Abstract
N-Acetylcysteine is the drug of choice for the treatment of an acetaminophen overdose. It is thought to provide cysteine for glutathione synthesis and possibly to form an adduct directly with the toxic metabolite of acetaminophen, N-acetyl-p-benzoquinoneimine. However, these hypothese have not been tested in vivo, and other mechanisms of action such as reduction of the quinoneimine might be responsible for the clinical efficacy of N-acetylcysteine. After the administration to rats of acetaminophen (1 g/kg) intraduodenally (i.d.) and of [35S]-N-acetylcysteine (1.2 g/kg i.d.), the specific activity of the N-acetylcysteine adduct of acetaminophen (mercapturic acid) isolated from urine and assayed by high pressure liquid chromatography averaged 76±6% of the specific activity of the glutathione-acetaminophen adduct excreted in bile, indicating that virtually all N-acetylcysteine-acetaminophen originated from the metabolism of the glutathione-acetaminophen adduct rather than from a direct reaction with the toxic metabolite. N-Acetylcysteine promptly reversed the acetaminophen-induced depletion of glutathione by increasing glutathione synthesis from 0.54 to 2.69 μmol/g per h. Exogenous N-acetylcysteine did not increase the formation of the N-acetylcysteine and glutathione adducts of acetaminophen in fed rats. However, when rats were fasted before the administration of acetaminophen, thereby increasing the stress on the glutathione pool, exogenous N-acetylcysteine significantly increased the formation of the acetaminophen-glutathione adduct from 57 to 105 nmol/min per 100 g. Although the excretion of acetaminophen sulfate increased from 85±15 to 211±17 μmol/100 g per 24 h after N-acetylcysteine, kinetic simulations showed that increased sulfation does not significantly decrease formation of the toxic metabolite. Reduction of the benzoquinoneimine by N-acetylcysteine should result in the formation of N-acetylcysteine disulfides and glutathione disulfide via thiol-disulfide exchange. Acetaminophen alone depleted intracellular glutathione, and led to a progressive decrease in the biliary excretion of glutathione and glutathione disulfide. N-Acetylcysteine alone did not affect the biliary excretion of glutathione disulfide. However, when administered after acetaminophen. N-acetylcysteine produced a marked increase in the biliary excretion of glutathione disulfide from 1.2±0.3 nmol/min per 100 g in control animals to 5.7±0.8 nmol/min per 100 g. Animals treated with acetaminophen and N-acetylcysteine excreted 2.7±0.8 nmol/min per 100 g of N-acetylcysteine disulfides (measured by high performance liquid chromatography) compared to 0.4±0.1 nmol/min per 100 g in rats treated with N-acetylcysteine alone. In conclusion, exogenous N-acetylcysteine does not form significant amounts of conjugate with the reactive metabolite of acetaminophen in the rat in vivo but increases glutathione synthesis, thus providing more substrate for the detoxification of the reactive metabolite in the early phase of an acetaminophen intoxication when the critical reaction with vital macromolecules occurs.
Full text
PDF

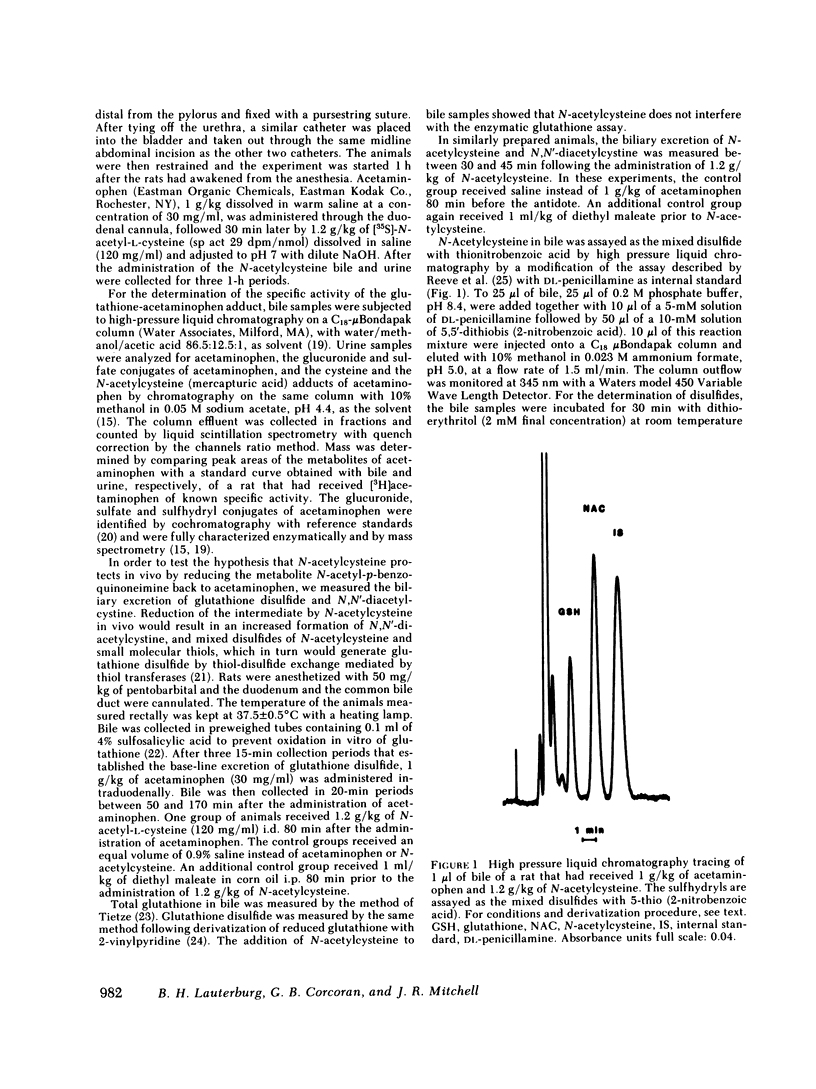

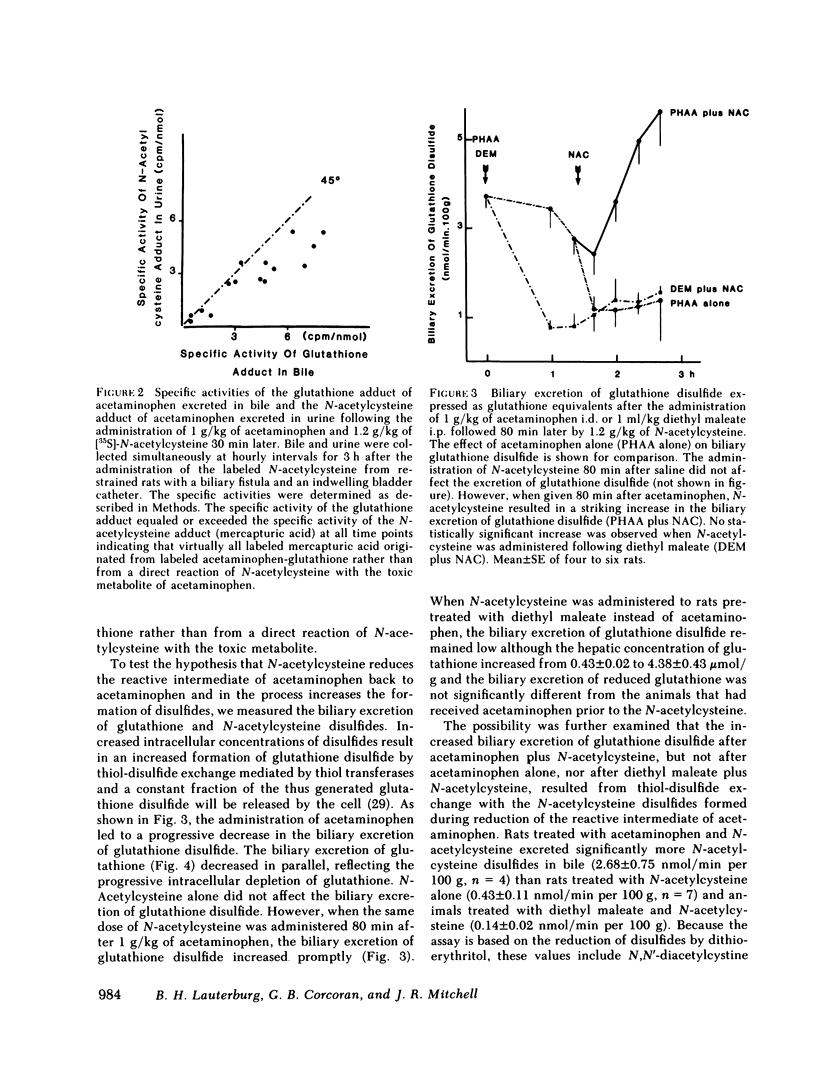

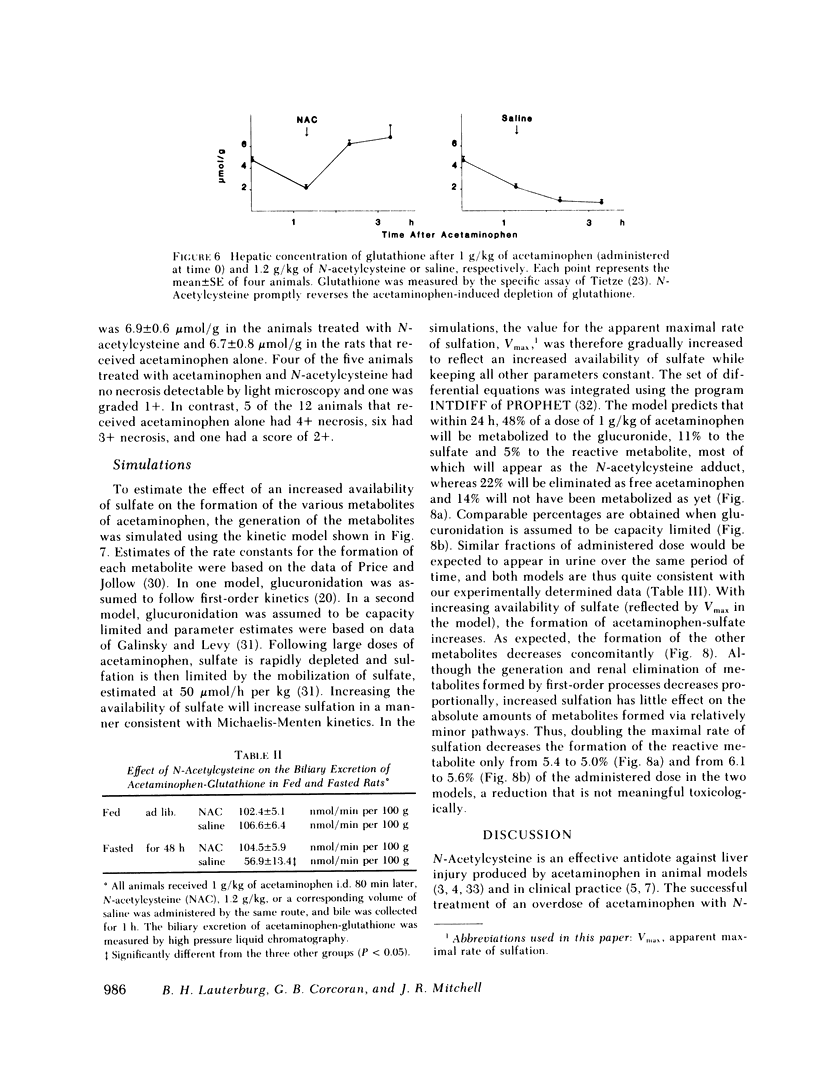
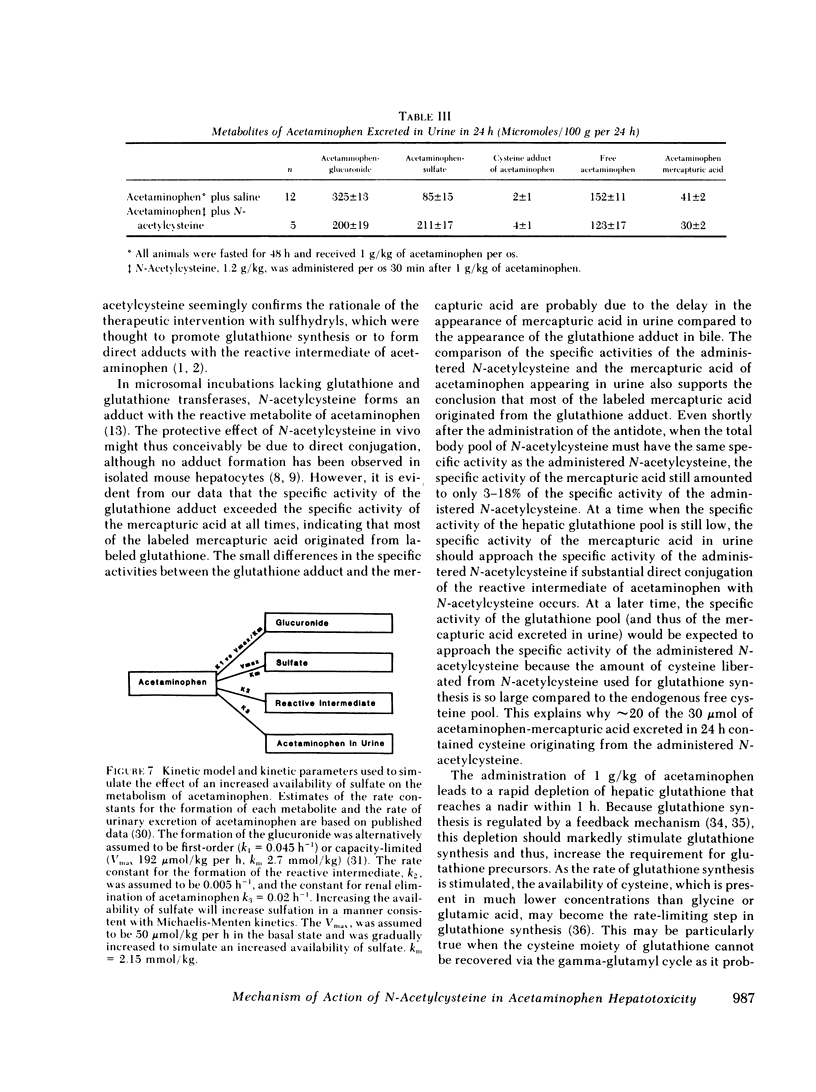



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckpitt A. R., Rollins D. E., Mitchell J. R. Varying effects of sulfhydryl nucleophiles on acetaminophen oxidation and sulfhydryl adduct formation. Biochem Pharmacol. 1979 Oct 1;28(19):2941–2946. doi: 10.1016/0006-2952(79)90590-2. [DOI] [PubMed] [Google Scholar]
- Buckpitt A. R., Rollins D. E., Nelson S. D., Franklin R. B., Mitchell J. R. Quantitative determination of the glutathione, cysteine, and N-acetyl cysteine conjugates of acetaminophen by high-pressure liquid chromatography. Anal Biochem. 1977 Nov;83(1):168–177. doi: 10.1016/0003-2697(77)90522-x. [DOI] [PubMed] [Google Scholar]
- Corcoran G. B., Mitchell J. R., Vaishnav Y. N., Horning E. C. Evidence that acetaminophen and N-hydroxyacetaminophen form a common arylating intermediate, N-acetyl-p-benzoquinoneimine. Mol Pharmacol. 1980 Nov;18(3):536–542. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Eberle D., Clarke R., Kaplowitz N. Rapid oxidation in vitro of endogenous and exogenous glutathione in bile of rats. J Biol Chem. 1981 Mar 10;256(5):2115–2117. [PubMed] [Google Scholar]
- Forrest J. A., Clements J. A., Prescott L. F. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet. 1982 Mar-Apr;7(2):93–107. doi: 10.2165/00003088-198207020-00001. [DOI] [PubMed] [Google Scholar]
- Galinsky R. E., Levy G. Dose- and time-dependent elimination of acetaminophen in rats: pharmacokinetic implications of cosubstrate depletion. J Pharmacol Exp Ther. 1981 Oct;219(1):14–20. [PubMed] [Google Scholar]
- Galinsky R. E., Levy G. Effect of N-acetylcysteine on the pharmacokinetics of acetaminophen in rats. Life Sci. 1979 Aug 20;25(8):693–699. doi: 10.1016/0024-3205(79)90511-3. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Jollow D. J., Thorgeirsson S. S., Potter W. Z., Hashimoto M., Mitchell J. R. Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology. 1974;12(4-5):251–271. doi: 10.1159/000136547. [DOI] [PubMed] [Google Scholar]
- Lake B. G., Harris R. A., Phillips J. C., Gangolli S. D. Studies on the effects of L-ascorbic acid on acetaminophen-induced hepatotoxicity. 1. Inhibition of the covalent binding of acetaminophen metabolites to hepatic microsomes in vitro. Toxicol Appl Pharmacol. 1981 Sep 15;60(2):229–240. doi: 10.1016/0041-008x(91)90227-6. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Mitchell J. R. Regulation of hepatic glutathione turnover in rats in vivo and evidence for kinetic homogeneity of the hepatic glutathione pool. J Clin Invest. 1981 May;67(5):1415–1424. doi: 10.1172/JCI110170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterburg B. H., Mitchell J. R. Toxic doses of acetaminophen suppress hepatic glutathione synthesis in rats. Hepatology. 1982 Jan-Feb;2(1):8–12. doi: 10.1002/hep.1840020103. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Vaishnav Y., Stillwell W. G., Mitchell J. R. The effects of age and glutathione depletion on hepatic glutathione turnover in vivo determined by acetaminophen probe analysis. J Pharmacol Exp Ther. 1980 Apr;213(1):54–58. [PubMed] [Google Scholar]
- Mannervik B., Axelsson K. Reduction of disulphide bonds in proteins mixed disulphides catalysed by a thioltransferase in rat liver cytosol. Biochem J. 1975 Sep;149(3):785–788. doi: 10.1042/bj1490785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey T. E., Racz W. J. Effects of N-acetylcysteine on metabolism, covalent binding, and toxicity of acetaminophen in isolated mouse hepatocytes. Toxicol Appl Pharmacol. 1981 Sep 15;60(2):220–228. doi: 10.1016/0041-008x(91)90226-5. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973 Oct;187(1):185–194. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211–217. [PubMed] [Google Scholar]
- Mitchell J. R., Thorgeirsson S. S., Potter W. Z., Jollow D. J., Keiser H. Acetaminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther. 1974 Oct;16(4):676–684. doi: 10.1002/cpt1974164676. [DOI] [PubMed] [Google Scholar]
- Peterson R. G., Rumack B. H. Treating acute acetaminophen poisoning with acetylcysteine. JAMA. 1977 May 30;237(22):2406–2407. [PubMed] [Google Scholar]
- Piperno E., Berssenbruegge D. A. Reversal of experimental paracetamol toxicosis with N-acetylcysteine. Lancet. 1976 Oct 2;2(7988):738–739. doi: 10.1016/s0140-6736(76)90030-1. [DOI] [PubMed] [Google Scholar]
- Piperno E., Mosher A. H., Berssenbruegge D. A., Winkler J. D., Smith R. B. Pathophysiology of acetaminophen overdosage toxicity: implications for management. Pediatrics. 1978 Nov;62(5 Pt 2 Suppl):880–889. [PubMed] [Google Scholar]
- Prescott L. F., Park J., Ballantyne A., Adriaenssens P., Proudfoot A. T. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977 Aug 27;2(8035):432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- Prescott L. F., Sutherland G. R., Park J., Smith I. J., Proudfoot A. T. Cysteamine, methionine, and penicillamine in the treatment of paracetamol poisoning. Lancet. 1976 Jul 17;2(7977):109–113. doi: 10.1016/s0140-6736(76)92842-7. [DOI] [PubMed] [Google Scholar]
- Price V. F., Jollow D. J. Increased resistance of diabetic rats to acetaminophen-induced hepatotoxicity. J Pharmacol Exp Ther. 1982 Mar;220(3):504–513. [PubMed] [Google Scholar]
- Reeve J., Kuhlenkamp J., Kaplowitz N. Estimation of glutathione in rat liver by reversed-phase high-performance liquid chromatography: separation from cysteine and gamma-glutamylcysteine. J Chromatogr. 1980 Jun 27;194(3):424–428. doi: 10.1016/s0021-9673(00)81435-1. [DOI] [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Rollins D. E., Buckpitt A. R. Liver cytosol catalyzed conjugation of reduced glutathione with a reactive metabolite of acetaminophen. Toxicol Appl Pharmacol. 1979 Feb;47(2):331–339. doi: 10.1016/0041-008x(79)90328-4. [DOI] [PubMed] [Google Scholar]
- Sheffner A. L., Medler E. M., Bailey K. R., Gallo D. G., Mueller A. J., Sarett H. P. Metabolic studies with acetylcysteine. Biochem Pharmacol. 1966 Oct;15(10):1523–1535. doi: 10.1016/0006-2952(66)90197-3. [DOI] [PubMed] [Google Scholar]
- Sies H., Gerstenecker C., Menzel H., Flohé L. Oxidation in the NADP system and release of GSSG from hemoglobin-free perfused rat liver during peroxidatic oxidation of glutathione by hydroperoxides. FEBS Lett. 1972 Oct 15;27(1):171–175. doi: 10.1016/0014-5793(72)80434-4. [DOI] [PubMed] [Google Scholar]
- Sies H., Summer K. H. Hydroperoxide-metabolizing systems in rat liver. Eur J Biochem. 1975 Sep 15;57(2):503–512. doi: 10.1111/j.1432-1033.1975.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Tateishi N., Higashi T., Shinya S., Naruse A., Sakamoto Y. Studies on the regulation of glutathione level in rat liver. J Biochem. 1974 Jan;75(1):93–103. doi: 10.1093/oxfordjournals.jbchem.a130387. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Viña J., Romero F. J., Estrela J. M., Viña J. R. Effect of acetaminophen (paracetamol) and its antagonists on glutathione (GSH) content in rat liver. Biochem Pharmacol. 1980 Jul 1;29(13):1968–1970. doi: 10.1016/0006-2952(80)90113-6. [DOI] [PubMed] [Google Scholar]
- Whitehouse L. W., Wong L. T., Solomonraj G., Paul C. J., Thomas B. H. N-acetylcysteine-induced inhibition of gastric emptying: a mechanism affording protection to mice from the hepatotoxicity of concomitantly administered acetaminophen. Toxicology. 1981;19(2):113–125. doi: 10.1016/0300-483x(81)90093-7. [DOI] [PubMed] [Google Scholar]



