Abstract
We have studied 5′-deiodination of thyroxine (T4) and 3,3′,5′-triiodothyronine (rT3) in rat pituitary tissue in vitro, with respect to substrate specificity, reaction kinetics, effects of 6-n-propyl-2-thiouracil (PTU), and the time course of effects of thyroid hormone depletion and repletion. Removal of one phenolic iodine or both tyrosyl iodines from the T4 molecule resulted in compounds that were not deiodinated, but alterations in the alanine side chain had little effect.
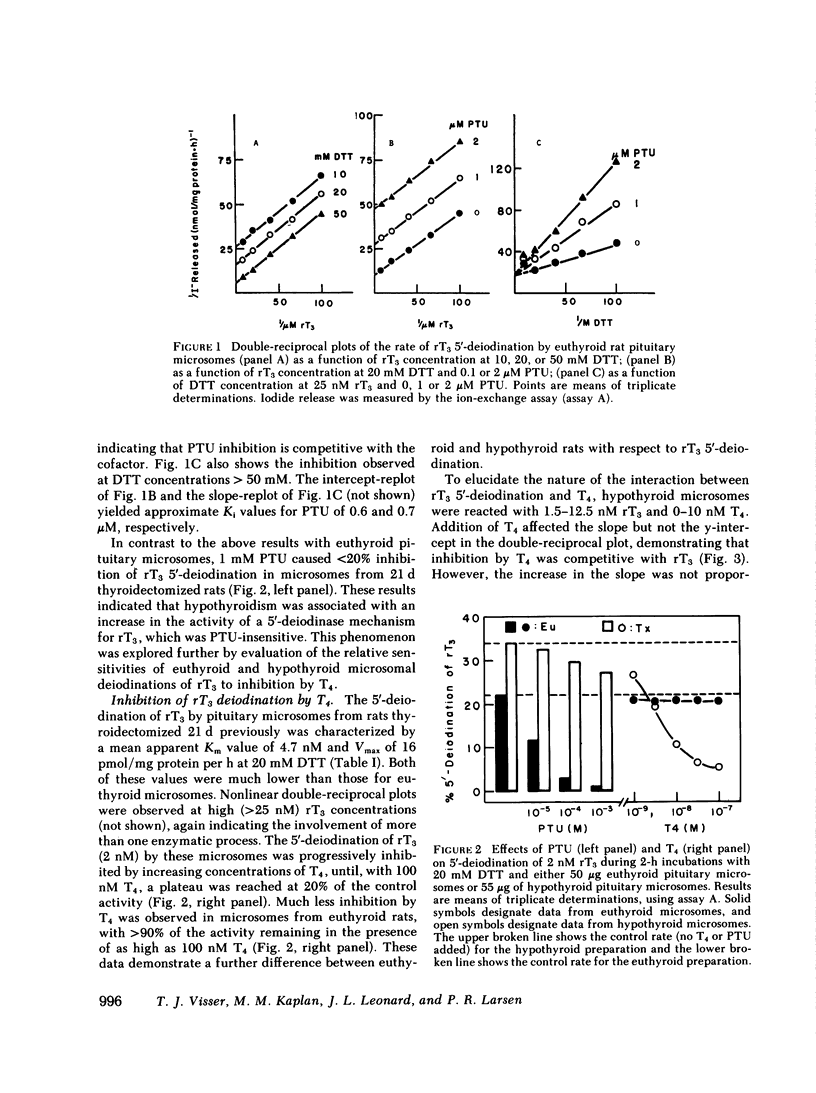
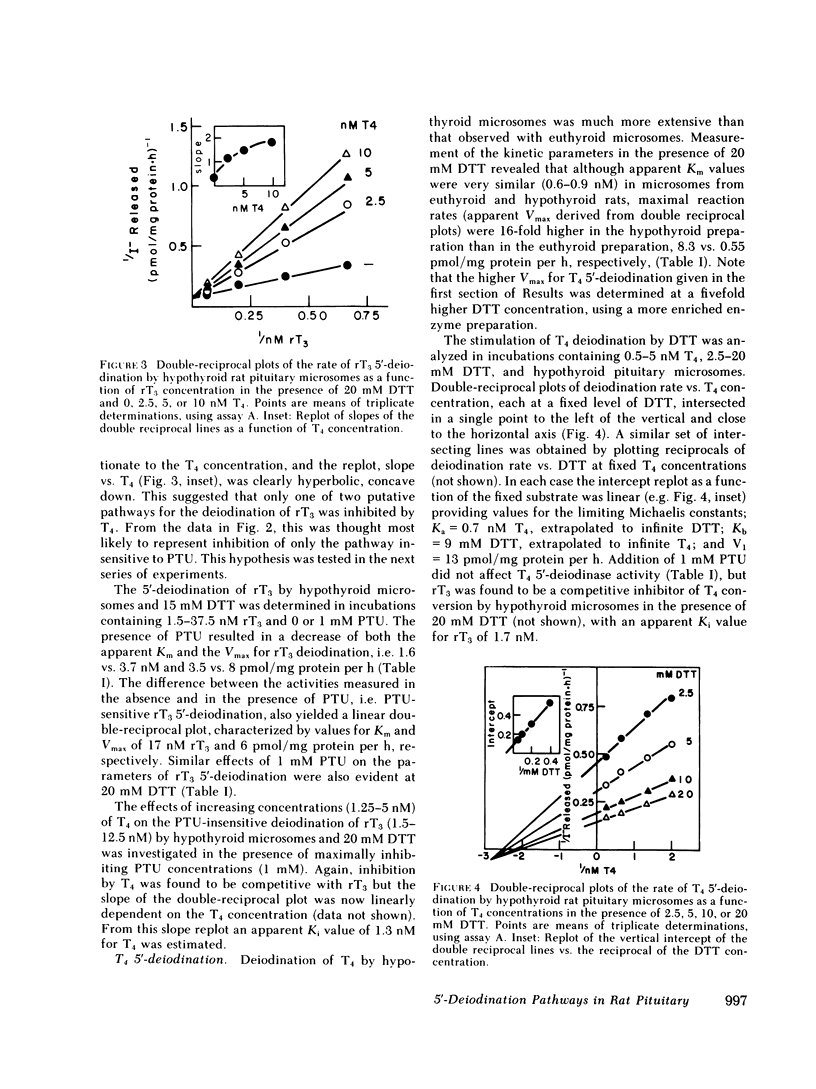
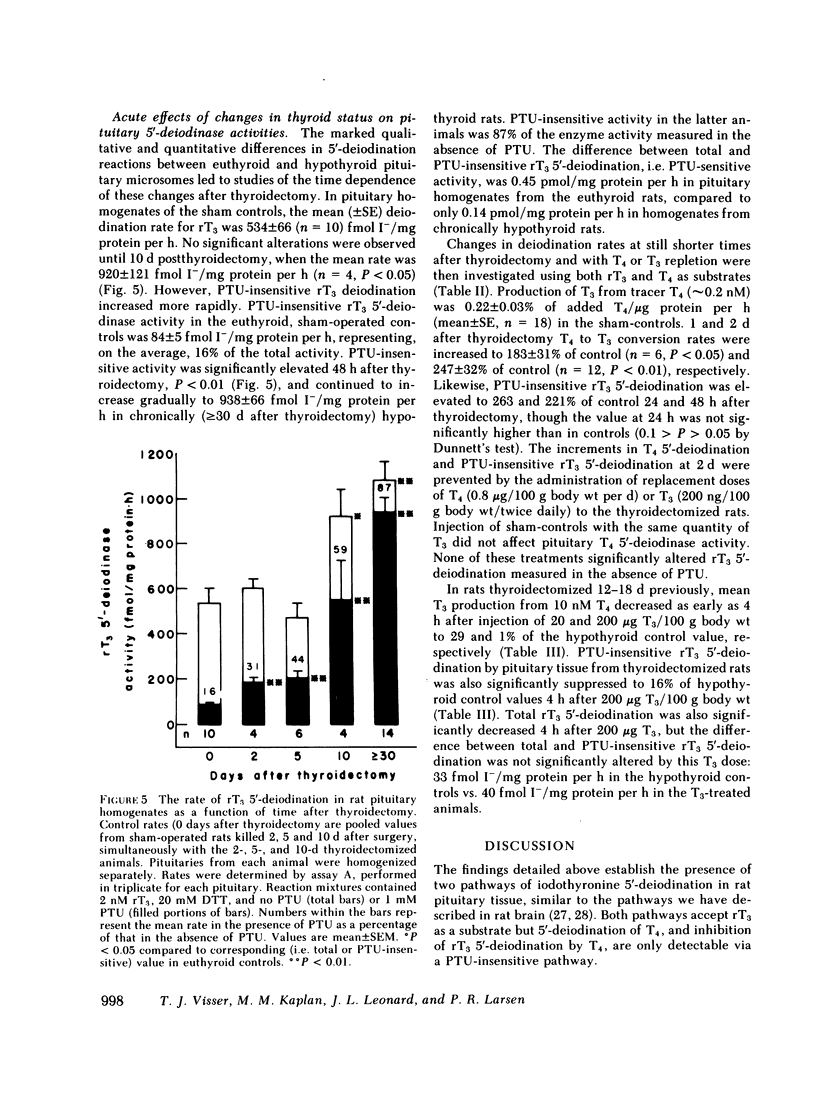
5′-Deiodination of 2 nM rT3 by pituitary microsomes from euthyroid rats was inhibited >90% by 1 mM PTU, but was inhibited <10% by 100 nM T4. The apparent Michaelis constant (Km) and maximum velocity (Vmax) for rT3 at 20 mM dithiothreitol (DTT) were 33 nM and 84 pmol/mg protein per h. This reaction followed ping-pong type reaction kinetics when concentrations of DTT were varied. PTU inhibition was competitive with DTT and uncompetitive with rT3. In contrast, when pituitary microsomes from hypothyroid rats (21 d postthyroidectomy) were used, deiodination of 2 nM rT3 was inhibited only 20% by 1 mM PTU and up to 80% by 100 nM T4. At 20 mM DTT, the apparent Km and Vmax in hypothyroid microsomes were 4.7 nM rT3 and 16 pmol/mg protein per h. T4 was a competitive inhibitor of PTU-insensitive rT3 5′-deiodination (Ki = 1.3 nM). T4 5′-deiodination by hypothyroid microsomes was not affected by PTU, was competitively inhibited by rT3 (Ki, 1.7 nM), and exhibited sequential type reaction kinetics with DTT as cosubstrate. When T4 5′-deiodination was measured in euthyroid and hypothyroid microsomes, respectively, the apparent Km and Vmax for T4 at 20 mM DTT, were 0.9 nM and 0.55 pmol/mg protein per h (euthyroid), and 0.8 nM and 6.9 pmol/mg protein per h (hypothyroid).
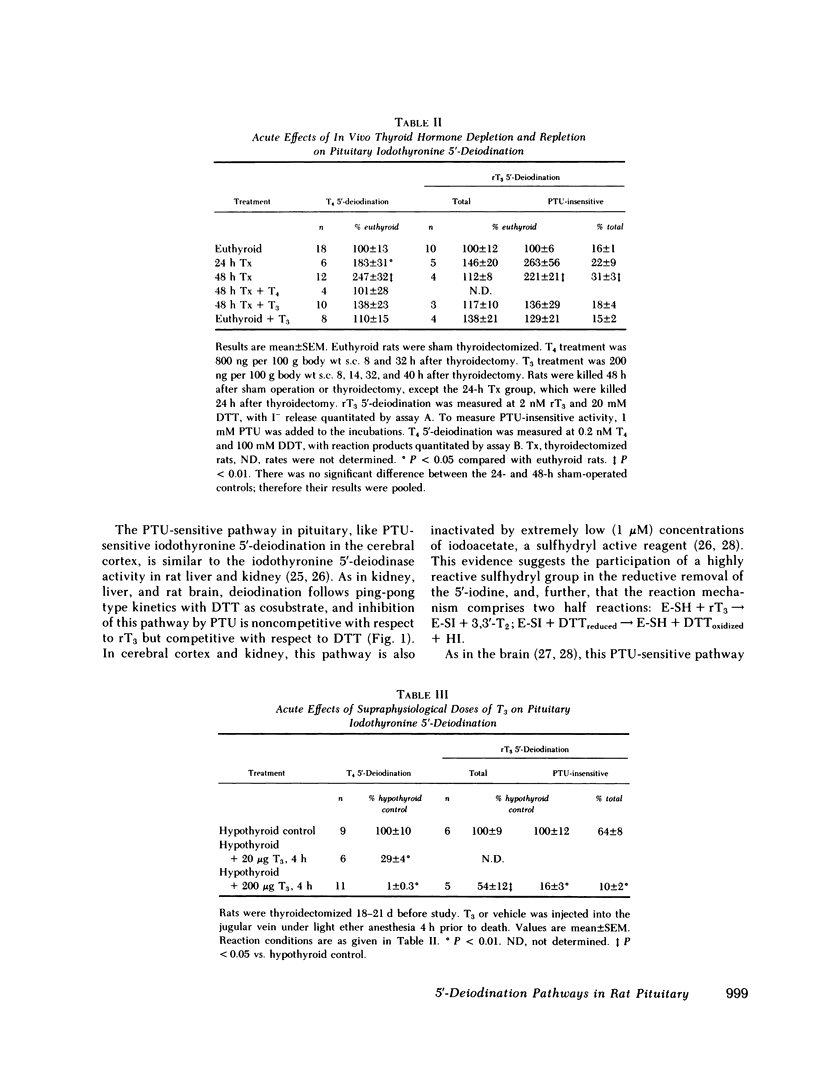
The T4 5′-deiodination rate and the PTU-insensitive, but not total, rT3 5′-deiodination rate (i.e. measured in the presence and the absence of 1 mM PTU, respectively) in pituitary homogenates were significantly elevated 24 h after thyroidectomy. PTU-insensitive activity continued to increase until at ≥30 d after thyroidectomy it was 11 times the PTU-insensitive activity in controls. At the latter time, PTU-sensitive rT3 5′-deiodinase activity appeared to be decreased. The increase in PTU-insensitive T4 and rT3 5′-deiodination observed 48 h after thyroidectomy was prevented by replacement doses of T4 or T3. The PTU-insensitive activity of long term hypothyroid pituitaries was decreased by 71% and ≥84% 4 h after injection of 20 and 200 μg T3, respectively, with no change in PTU-sensitive rT3 deiodination.
These data show that rat pituitary tissue contains two distinct iodothyronine 5′-deiodinating pathways that differ with respect to substrate specificity, PTU sensitivity, reaction kinetics, and regulation by thyroid hormone. One of these resembles the 5′-deiodinase of liver and kidney, and predominates in euthyroid pituitary tissue in vitro. The other, also found in rat brain, predominates in hypothyroid pituitary tissue, is rapidly responsive to changes in thyroid hormone availability, and, as judged by previous, in vivo studies, appears to account for all the T3 produced locally in the pituitary and, thereby, 50% of the intracellular T3 in this tissue.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheron R. G., Kaplan M. M., Larsen P. R. Physiological and pharmacological influences on thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat anterior pituitary. J Clin Invest. 1979 Nov;64(5):1402–1414. doi: 10.1172/JCI109598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conversion of thyroxine into tri-iodothyronine by rat liver homogenate. Biochem J. 1975 Sep;150(3):489–493. doi: 10.1042/bj1500489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crantz F. R., Larsen P. R. Rapid thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat cerebral cortex and cerebellum. J Clin Invest. 1980 Apr;65(4):935–938. doi: 10.1172/JCI109749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zaheri M. M., Braverman L. E., Vagenakis A. G. Enhanced conversion of thyroxine to triiodothyronine by the neonatal rat pituitary. Endocrinology. 1980 Jun;106(6):1735–1739. doi: 10.1210/endo-106-6-1735. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Baxter J. D., Morris J. A. Interaction of thyroid and glucocorticoid hormones in rat pituitary tumor cells. Specificity and diversity of the responses analyzed by two-dimensional gel electrophoresis. J Biol Chem. 1981 May 10;256(9):4520–4528. [PubMed] [Google Scholar]
- Kaplan M. M. Thyroxine 5'-monodeiodination in rat anterior pituitary homogenates. Endocrinology. 1980 Feb;106(2):567–576. doi: 10.1210/endo-106-2-567. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in liver and kidney homogenates from hyperthyroid and hypothyroid rats. Endocrinology. 1978 Jul;103(1):156–161. doi: 10.1210/endo-103-1-156. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J Clin Invest. 1980 Sep;66(3):551–562. doi: 10.1172/JCI109887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumara-Siri M. H., Lee K., Surks M. I. Regulation of thyrotropin secretion in rats bearing the Walker 256 carcinoma. Endocrinology. 1981 Nov;109(5):1760–1768. doi: 10.1210/endo-109-5-1760. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Dick T. E., Markovitz B. P., Kaplan M. M., Gard T. G. Inhibition of intrapituitary thyroxine to 3.5.3'-triiodothyronine conversion prevents the acute suppression of thyrotropin release by thyroxine in hypothyroid rats. J Clin Invest. 1979 Jul;64(1):117–128. doi: 10.1172/JCI109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Silva J. E., Kaplan M. M. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev. 1981 Winter;2(1):87–102. doi: 10.1210/edrv-2-1-87. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Kaplan M. M., Visser T. J., Silva J. E., Larsen P. R. Cerebral cortex responds rapidly to thyroid hormones. Science. 1981 Oct 30;214(4520):571–573. doi: 10.1126/science.7291997. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rennke H., Kaplan M. M., Larsen P. R. Subcellular distribution of iodothyronine 5'-deiodinase in cerebral cortex from hypothyroid rats. Biochim Biophys Acta. 1982 Sep 17;718(1):109–119. doi: 10.1016/0304-4165(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Iodothyronine 5'-deiodinase from rat kidney: substrate specificity and the 5'-deiodination of reverse triiodothyronine. Endocrinology. 1980 Nov;107(5):1376–1383. doi: 10.1210/endo-107-5-1376. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Thyroxine 5'-deiodinase activity of rat kidney: observations on activation by thiols and inhibition by propylthiouracil. Endocrinology. 1978 Dec;103(6):2137–2144. doi: 10.1210/endo-103-6-2137. [DOI] [PubMed] [Google Scholar]
- Maeda M., Ingbar S. H. Effect of alterations in thyroid status on the metabolism of thyroxine and triiodothyronine by rat pituitary gland in vitro. J Clin Invest. 1982 Apr;69(4):799–808. doi: 10.1172/JCI110519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S., Kurtzman G., Reed A., Hershman J. M. Non-thyrotropic pituitary cells in culture convert T4 to T3. Life Sci. 1979 May 21;24(21):1947–1952. doi: 10.1016/0024-3205(79)90304-7. [DOI] [PubMed] [Google Scholar]
- Melmed S., Nelson M., Kaplowitz N., Yamada T., Hershman J. M. Glutathione-dependent thyroxine 5'-monodeiodination modulates growth hormone production by cultured nonthyrotropic rat pituitary cells. Endocrinology. 1981 Mar;108(3):970–976. doi: 10.1210/endo-108-3-970. [DOI] [PubMed] [Google Scholar]
- Obregon M. J., Pascual A., Mallol J., Morreale de Escobar G., Escobar del Rey F. Evidence against a major role of L-thyroxine at the pituitary level: studies in rats treated with iopanoic acid (telepaque). Endocrinology. 1980 Jun;106(6):1827–1836. doi: 10.1210/endo-106-6-1827. [DOI] [PubMed] [Google Scholar]
- Rosenthal H. E. A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem. 1967 Sep;20(3):525–532. doi: 10.1016/0003-2697(67)90297-7. [DOI] [PubMed] [Google Scholar]
- Seo H., Vassart G., Brocas H., Refetoff S. Triiodothyronine stimulates specifically growth hormone mRNA in rat pituitary tumor cells. Proc Natl Acad Sci U S A. 1977 May;74(5):2054–2058. doi: 10.1073/pnas.74.5.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Kaplan M. M., Cheron R. G., Dick T. E., Larsen P. R. Thyroxine to 3,5,3'-triiodothyronine conversion by rat anterior pituitary and liver. Metabolism. 1978 Nov;27(11):1601–1607. doi: 10.1016/0026-0495(78)90282-2. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Peripheral metabolism of homologous thyrotropin in euthyroid and hypothyroid rats: acute effects of thyrotropin-releasing hormone, triiodothyronine, and thyroxine. Endocrinology. 1978 Jun;102(6):1783–1796. doi: 10.1210/endo-102-6-1783. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Leonard J. L., Crantz F. R., Larsen P. R. Evidence for two tissue-specific pathways for in vivo thyroxine 5'-deiodination in the rat. J Clin Invest. 1982 May;69(5):1176–1184. doi: 10.1172/JCI110554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Fekkes D., Docter R., Hennemann G. Kinetics of enzymic reductive deiodination of iodothyronines. Effect of pH. Biochem J. 1979 Jun 1;179(3):489–495. doi: 10.1042/bj1790489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Leonard J. L., Kaplan M. M., Larsen P. R. Different pathways of iodothyronine 5'-deiodination in rat cerebral cortex. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1297–1304. doi: 10.1016/0006-291x(81)91588-6. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Leonard J. L., Kaplan M. M., Larsen P. R. Kinetic evidence suggesting two mechanisms for iodothyronine 5'-deiodination in rat cerebral cortex. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5080–5084. doi: 10.1073/pnas.79.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke J., Orskov H. Synthesis of 125I monolabelled 3, 5, 3'-triiodothyronine and thyroxine of maximum specific activity for radioimmunoassay. Scand J Clin Lab Invest. 1973 Dec;32(4):357–360. doi: 10.3109/00365517309084359. [DOI] [PubMed] [Google Scholar]


