necessary. Such diagnostics could include fingerstick testing for disseminated bacterial infections, allowing for rapid detection of the presence or absence of bacterial antigens with simultaneous genetic analysis of the bacterium for resistance genes. More readily accessible strategies could include use of ultrasound to diagnose pneumonia, and transfer of molecular platforms requiring minimal sample preparation for bacterial analysis directly to LMICs.
One successful example of dissemination of bedside diagnostic support in LMICs is rapid diagnostic fingerstick tests for malaria. These tests are now in widespread use despite having little application in resource-rich nations. While necessary, creation and implementation of new diagnostics may not be the panacea. For example, after the introduction of the low-cost, rapid malaria test which is perfectly poised to help reduce unnecessary antimalarial use, studies showed that some community health workers continued to administer antimalarials in patients despite testing negative[81, 82]. Alongside new bedside diagnostics, will be the need for extensive education, and monitoring and guidance on use once technologies are developed and disseminated.
Turning the tide of antimicrobial resistance: interventions that work—hand hygiene and antimicrobial stewardship
Since its recognition by Semmelweis in the 1800s[83], hand hygiene is judged the most important measure for prevention of microbial transmission during patient care. However, hand hygiene is in irregular practice in low-resource settings, historically reported at rates of less than 20%[84–86], though new data now suggest that regular hand cleansing practices may now be on the rise[86–88]. Multidimensional hand hygiene programs incorporating education, observation, feedback and incentives have been shown to at least transiently improve hand hygiene compliance[86]. To improve hang hygiene globally, the WHO developed international guidelines. Implementation studies[89] show that the guidelines have improved overall compliance with handwashing from 51% to 67.2% across all sites where implemented, with greater improvements in LMIC sites than in wealthier nations[89]. Increasing education around hand hygiene practices must be coupled with supplying the means to perform hygiene easily, and seems to be a reasonable first step forward in LMICs to control the spread of resistant organisms and reduce HCAIs. Importantly, increased hand hygiene has been shown to correlate with a reduction in antimicrobial resistance[90] and HCAIs[91].
The ideal infection control program to progress on stemming the tide of antimicrobial resistance and decreasing HCAIs would pair comprehensive hand hygiene efforts with antimicrobial stewardship. Antimicrobial stewardship programs (ASP)s are increasingly considered essential in resource-rich countries, and the WHO, Infectious Diseases Society of America (IDSA) and INICC have called for the development of ASPs worldwide[44, 63, 65, 68]. ASPs are associated with improved clinical outcomes and reduced antimicrobial resistance[92]. They achieve their effect through several mechanisms. In general, ASPs restrict the use of antibiotics to ones approved by the ASP program, appropriate to their setting as judged by the ASP and then labeled as ‘formulary’ drugs and often acquired and used at lower cost due to bulk purchasing practices. ASPs generally require ‘prior authorization’ for clinicians to prescribe restricted or non-formulary antibiotics, making the use of such medications more difficult and also more transparent. Lastly, ASPs commonly perform post-prescription auditing, ensuring that the right antibiotics have been used for every infection at an appropriate dose and duration to effectively treat the disease.
Unfortunately, ASPs require significant up-front investment in human capital through training. They also depend on specific infrastructure needs, including the ability to perform surveillance on a proportion—if not all—clinical samples, and perform microbiology testing on bacterial isolates to determine resistance patterns. Because of these requirements, cost can become a barrier to implementation, particularly in low-resource settings. Modified ASPs though should be considered which could be scaled-down to the capacity of an individual institution.
Investing in ASPs in LMICs is worthwhile, as they have been shown to be effective and cost-saving. A 2012 review summarized recent studies in high-income settings [14] demonstrating in detail the financial offset of implementing an ASP program[92, 93]. The review described substantial savings sustained over multi-year ASP life spans, showing ASPs to be self-sustainable and cost-saving in high-resource settings[15]. One study examined the before-and-after effect from when an ASP program was discontinued; it found a temporal association with substantial increased costs driven by higher antibiotic utilization[93]. Although the cost-savings goals from these programs were moderate, they more than paid for the program itself. Similar studies conducted in LMICs could help establish the cost-benefit balance of ASPs in these settings. If proven to be as cost-saving or even cost-effective outside of resource-rich countries, it would help motivate resources towards their implementation in LMIC setting. ASP teams in LMICs could have a role in encouraging the switch from intravenous to oral antibiotics based on available clinical and microbiology data, which could lead to substantial savings[93]. While there is a need for consistently available and reliable microbiology and laboratory data to de-escalate therapy safely, it is also possible that ASP teams could safely tailor therapy without such data— a hypothesis worth testing. Once cost savings are established, ideally they could fund additional research and implementation strategies in this area. Given substantial evidence demonstrating a causal link between antimicrobial use and the emergence of antimicrobial resistance[14–19], implementation of ASPs in LMICs should, in theory, lead to a significant decrease in antimicrobial resistance over time[94]. There is some evidence to suggest that when specific antibiotic classes are restricted, bacterial resistance selection pressure is lifted, and antimicrobial resistance can once again regress[95], giving hope for ASPs to have a significant impact, even in LMICs.
Other interventions routinely used in the high-resource settings to reduce antimicrobial resistance and HCAIs in conjunction with a functional ASP include isolation and barrier precautions, selective de-contamination of asymptomatic resistant bacterial carriage, and monitoring and reinforcement of hand hygiene. None of these measures have been studied adequately in LMIC settings, with the exception of hand hygiene monitoring, a recent focus of the WHO[85, 96]. Each of these potential interventions merits further study in LMICs.
Healthcare-associated infections and antimicrobial resistance—more data needed
The bulk of published data reporting high rates of antimicrobial resistance from LMICs are from ICU settings and vulnerable maternal-child populations—but no population is immune to resistant bacteria. Despite the rapid rise of antibiotic resistance and its potential for global implications, to date, the medical and scientific literature has focused on treatment and management of specific infections, including tuberculosis, malaria and HIV. This phenomenon of focus on the “big three” is especially true in countries where less than 5% of GDP is spent on healthcare and healthcare workforce density is less than five per 100,000[61]; in these settings, far less attention has been paid to antimicrobial resistance, infection control and HCAI despite growing implications of these complications. Without this much-needed data, populations and the health care systems in these countries, and worldwide, are at risk of high morbidity and mortality due to infections from emerging antibiotic-resistant bacteria.
Where sparse data exist, they often come from small studies with poor data quality, especially data originating from Africa and the western Pacific, two of the six WHO-recognized world regions[28]. The aforementioned 2011 review and meta-analysis by Allegranzi et al. published in The Lancet compiled all data on HCAIs in LMICs between 1995 and 2008 is the most comprehensive review of the topic to date [28]. However, in this study, only 271 studies from LMICs had sufficiently complete data to merit inclusion in the analysis. Furthermore, 54% of those 271 included studies were judged to be of low quality. Among the high quality studies analyzed in the review, the prevalence of HCAIs was 15.5 infections per 100 patients in LMICs, three times the ratio reported over the same time period in the US (4.5 per 100 patients in 2002)[28]. Another recent report from a neonatal ICU in Brazil estimated infection density at up to nine times higher than in the US (15.2–62.0 infections per 1000 patient days vs. 6.9 per 1000)[28]. These reports strongly suggest that the burden of HCAIs in LMICs may be under-recognized, highlighting the need for continued study in this arena.
Despite the significant worldwide burden of antimicrobial resistance and HCAIs, very little funding from either public or private sources is available for research (or capacity building to train professionals) in antimicrobial stewardship and best practices to prevent HCAIs. For example, HCAIs attracted only 2.0% of United Kingdom research funding spent overseas, despite constituting a much higher percentage of the worldwide burden of disease[97]. Historically, it has been challenging to justify high-level spending on antimicrobial resistance and HCAIs as data on the incidence and prevalence to drive increased global spending on of HCAIs and antibiotic resistance are lacking.
To address the lack of HCAI and antimicrobial resistance data from LMICs, the International Nosocomial Infection Control Consortium (INICC) was created, an international nonprofit, open, multicenter, collaborative health care-associated infection control program with a surveillance system based on that of the US National Healthcare Safety Network (NHSN)[98]. Founded in 1988, the INICC is the first multinational research network established to control and reduce device-associated infections which publishes their research and implementation activities in semi-regular manuscripts. In their 2009 report, 173 ICUs from 29 countries were included, 68% of which were located in LMICs[59]. Although antimicrobial resistance rates were lower than in US-based ICUs for some organisms, rates of high-level carbapenem resistance for Klebsiella were nearly 3 times higher in LMICs than US ICUs[59]. Rates of surgical site infection were also reported to be significantly higher in INICC hospitals compared with NHSN data[99]. According to the INICC data, determinants of a high burden of HCAI in LMIC include inadequate environmental hygienic conditions, poor infrastructure, insufficient equipment, understaffing, overcrowding, lack of knowledge and application of basic infection control principles, prolonged and inappropriate use of antimicrobials and devices, and lack of local and national guidelines, policies and monitoring[28].
The INICC requires member hospitals to have an infection control team comprised of a physician and an infection control practitioner, and a microbiology laboratory that can isolate and identify aerobic pathogens from clinical cultures and perform in vitro susceptibility using standard methods[98]. The person responsible for surveillance must have had at least three years experience, and in most hospitals, teams had access to electronic data[87, 98]. Forty-six LMICs on four continents are current members, but there are no countries represented from sub-Saharan Africa except Nigeria[100]. Low African participation may due to the personnel requirements for participation. By far the world’s poorest region, Africa represents one-seventh of the world’s population. It will record the largest amount of population growth of any world region between now and 2050, and is expected to more than double from 1.1 billion today to at least 2.4 billion by 2050, with nearly all the growth in the 51 countries of sub-Saharan Africa[100]. Given the population growth and with it the likely rise in infectious disease and concomitant disease resistance, Africa will be challenged to increase its contributions and participation in efforts like the INICC. Research, diagnostic development, and stewardship efforts will need to be increased in this region to develop the capacity for sub-Saharan Africa to participate in global research and surveillance methods.
More reliable and systematic data—specific to country and setting—are needed urgently globally, including cost-effectiveness of antimicrobial stewardship and how this could be incorporated into LMICs financial strategy. These data can inform policymakers and country officials to make appropriate decisions for their setting that will decrease the rate of development of antimicrobial resistance help protect their populations from infections that they may not be able to effectively or affordably treat[28]. Future research should include collection of data on antimicrobial resistance with respect to HCAIs, as susceptibility patterns and degree of antibiotic resistance have almost never been included in such studies[28]. The absence of high-quality studies to evaluate antibiotic non-use or de-escalation using the support of diagnostic tools is also a hindrance to forward progress in changing antibiotic use practices as one avenue to mitigate the spread of antibiotic resistance. Innovation and research on bedside or point-of-care diagnostics is stymied by inadequate funding to invent new devices, study the use of old devices in new ways, cost containment concerns, lack of reliable electricity, clean water and cold chains necessary for many diagnostics to function, and concerns over adequate training and staffing of personnel. Research on diagnostics and their potential to reduce antibiotic use and assist with appropriate antibiotic selection based on antimicrobial resistance patterns should be an urgent priority. There should also be a call for research into antimicrobial drug resistance globally, with an increased investment from the public and private sector in every sector to combat this global problem.
The profound lack of data on health care-associated infections and prevalence of antimicrobial resistance in LMICs calls for rigorous surveillance to better define the problem. The most effective surveillance would involve horizontally integrated programs including ASPs, pharmacy management, microbiology and laboratory quality control, creation and dissemination of standardized antibiograms, and additional decision support tools such as enhanced, accessible bedside diagnostic tools. Encouragingly, the WHO has made an effort to help highlight the problem. Starting in 2005, the WHO announced the first Global Patient Safety Challenge. Since its inception, 88 UN member states, 147 resource-limited countries and 36 resource-rich countries have committed to reducing HCAIs by signing up for this endeavor[28, 31]. The goal of the Safety Challenge is to ensure that infection control is acknowledged universally as a solid and essential basis towards patient safety and recognize that infection control, including improved hand hygiene among healthcare workers, supports the reduction of health care-associated infections and their consequences. The hope is that the convening power of the WHO and its global visibility will help set worldwide priorities and align global healthcare agendas, promoting additional investments in research about and interventions to counteract antimicrobial resistance across all infection types. Most importantly, future research spending in this area will need to be better aligned with the rapidly increasing sequelae of the burden of disease from resistance and HCAI.
A road map for the future
Antimicrobial resistance has emerged as a global problem and a threat to our collective future. Despite being identified as a worldwide public health priority by the WHO and other international organizations, data on antimicrobial resistance and hospital-associated infections in low-resource settings remain extremely limited. Without the infrastructure to collect surveillance and antibiotic use data, the extent of the problem and impact of interventions cannot be accurately measured. Surveillance and research efforts in LMICs should extend to inpatient as well as outpatient settings in LMICs to ensure that antimicrobial resistance is adequately monitored and addressed. Policy-makers should consider the model of ASPs from developed countries, adapt these models to the resources and needs of LMICs, and test their effectiveness and potential for cost savings. Major difficulties exist for implementation of antimicrobial and resistance surveillance, including lack of expertise in infection surveillance and control practices, inadequate human and financial resources[28], poor diagnostic infrastructure, lack of equipment and cold chain for appropriate diagnosis and surveillance of antibiotic-resistant infections. Because of these challenging conditions, some aspects of monitoring and intervention will be out of reach for LMICs. However, others are likely to be feasible and should be tested and implemented, including the development of easier and better tools to diagnose bacterial infections and assess for antimicrobial resistance at the bedside and modified ASPs.
The solution to increasing antibiotic resistance will require comprehensive antibiotic stewardship in low-income countries as its cornerstone, and this should be done with the financial assistance and collegial partnership wealthier nations[101], capitalizing on the political and regulatory willpower of international partnerships. Resource-rich nations should share their expertise in development of ASPs, training healthcare personnel from LMICs interested ASP and other interventions, encouraging trained staff to return to their home countries to implement these skills, transferring this learning to their colleagues to create a brighter future beyond the postantibiotic era.
Highlights.
Antibiotic resistance is a global crisis driven by appropriate and inappropriate antibiotic use
Resistance is driven by unfettered access and lack of product regulation and clinical diagnostic tools
Research and surveillance in low-resource settings is extremely limited and should be intensified
Antimicrobial stewardship and new bedside diagnostic tools are also needed to turn the tide
Case-in-point: Carbapenems—antibiotic resistance in mainstream news.
The globalization of antibiotic resistance made popular media headlines in 2010 when the New York Times published an article on NDM-1 (New Delhi metallo-β-lactamase-1), a broad-spectrum broad-carbapenem-active metallo-β-lactamase mutation moving from southeast Asia to the United States[45]. NDM-1 mutations are a frightening development in recent medical history, as they confer resistance to some of the most powerful and broad-spectrum antibiotics known. These mutations are becoming more prevalent in E. Coli and Klebsiella isolates worldwide[46], increasing from 0% in 2001 to 1.4% in 2010[47]. Acquisition of this mutation by bacteria, as with many β-lactam resistance genes among gram-negative bacteria, occurs through a relatively simple horizontal plasmid transfer[48]. These mobile genes on plasmids can rapidly spread through bacterial populations[23], an evolution facilitated by population overcrowding and lack of adequate sanitation. Acquisition of these plasmids by gram negative rods (GNRs) decreases carbapenem effectiveness markedly, or eliminates the potential use of this highly-effective antibiotic class of altogether, sometimes leaving no remaining treatment option. Bacteria expressing the NDM-1 mutation are resistant to nearly all antibiotics including the potent carbapenems, leaving the highly toxic aminoglycoside colistin, and black-box-warning-labeled drug tigecycline as the only remaining antibiotics with guaranteed activity[23]. While the mutation made headlines in hospitals reporting the bacteria in HCAIs, what is especially alarming is the commonness of this superbug mutation amongst routine hospital surveillance samples and outpatients. A recent study in Bangladesh reported 9% prevalence of NDM-1 mutations amongst 100 patients seeking care for diarrhea from the Dhaka slums, where the population density is up to 100,000 people per square mile[49], resulting in high potential for human-human transmission to become carriers of the resistant bacterium. In addition to the potential for transmission between humans in crowded settings with sanitation challenges and contaminated water supplies such as these informal settlements, bacteria carrying NDM-1 mutations have been found in the food supply, including livestock, companion animals and wildlife[50], which may result from environmental contamination or use of antibiotics as growth promoters[51]. A recent study from New Delhi found NDM-1 mutant bacteria in 4% of drinking water and 30% of ground seepage samples[52], and multiple other studies have found that a substantial proportion of healthy children and adults across the world carry the resistant bacterium [53–56]. A study to be published in The Lancet now reports that to 95% of adults in India and Pakistan carry some bacteria resistant to β-lactams, including cabapenems[57]. Highly-antibiotic-resistant bacteria have been proven to cross international borders via human travelers, insect and animal vectors, water and farm products[58]. Often, transmission is not noticed, because the bacteria may not lead to clinically significant disease and routine surveillance does not detect them[58, 59]. NDM-1 mutations are a sobering illustration of the prevalence, ease of acquisition, and mobility of potentially devastating antimicrobial resistance.
Acknowledgements
The authors are grateful to Dr. Vanessa Bradford-Kerry for her editorial expertise in reviewing this manuscript, and to Dr. Erica Shenoy for consulting on the framework of the manuscript and providing key edits. Dr. Bebell is supported through a T32 training grant from the NIH (NIAID AI 007433, PI Dr. Kenneth Freedberg).
Abbreviations
- HCAI
healthcare-associated infection
- LMIC(s)
lower and middle-income country(ies)
- WHO
World Health Organization
- ESBL
extended-spectrum β-lactamase
- NDM-1
New Delhi metallo-β-lactamase-1
- US
United Stated
- GNRs
gram negative rods
- ASP
antimicrobial stewardship program
- IDSA
Infectious Diseases Society of America
- INICC
International Nosocomial Infection Control Consortium
- NHSN
National Healthcare Safety Network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors disclose no conflicts of interest.
REFERENCES
- 1.Col NF, O'Connor RW. Estimating worldwide current antibiotic usage: report of Task Force 1. Rev Infect Dis. 1987;9(Suppl 3):S232–S243. doi: 10.1093/clinids/9.supplement_3.s232. [DOI] [PubMed] [Google Scholar]
- 2.Hardin G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- 3.Talukdar PK, Rahman M, Nabi A, Islam Z, Hoque MM, Endtz HP, et al. Antimicrobial resistance, virulence factors and genetic diversity of Escherichia coli isolates from household water supply in Dhaka, Bangladesh. PLoS One. 2013;8:e61090. doi: 10.1371/journal.pone.0061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueira V, Serra EA, Vaz-Moreira I, Brandao TR, Manaia CM. Comparison of ubiquitous antibiotic-resistant Enterobacteriaceae populations isolated from wastewaters, surface waters and drinking waters. J Water Health. 2012;10:1–10. doi: 10.2166/wh.2011.002. [DOI] [PubMed] [Google Scholar]
- 5.Coleman BL, Salvadori MI, McGeer AJ, Sibley KA, Neumann NF, Bondy SJ, et al. The role of drinking water in the transmission of antimicrobial-resistant E. coli. Epidemiol Infect. 2012;140:633–642. doi: 10.1017/S0950268811001038. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Migura L, Hendriksen RS, Fraile L, Aarestrup FM. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: the missing link between consumption and resistance in veterinary medicine. Vet Microbiol. 2014;170:1–9. doi: 10.1016/j.vetmic.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Oluyege JO, Dada AC, Odeyemi AT. Incidence of multiple antibiotic resistant Gram-negative bacteria isolated from surface and underground water sources in south western region of Nigeria. Water Sci Technol. 2009;59:1929–1936. doi: 10.2166/wst.2009.219. [DOI] [PubMed] [Google Scholar]
- 8.Coleman BL, Louie M, Salvadori MI, McEwen SA, Neumann N, Sibley K, et al. Contamination of Canadian private drinking water sources with antimicrobial resistant Escherichia coli. Water Res. 2013;47:3026–3036. doi: 10.1016/j.watres.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Yu T, Wu N, Meng H, Shi L. Detection of qnr, aac(6')-Ib-cr and qepA genes in Escherichia coli isolated from cooked meat products in Henan, China. Int J Food Microbiol. 2014;187c:22–25. doi: 10.1016/j.ijfoodmicro.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Vogt D, Overesch G, Endimiani A, Collaud A, Thomann A, Perreten V. Occurrence and Genetic Characteristics of Third-Generation Cephalosporin-Resistant Escherichia coli in Swiss Retail Meat. Microb Drug Resist. 2014 doi: 10.1089/mdr.2013.0210. [DOI] [PubMed] [Google Scholar]
- 11.Abgottspon H, Stephan R, Bagutti C, Brodmann P, Hachler H, Zurfluh K. Characteristics of extended-spectrum cephalosporin-resistant Escherichia coli isolated from Swiss and imported poultry meat. J Food Prot. 2014;77:112–115. doi: 10.4315/0362-028X.JFP-13-120. [DOI] [PubMed] [Google Scholar]
- 12.Jana A, Mondal A. Serotyping, pathogenicity and antibiogram of Escherichia coli isolated from raw poultry meat in West Bengal, India. Vet Ital. 2013;49:361–365. doi: 10.12834/VetIt.1215.10. [DOI] [PubMed] [Google Scholar]
- 13.Ingram PR, Rogers BA, Sidjabat HE, Gibson JS, Inglis TJ. Co-selection may explain high rates of ciprofloxacin non-susceptible Escherichia coli from retail poultry reared without prior fluoroquinolone exposure. J Med Microbiol. 2013;62:1743–1746. doi: 10.1099/jmm.0.062729-0. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson KB, Balada-Llasat JM, Bauer K, Deutscher M, Goff D, Lustberg M, et al. The economics of antimicrobial stewardship: the current state of the art and applying the business case model. Infect Control Hosp Epidemiol. 2012;33:389–397. doi: 10.1086/664910. [DOI] [PubMed] [Google Scholar]
- 15.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 16.McGowan JE., Jr Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983;5:1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- 17.Courcol RJ, Pinkas M, Martin GR. A seven year survey of antibiotic susceptibility and its relationship with usage. J Antimicrob Chemother. 1989;23:441–451. doi: 10.1093/jac/23.3.441. [DOI] [PubMed] [Google Scholar]
- 18.Seppala H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 19.Muller AA, Mauny F, Bertin M, Cornette C, Lopez-Lozano JM, Viel JF, et al. Relationship between spread of methicillin-resistant Staphylococcus aureus and antimicrobial use in a French university hospital. Clin Infect Dis. 2003;36:971–978. doi: 10.1086/374221. [DOI] [PubMed] [Google Scholar]
- 20.Chancey ST, Zahner D, Stephens DS. Acquired inducible antimicrobial resistance in Gram-positive bacteria. Future Microbiol. 2012;7:959–978. doi: 10.2217/fmb.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 22.Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Infect Control. 2006;34:S3–S10. doi: 10.1016/j.ajic.2006.05.219. discussion S64–73. [DOI] [PubMed] [Google Scholar]
- 23.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hede K. Antibiotic resistance: An infectious arms race. Nature. 2014;509:S2–S3. doi: 10.1038/509S2a. [DOI] [PubMed] [Google Scholar]
- 25.Organization WH. Antimicrobial resistance: global report on surveillance. Geneva: WHO Press; 2014. [Google Scholar]
- 26.CDC. Types of Healthcare-associated Infections. Atlanta: Centers for Disease Control; 2014. [Google Scholar]
- 27.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 28.Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 29.Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348:651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 30.Bates DW, Larizgoitia I, Prasopa-Plaizier N, Jha AK. Global priorities for patient safety research. Bmj. 2009;338:b1775. doi: 10.1136/bmj.b1775. [DOI] [PubMed] [Google Scholar]
- 31.Pittet D, Allegranzi B, Storr J, Donaldson L. 'Clean Care is Safer Care': the Global Patient Safety Challenge 2005–2006. Int J Infect Dis. 2006;10:419–424. doi: 10.1016/j.ijid.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O'Brien TF, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5:481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 33.Rhomberg PR, Fritsche TR, Sader HS, Jones RN. Antimicrobial susceptibility pattern comparisons among intensive care unit and general ward Gram-negative isolates from the Meropenem Yearly Susceptibility Test Information Collection Program (USA) Diagn Microbiol Infect Dis. 2006;56:57–62. doi: 10.1016/j.diagmicrobio.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med. 2010;38:S335–S344. doi: 10.1097/CCM.0b013e3181e6ab12. [DOI] [PubMed] [Google Scholar]
- 35.Zarrilli R, Giannouli M, Tomasone F, Triassi M, Tsakris A. Carbapenem resistance in Acinetobacter baumannii: the molecular epidemic features of an emerging problem in health care facilities. J Infect Dev Ctries. 2009;3:335–341. doi: 10.3855/jidc.240. [DOI] [PubMed] [Google Scholar]
- 36.Wattal C, Goel N, Oberoi JK, Raveendran R, Datta S, Prasad KJ. Surveillance of multidrug resistant organisms in tertiary care hospital in Delhi, India. J Assoc Physicians India. 2010;58(Suppl):32–36. [PubMed] [Google Scholar]
- 37.Daneman N, Sarwar S, Fowler RA, Cuthbertson BH. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:328–341. doi: 10.1016/S1473-3099(12)70322-5. [DOI] [PubMed] [Google Scholar]
- 38.Madani N, Rosenthal VD, Dendane T, Abidi K, Zeggwagh AA, Abouqal R. Health-care associated infections rates, length of stay, and bacterial resistance in an intensive care unit of Morocco: findings of the International Nosocomial Infection Control Consortium (INICC) Int Arch Med. 2009;2:29. doi: 10.1186/1755-7682-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leblebicioglu H, Rosenthal VD, Arikan OA, Ozgultekin A, Yalcin AN, Koksal I, et al. Device-associated hospital-acquired infection rates in Turkish intensive care units. Findings of the International Nosocomial Infection Control Consortium (INICC) J Hosp Infect. 2007;65:251–257. doi: 10.1016/j.jhin.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365:1175–1188. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 41.Waters D, Jawad I, Ahmad A, Lukšić I, Nair H, Zgaga L, et al. Aetiology of community-acquired neonatal sepsis in low and middle income countries. J Glob Health. 2011;1:154–170. [PMC free article] [PubMed] [Google Scholar]
- 42.Viswanathan R, Singh AK, Ghosh C, Dasgupta S, Mukherjee S, Basu S. Profile of neonatal septicaemia at a district-level sick newborn care unit. J Health Popul Nutr. 2012;30:41–48. doi: 10.3329/jhpn.v30i1.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollock MR. Origin and function of penicillinase: a problem in biochemical evolution. Br Med J. 1967;4:71–77. doi: 10.1136/bmj.4.5571.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO. The World Medicines Situation 2011. Geneva: The World Health Organization; 2011. [Google Scholar]
- 45.McNeil DG., Jr . The New York Times. New York: The New York Times; 2010. Antibiotic-Resistant Bacteria Moving From South Asia to U.S. [Google Scholar]
- 46.Nordmann P. Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect. 2014;44:51–56. doi: 10.1016/j.medmal.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 48.Hudson CM, Bent ZW, Meagher RJ, Williams KP. Resistance Determinants and Mobile Genetic Elements of an NDM-1-Encoding Klebsiella pneumoniae Strain. PLoS One. 2014;9:e99209. doi: 10.1371/journal.pone.0099209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam MA, Nabi A, Rahman M, Islam M, Ahmed D, Faruque AS, et al. J Med Microbiol. England; 2014. Prevalence of faecal carriage of NDM-1-producing bacteria among patients with diarrhoea in Bangladesh; pp. 620–622. [DOI] [PubMed] [Google Scholar]
- 50.Guerra B, Fischer J, Helmuth R. An emerging public health problem: Acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet Microbiol. 2014;171:290–297. doi: 10.1016/j.vetmic.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Singer RS, Finch R, Wegener HC, Bywater R, Walters J, Lipsitch M. Antibiotic resistance--the interplay between antibiotic use in animals and human beings. Lancet Infect Dis. 2003;3:47–51. doi: 10.1016/s1473-3099(03)00490-0. [DOI] [PubMed] [Google Scholar]
- 52.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 53.Stelling JM, Travers K, Jones RN, Turner PJ, O'Brien TF, Levy SB. Integrating Escherichia coli antimicrobial susceptibility data from multiple surveillance programs. Emerg Infect Dis. 2005;11:873–882. doi: 10.3201/eid1106.041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lester SC, del Pilar Pla M, Wang F, Perez Schael I, Jiang H, O'Brien TF. The carriage of Escherichia coli resistant to antimicrobial agents by healthy children in Boston, in Caracas, Venezuela, and in Qin Pu, China. N Engl J Med. 1990;323:285–289. doi: 10.1056/NEJM199008023230501. [DOI] [PubMed] [Google Scholar]
- 55.Executive summary: select findings, conclusions, and policy recommendations. Clin Infect Dis. 2005;41(Suppl 4):S224–S227. doi: 10.1086/430781. [DOI] [PubMed] [Google Scholar]
- 56.Bartoloni A, Pallecchi L, Benedetti M, Fernandez C, Vallejos Y, Guzman E, et al. Multidrug-resistant commensal Escherichia coli in children, Peru and Bolivia. Emerg Infect Dis. 2006;12:907–913. doi: 10.3201/eid1206.051258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reardon S. Nature. England: 2014. Antibiotic resistance sweeping developing world; pp. 141–142. [DOI] [PubMed] [Google Scholar]
- 58.Okeke IN, Edelman R. Dissemination of antibiotic-resistant bacteria across geographic borders. Clin Infect Dis. 2001;33:364–369. doi: 10.1086/321877. [DOI] [PubMed] [Google Scholar]
- 59.Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003–2008, issued June 2009. Am J Infect Control. 2010;38:95–104. e102. doi: 10.1016/j.ajic.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Ruppe E, Armand-Lefevre L, Estellat C, El-Mniai A, Boussadia Y, Consigny PH, et al. Acquisition of carbapenemase-producing Enterobacteriaceae by healthy travellers to India, France, February 2012 to March 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.14.20768. [DOI] [PubMed] [Google Scholar]
- 61.organization Wh. The World Health Report 2006 - working together for health. Geneva: 2006. [Google Scholar]
- 62.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leung E, Weil DE, Raviglione M, Nakatani H. The WHO policy package to combat antimicrobial resistance. Bull World Health Organ. 2011;89:390–392. doi: 10.2471/BLT.11.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WHO. WHO global strategy for containment of antimicrobial resistance. Geneva: 2001. [Google Scholar]
- 65.Holloway K, Mathai E, Gray A. Surveillance of antimicrobial resistance in resource-constrained settings - experience from five pilot projects. Trop Med Int Health. 2011;16:368–374. doi: 10.1111/j.1365-3156.2010.02696.x. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen KV, Thi Do NT, Chandna A, Nguyen TV, Pham CV, Doan PM, et al. Antibiotic use and resistance in emerging economies: a situation analysis for Viet Nam. BMC Public Health. 2013;13:1158. doi: 10.1186/1471-2458-13-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenthal VD, Todi SK, Alvarez-Moreno C, Pawar M, Karlekar A, Zeggwagh AA, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC) Infection. 2012;40:517–526. doi: 10.1007/s15010-012-0278-x. [DOI] [PubMed] [Google Scholar]
- 68.Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004–2009. Am J Infect Control. 2012;40:396–407. doi: 10.1016/j.ajic.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 69.Rosenthal VD, Pawar M, Leblebicioglu H, Navoa-Ng JA, Villamil-Gomez W, Armas-Ruiz A, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34:415–423. doi: 10.1086/669860. [DOI] [PubMed] [Google Scholar]
- 70.Country pharmaceutical situations. Geneva: World Health Organization; 2009. [Google Scholar]
- 71.Medicines use in primary care in developing and transitional countries. Geneva: World Health Organization; 2009. [Google Scholar]
- 72.Goossens H. Antibiotic consumption and link to resistance. Clin Microbiol Infect. 2009;15(Suppl 3):12–15. doi: 10.1111/j.1469-0691.2009.02725.x. [DOI] [PubMed] [Google Scholar]
- 73.WHO. Using indicators to measure country pharmaceutical situations: Fact book on WHO Level I and Level II monitoring indicator. Geneva: 2006. [Google Scholar]
- 74.Bavestrello L, Cabello A, Casanova D. [Impact of regulatory measures in the trends of community consumption of antibiotics in Chile] Rev Med Chil. 2002;130:1265–1272. [PubMed] [Google Scholar]
- 75.Okeke IN, Peeling RW, Goossens H, Auckenthaler R, Olmsted SS, de Lavison JF, et al. Diagnostics as essential tools for containing antibacterial resistance. Drug Resist Updat. 2011;14:95–106. doi: 10.1016/j.drup.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Berkelman R, Cassell G, Specter S, Hamburg M, Klugman K. Clin Infect Dis. United States; 2006. The "Achilles heel" of global efforts to combat infectious diseases; pp. 1503–1504. [DOI] [PubMed] [Google Scholar]
- 77.Dryden MS, Cooke J, Davey P. Antibiotic stewardship--more education and regulation not more availability? J Antimicrob Chemother. 2009;64:885–888. doi: 10.1093/jac/dkp305. [DOI] [PubMed] [Google Scholar]
- 78.Seale AC, Mwaniki M, Newton CR, Berkley JA. Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis. 2009;9:428–438. doi: 10.1016/S1473-3099(09)70172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.B NJ, J RK, B WS, A AA, Eric T, Anita C, et al. Diagnostics for Global Biosurveillance: Turning Promising Science into the Tools Needed in the Field. 2011 [Google Scholar]
- 80.Caliendo AM, Gilbert DN, Ginocchio CC, Hanson KE, May L, Quinn TC, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis. 2013;57(Suppl 3):S139–S170. doi: 10.1093/cid/cit578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lubell Y, Reyburn H, Mbakilwa H, Mwangi R, Chonya K, Whitty CJ, et al. The cost-effectiveness of parasitologic diagnosis for malaria-suspected patients in an era of combination therapy. Am J Trop Med Hyg. 2007;77:128–132. [PubMed] [Google Scholar]
- 82.Bell D, Perkins MD. Making malaria testing relevant: beyond test purchase. Trans R Soc Trop Med Hyg. 2008;102:1064–1066. doi: 10.1016/j.trstmh.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 83.Iffy L. Contribution of Semmelweis to problem of puerperal fever. Am J Obstet Gynecol. 1968;102:1180–1181. doi: 10.1016/0002-9378(68)90417-1. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen D, MacLeod WB, Phung DC, Cong QT, Nguy VH, Van Nguyen H, et al. Incidence and predictors of surgical-site infections in Vietnam. Infect Control Hosp Epidemiol. 2001;22:485–492. doi: 10.1086/501938. [DOI] [PubMed] [Google Scholar]
- 85.Allegranzi B, Sax H, Bengaly L, Richet H, Minta DK, Chraiti MN, et al. Successful implementation of the World Health Organization hand hygiene improvement strategy in a referral hospital in Mali, Africa. Infect Control Hosp Epidemiol. 2010;31:133–141. doi: 10.1086/649796. [DOI] [PubMed] [Google Scholar]
- 86.Rosenthal VD, McCormick RD, Guzman S, Villamayor C, Orellano PW. Effect of education and performance feedback on handwashing: the benefit of administrative support in Argentinean hospitals. Am J Infect Control. 2003;31:85–92. doi: 10.1067/mic.2003.63. [DOI] [PubMed] [Google Scholar]
- 87.Rosenthal VD, Maki DG, Salomao R, Moreno CA, Mehta Y, Higuera F, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006;145:582–591. doi: 10.7326/0003-4819-145-8-200610170-00007. [DOI] [PubMed] [Google Scholar]
- 88.Barahona-Guzman N, Rodriguez-Calderon ME, Rosenthal VD, Olarte N, Villamil-Gomez W, Rojas C, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach in three cities of Colombia. Int J Infect Dis. 2014;19:67–73. doi: 10.1016/j.ijid.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 89.Allegranzi B, Gayet-Ageron A, Damani N, Bengaly L, McLaws ML, Moro ML, et al. Global implementation of WHO's multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13:843–851. doi: 10.1016/S1473-3099(13)70163-4. [DOI] [PubMed] [Google Scholar]
- 90.Pires dos Santos R, Jacoby T, Pires Machado D, Lisboa T, Gastal SL, Nagel FM, et al. Hand hygiene, and not ertapenem use, contributed to reduction of carbapenem-resistant Pseudomonas aeruginosa rates. Infect Control Hosp Epidemiol. 2011;32:584–590. doi: 10.1086/660100. [DOI] [PubMed] [Google Scholar]
- 91.Al-Tawfiq JA, Abed MS, Al-Yami N, Birrer RB. Promoting and sustaining a hospital-wide, multifaceted hand hygiene program resulted in significant reduction in health care-associated infections. Am J Infect Control. 2013;41:482–486. doi: 10.1016/j.ajic.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 92.Beardsley JR, Williamson JC, Johnson JW, Luther VP, Wrenn RH, Ohl CC. Show me the money: long-term financial impact of an antimicrobial stewardship program. Infect Control Hosp Epidemiol. 2012;33:398–400. doi: 10.1086/664922. [DOI] [PubMed] [Google Scholar]
- 93.Standiford HC, Chan S, Tripoli M, Weekes E, Forrest GN. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7- year program. Infect Control Hosp Epidemiol. 2012;33:338–345. doi: 10.1086/664909. [DOI] [PubMed] [Google Scholar]
- 94.van de Sande-Bruinsma N, Grundmann H, Verloo D, Tiemersma E, Monen J, Goossens H, et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14:1722–1730. doi: 10.3201/eid1411.070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis GJ, Fang X, Gooch M, Cook PP. Decreased resistance of Pseudomonas aeruginosa with restriction of ciprofloxacin in a large teaching hospital's intensive care and intermediate care units. Infect Control Hosp Epidemiol. 2012;33:368–373. doi: 10.1086/664763. [DOI] [PubMed] [Google Scholar]
- 96.Save lives: clean your hands - WHO's annual campaign. World health organization; 2014. [Google Scholar]
- 97.Head MG, Fitchett JR, Cooke MK, Wurie FB, Hayward AC, Atun R. UK investments in global infectious disease research 1997–2010: a case study. Lancet Infect Dis. 2013;13:55–64. doi: 10.1016/S1473-3099(12)70261-X. [DOI] [PubMed] [Google Scholar]
- 98.Rosenthal VD, Maki DG, Graves N. The International Nosocomial Infection Control Consortium (INICC): goals and objectives, description of surveillance methods, and operational activities. Am J Infect Control. 2008;36:e1–e12. doi: 10.1016/j.ajic.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 99.Rosenthal VD, Richtmann R, Singh S, Apisarnthanarak A, Kubler A, Viet-Hung N, et al. Surgical site infections, International Nosocomial Infection Control Consortium (INICC) report, data summary of 30 countries, 2005–2010. Infect Control Hosp Epidemiol. 2013;34:597–604. doi: 10.1086/670626. [DOI] [PubMed] [Google Scholar]
- 100.board Pr. 2013 World Population Data Sheet. USAID. 2013 [Google Scholar]
- 101.Safdar N, Anderson DJ, Braun BI, Carling P, Cohen S, Donskey C, et al. The evolving landscape of healthcare-associated infections: recent advances in prevention and a road map for research. Infect Control Hosp Epidemiol. 2014;35:480–493. doi: 10.1086/675821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Widdowson CA, Klugman KP. Molecular mechanisms of resistance to commonly used non-betalactam drugs in Streptococcus pneumoniae. Semin Respir Infect. 1999;14:255–268. [PubMed] [Google Scholar]
- 103.Labby KJ, Garneau-Tsodikova S. Strategies to overcome the action of aminoglycoside-modifying enzymes for treating resistant bacterial infections. Future Med Chem. 2013;5:1285–1309. doi: 10.4155/fmc.13.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tupin A, Gualtieri M, Roquet-Baneres F, Morichaud Z, Brodolin K, Leonetti JP. Resistance to rifampicin: at the crossroads between ecological, genomic and medical concerns. Int J Antimicrob Agents. 2010;35:519–523. doi: 10.1016/j.ijantimicag.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 105.Rossolini GM, Mantengoli E, Montagnani F, Pollini S. Epidemiology and clinical relevance of microbial resistance determinants versus anti-Gram-positive agents. Curr Opin Microbiol. 2010;13:582–588. doi: 10.1016/j.mib.2010.08.006. [DOI] [PubMed] [Google Scholar]