SUMMARY
The adipocyte hormone leptin has potent beneficial effects on glucose metabolism via actions in the arcuate nucleus of the hypothalamus (ARC). However, the requirement of specific subgroups of neurons within the ARC in mediating leptin's anti-diabetic actions is unknown. Here we generated diabetic Lepob/ob or Leprdb/db mice lacking or re-expressing leptin receptors only in selected subgroups of neurons to test the sufficiency and requirement of these neurons in the glucose-lowering effects of leptin. Our results show that agouti-related peptide (AgRP)-expressing neurons are both required and sufficient to normalize serum glucose levels by leptin. Leptin receptors in pro-opiomelanocortin (POMC) neurons or SF1 neurons are not required. Furthermore, glucose normalization by leptin is blunted in diabetic Lepob/ob;MC4R-null mice, but not in Lepob/ob mice lacking NPY or GABA in AgRP neurons. Collectively, our data suggests that AgRP neurons play a key role in mediate the glucose-lowering actions of leptin and that these beneficial actions require the melanocortin system, but not NPY and GABA.
INTRODUCTION
The adipocyte hormone leptin, besides its well-known effects on body weight, also plays a key role in the control of glucose homeostasis. Lepob/ob and Leprdb/db mice that lack leptin or its receptors, respectively, are severely hyperglycemic and hyperinsulinemic (Chen et al., 1996; Coleman, 1978; Lee et al., 1996; Spiegelman and Flier, 2001; Zhang et al., 1994). Although this impairment in serum glucose balance could be secondary to the massive obesity observed in those mice, several studies support the possibility that the glucose-lowering actions of leptin are independent of its effects on body weight (Hedbacker et al., 2010; Pelleymounter et al., 1995; Schwartz et al., 1996). In addition, leptin therapy is effective to improve insulin sensitivity in mice and humans suffering from lipodystrophy (Oral et al., 2002; Petersen at al., 2002; Shimomura et al., 1999). Rodent studies suggest that these beneficial effects of leptin in control of glucose metabolism are mediated mainly by its actions in the brain, more specifically by its action in the hypothalamus (Coppari and Bjorbaek, 2012).
The arcuate nucleus of the hypothalamus (ARC) have been shown to play a pivotal role in mediating the anti-diabetic effects of leptin because restoration of leptin receptor (LEPR) expression only in ARC neurons of the otherwise very diabetic LEPR-deficient animals is sufficient to fully normalize the circulating glucose levels (Coppari et al., 2005). The ARC has two major subsets of neurons that express leptin receptors with opposite effects in the control of energy homeostasis: pro-opiomelanocortin (POMC) neurons that release α-melanocyte stimulating hormone (α-MSH), an anorexigenic neuropeptide that activates the melanocortin receptor 3 and 4 (MC3R and MC4R), and agouti-related peptide (AgRP)-expressing neurons that in addition of being GABAergic, also releases the orexigenic neuropeptides AgRP (an antagonist of MC3R and MC4R) and neuropeptide Y (NPY) (Cone et al., 2005; Horvath et al., 1997; Ollmann et al., 1997; Schwartz et al., 2000; Vong et al., 2011). Leptin promotes its effects on body weight partly by activating POMC neurons and inhibiting AgRP neurons (Balthasar et al., 2004; Cowley et al., 2001; van den Top et al., 2004; van de Wall et al., 2008; Huang et al., 2013). Recent evidence has demonstrated that POMC neurons can serve a sufficient role in mediating anti-diabetic effects of leptin: re-expression of the long signaling form of the leptin receptor, LepRb, only in POMC neurons normalized the severe hyperglycemia of hyperleptinemic Leprdb/db animals (Huo et al., 2009). However, the requirement of POMC neurons in diabetic mice with physiological serum leptin levels has not been investigated. In addition, animals that lack leptin receptors only in POMC neurons are normoglycemic (Balthasar et al., 2004). These data raise the possibility that other subsets of neurons might also play a critical role in mediating the beneficial glucose-lowering effects of leptin.
Of the diverse subgroups of neurons that express leptin receptors within the hypothalamus, two major populations could be potential targets of leptin to mediate its anti-diabetic actions: 1) Arcuate AgRP neurons; for example, AgRP neurons were likely targeted in LEPR-deficient mice where virally-mediated re-expression of LEPRs in the ARC corrected diabetes (Coppari et al., 2005); 2) neurons within the ventromedial nucleus of the hypothalamus (VMH); leptin microinjection into the VMH increases glucose uptake in peripheral tissues (Minokoshi et al., 1999). Also, it has been reported that intra-VMH leptin injections can rescue diabetes in STZ-diabetic rats (Meek et al., 2013).
To investigate the potential role of these different subsets of neurons in leptin's anti-diabetic actions in both high and low physiological levels of leptin, we generated different diabetic mouse models lacking or re-expressing leptin receptors only in selected subgroups of hypothalamic neurons known to be activated by leptin. In addition, to identify down-stream molecular mediators, we developed diabetic animal models that lack central nervous system (CNS) MC4Rs or NPY, or GABA release from AgRP neurons. Collectively, our data show that AgRP neurons, but not VMN or POMC neurons, are both sufficient and required to mediate the glucose-lowering action of leptin and that this effect requires the intact melanocortin system, but not NPY or GABA.
RESULTS
Sufficiency of AgRP neurons, but not SF1 neurons, in mediating leptin's anti-diabetic actions
To determine if AgRP or SF1 neurons of the hypothalamus are sufficient in mediating leptin's anti-diabetic actions, we generated Leprdb/db mice that only express LepRb in AgRP (HA-LepRbflox/AgRP-ires-cre/Leprdb/db) or SF1 (HALepRbflox/SF1-cre/Leprdb/db) neurons using our HA-LepRb flox transgenic mice which allow expression of LepRb in a cre-dependet manner (Huo et al., 2009). First, to verify the specificity of LepRb expression, we measured leptin-dependent STAT3 phosphorylation in the brain of those mice. As expected, STAT3 phosphorylation was only found in the ARC of the HA-LepRbflox/AgRP-ires-cre/Leprdb/db mice consistent with the known anatomical localization of AgRP neurons (Figure S1). In contrast, STAT3 phosphorylation was detected as expected in the ventromedial nucleus of the hypothalamus of HA-LepRbflox/SF1-cre/Leprdb/db mice (Figure S1). No STAT3 phosphorylation was found in brains of leptin-treated HA-LepRbflox/Leprdb/db control mice (Figure S1).
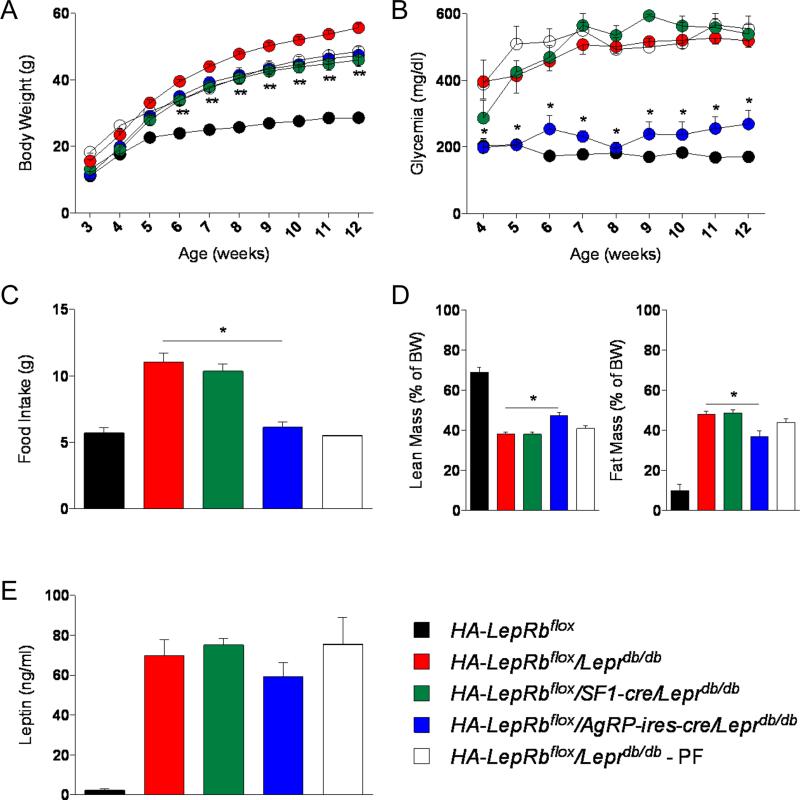
Both HA-LepRbflox/AgRP-ires-cre/Leprdb/db and HA-LepRbflox/SF1-cre/Leprdb/db male mice were slightly leaner compared to HA-LepRbflox/Leprdb/db control mice (Figure 1A). Surprisingly, despite being massively obese, food intake of HA-LepRbflox/AgRP-ires-cre/Leprdb/db mice was reduced to wild-type levels (Figure 1C). HA-LepRbflox/AgRP-ires-cre/Leprdb/db mice also had slightly reduced fat mass compared to Leprdb/db mice (Figure 1D). No effect on food intake or body composition was observed in HA-LepRbflox/SF1-cre/Leprdb/db mice compared to HA-LepRbflox/Leprdb/db mice (Figure 1C and 1D). Remarkably, expression of HA-LepRb only in AgRP neurons, but not in SF1 neurons, rescued the severe diabetes observed in the male HA-LepRbflox/Leprdb/db mice (Figure 1B). Similar results were found in HA-LepRbflox/AgRP-ires-cre/Leprdb/db females (Figure S2). To test if this normalization of the glucose levels could be secondary to the reduced food intake observed in the HA-LepRbflox/AgRP-ires-cre/Leprdb/db mice, we pair-fed (PF) HA-LepRbflox/Leprdb/db control mice to the food intake level of wild-type mice between 5 to 12 weeks of age. As shown in the figure 1B, the markedly reduced food intake of HA-LepRbflox/Leprdb/db - PF mice over the period of 7 weeks did not affect the severe hyperglycemia compared to that observed in the ad libitum HA-LepRbflox/Leprdb/db mice, suggesting that the normalization of blood glucose levels found in the HA-LepRbflox/AgRP-ires-cre/Leprdb/db mice was independent of their reduced food intake. These data suggest that AgRP neurons, but not SF1 neurons, are sufficient to mediate leptin's anti-diabetic actions, similar to what we have reported earlier for POMC neurons (Huo et al., 2009).
Figure 1. LepRbs in AgRP neurons, but not in SF1 neurons, are sufficient in mediating leptin's anti-diabetic actions.
(A-E) Body weight (A), glycemia (B), food intake (9 weeks of age) (C), body composition (10-12 weeks of age) (D) and serum leptin levels (E) in HA-LepRbflox, HA-LepRbflox/Leprdb/db, HA-LepRbflox/SF1-cre/Leprdb/db, HA-LepRbflox/AgRP-ires-cre/Leprdb/db and HA-LepRbflox/Leprdb/db - PF male mice. PF, pair-fed (5-12 weeks) to lean control mice. Errors bars are shown as SEM (n = 3-8/group). Statistical analyses were done using one-way or two-way ANOVA (Bonferroni post-hoc analyses). *p < 0.05 HA-LepRbflox/AgRP-ires-cre/Leprdb/db versus HA-LepRbflox/Leprdb/db mice; **p < 0.05 HA-LepRbflox/SF1-cre/Leprdb/db or HA-LepRbflox/AgRP-ires-cre/Leprdb/db versus HA-LepRbflox/Leprdb/db mice. See also Figure S1, S2 and S3.
Requirement of AgRP neurons, but not POMC or SF1 neurons, in mediating leptin's anti-diabetic actions
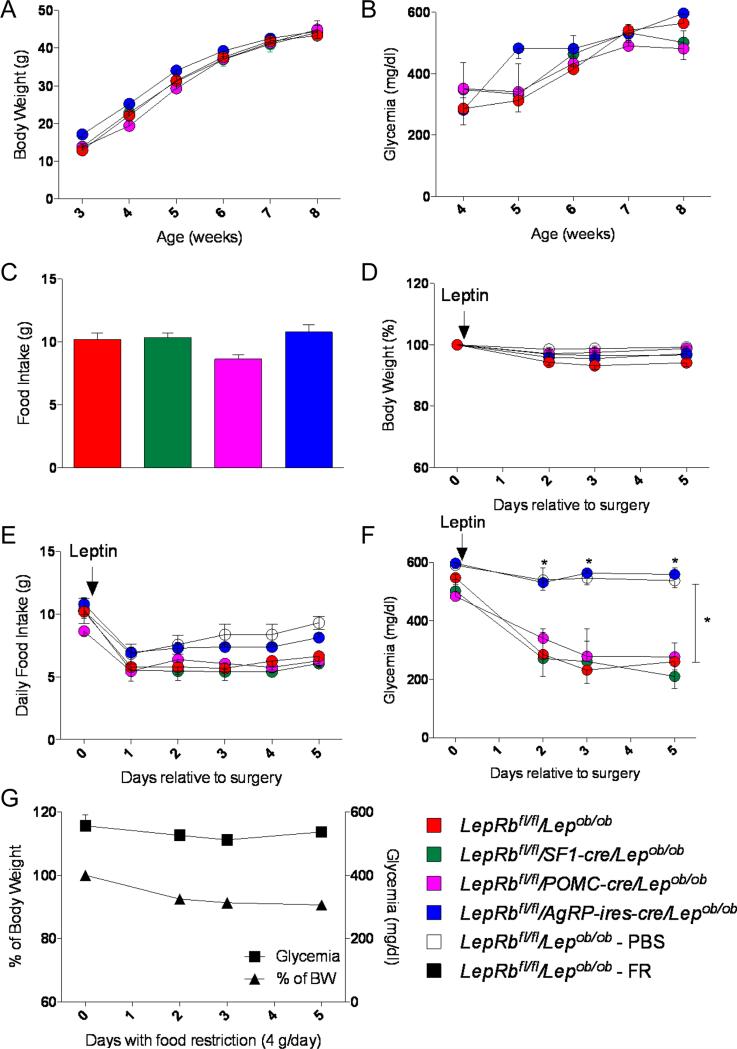
Next, to test the requirement of the different subsets of neurons in mediating leptin's anti-diabetic actions, we generated diabetic Lepob/ob mice lacking LepRb selectively in AgRP neurons (LepRbfl/fl/AgRP-ires-cre/Lepob/ob mice), POMC neurons (LepRbfl/fl/POMC-cre/Lepob/ob mice) or SF1 neurons (LepRbfl/fl/SF1-cre/Lepob/ob mice). As expected, LepRbfl/fl/AgRP-ires-cre/Lepob/ob, LepRbfl/fl/POMC-cre/Lepob/ob and LepRbfl/fl/SF1-cre/Lepob/ob were very hyperglycemic to the same extent, and had similar body weight curves and food intake compared to LepRbfl/fl/Lepob/ob control mice (Figures 2A-C).
Figure 2. LepRbs in AgRP neurons, but not in SF1 or POMC neurons, are required in mediating leptin's anti-diabetic actions.
(A-C) Body weight (A), glycemia (B) and food Intake (7 weeks of age) (C) in LepRbfl/fl/Lepob/ob, LepRbfl/fl/AgRP-ires-cre/Lepob/ob, LepRbfl/fl/POMC-cre/Lepob/ob and LepRbfl/fl/SF1-cre/Lepob/ob male mice. (D-F) Leptin infusion for 5 days: percent of initial body weight (D), daily food intake (E) and glycemia (F) in LepRbfl/fl/Lepob/ob - PBS (placebo group), LepRbfl/fl/Lepob/ob, LepRbfl/fl/AgRP-ires-cre/Lepob/ob, LepRbfl/fl/POMC-cre/Lepob/ob and LepRbfl/fl/SF1-cre/Lepob/ob male mice. (G) Mice were food restricted (FR) to 4 g of food/day: percent of initial body weight and glycemia in LepRbfl/fl/Lepob/ob - FR male mice. Errors bars are shown as SEM (n = 3-11/group). Statistical analyses were done using two-way ANOVA (Bonferroni post-hoc analyses). *p < 0.05 versus LepRbfl/fl/Lepob/ob mice. See also Figure S4.
To determine whether these different neuronal subgroups are required in mediating leptin's anti-diabetic actions, we implanted subcutaneously osmotic pumps delivering a low dose of leptin (25 ng/hr). This dose has been previously determined to improve glucose levels of diabetic Lepob/ob mice (Hedbacker et al., 2010). Also, we show that this rate of leptin delivery results in serum leptin levels that are comparable to that of normal wild-type mice (wt) (Figure S4). After mini-pump implantation (day 0), body weights, food intake and serum glucose concentrations were recorded.
As showed in Figure 2D, leptin treatment did not affect body weight, but caused a modest reduction of food intake in all groups, relative to mice receiving PBS (Figure 2E). As expected, leptin treatment normalized glucose levels of LepRbfl/fl/Lepob/ob control mice after 2-3 days (Figure 2F). In addition, leptin also fully corrected glycemia of LepRbfl/fl/POMC-cre/Lepob/ob and LepRbfl/fl/SF1-cre/Lepob/ob mice (Figure 2F). In contrast and remarkably, leptin did not have any effect on glucose levels of LepRbfl/fl/AgRP-ires-cre/Lepob/ob mice (Figure 2F). To rule out that the slightly reduced food intake in the LepRbfl/fl/Lepob/ob, LepRbfl/fl/POMC-cre/Lepob/ob and LepRbfl/fl/SF1-cre/Lepob/ob mice relative to the LepRbfl/fl/AgRP-ires-cre/Lepob/ob mice could affect the glycemic results, we food-restricted a new group of diabetic LepRbfl/fl/Lepob/ob mice to 4 grams/day (less than the daily food intake of any of the other groups) during 5 consecutive days. We found that this level of food restriction had no effect on lowering blood glucose levels (Figure 2G). Collectively, these data suggest that AgRP neurons, but not POMC or SF1 neurons, are required in mediating leptin's anti-diabetic actions.
Requirement of the melanocortin system on leptin's anti-diabetic actions
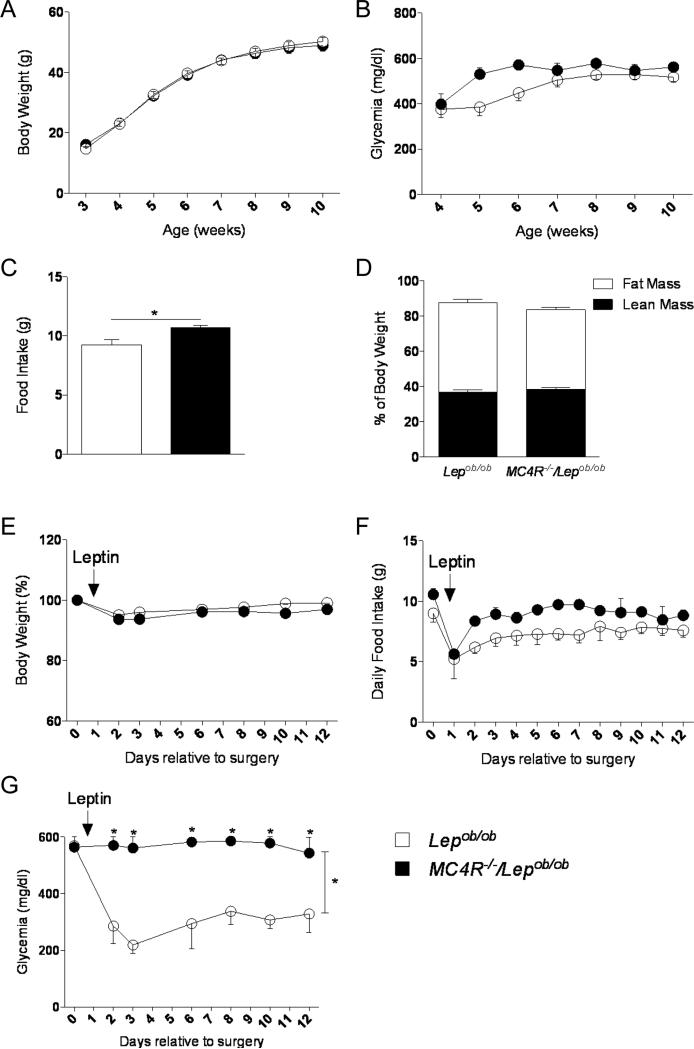
We next aimed to identify the downstream effectors of AgRP neurons responsible for leptin's anti-diabetic actions. First, we addressed the role of the AgRP peptide indirectly (Krashes et al., 2013) by generating diabetic Lepob/ob mice that lack MC4Rs (MC4R−/−/Lepob/ob mice) and by evaluating leptin-responsiveness. Deletion of MC4R in MC4R−/−/Lepob/ob mice did not affect body weight, blood glucose or body composition compared to control Lepob/ob mice (Figures 3A, 3B and 3D). However, despite having similar body weights, MC4R−/−/Lepob/ob mice ate slightly more than Lepob/ob mice (Figure 3C). To assess leptin's effects on glucose levels, we implanted subcutaneously osmotic pumps (day 0) with leptin (25 ng/h) for 12 days. As expected, body weight and food intake was similar in MC4R−/−/Lepob/ob and Lepob/ob mice (Figures 3E and 3F). Notably, glucose improvement by leptin was entirely blunted in diabetic MC4R−/−/Lepob/ob mice compared to the normalization observed Lepob/ob control mice (Figure 3G). These data show that leptin's beneficial action on glucose metabolism requires an intact melanocortin effector system.
Figure 3. Melanocortin receptors 4 (MC4Rs) are required for leptin's anti-diabetic actions.
(A-D) Body weight (A), glycemia (B), food Intake (9 weeks of age) (C) and body composition (9-10 weeks of age) (D) in Lepob/ob and MC4R−/−/Lepob/ob male mice. (E-G) Leptin infusion for 12 days at 10 weeks of age: percent of initial body weight (E), daily food intake (F) and glycemia (G) in Lepob/ob and MC4R−/−/Lepob/ob male mice. Errors bars are shown as SEM (n = 3-13/group). Statistical analyses were done using Student's t test or two-way ANOVA (Bonferroni post-hoc analyses). *p < 0.05 versus Lepob/ob mice.
NPY does not play a role on leptin's anti-diabetic actions
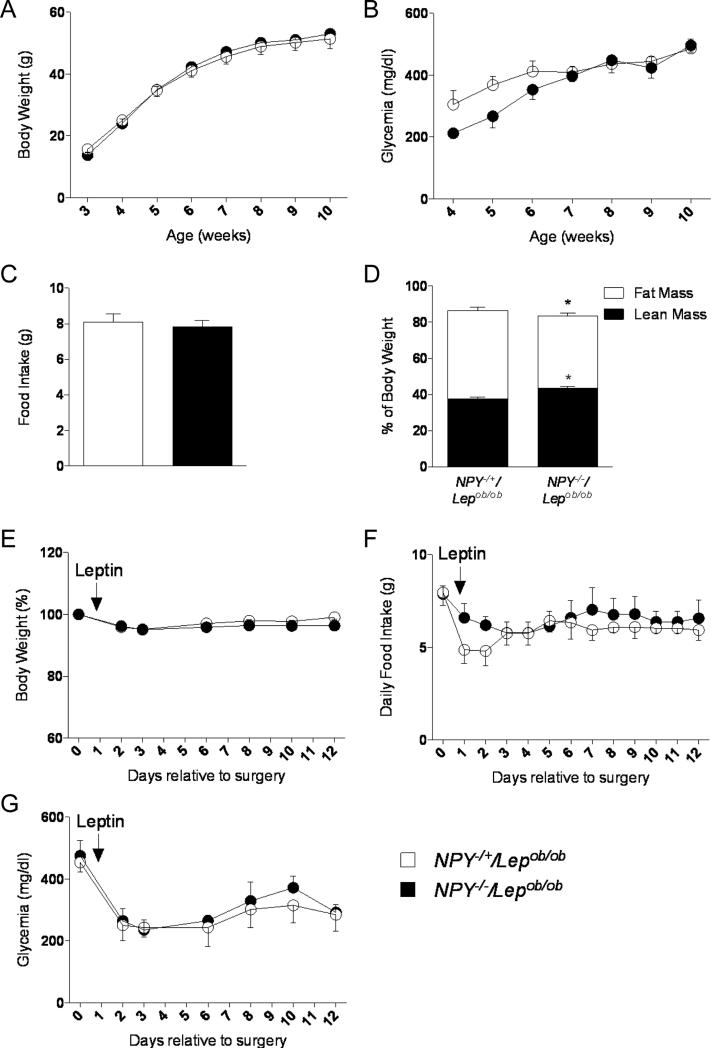
To determine whether NPY, a neuropeptide also known to be released by AgRP neurons (Hahn et al., 1998), plays a potential role in the glucose-lowering actions of leptin, we created diabetic Lepob/ob mice lacking NPY (NPY−/−/Lepob/ob mice). NPY−/−/Lepob/ob mice showed similar body weight curves, food intake and hyperglycemia as the control NPY−/+/Lepob/ob mice (Figures 4A, 4B and 4C). A slight increase in fat mass percentage was observed in NPY−/−/Lepob/ob mice compared to NPY−/+/Lepob/ob control mice (Figure 4D). Using similar approach as described above, we tested the effects of a low dose of leptin (25 ng/hr) using osmotic pumps. Again, this low dose of leptin did not significantly affect body weight or food intake (Figures 4E and 4F). In contrast, leptin treatment was able to fully ameliorate the diabetes of both study NPY−/−/Lepob/ob and control NPY−/+/Lepob/ob mice (Figure 4G). These results show that NPY is not required in the development of hyperglycemia observed in leptin-deficient mice and that it does not play a role in leptin's anti-diabetic actions in this model.
Figure 4. NPY is not required for leptin's anti-diabetic actions.
(A-D) Body weight (A), glycemia (B), food Intake (9 weeks of age) (C) and body composition (9-10 weeks of age) (D) in NPY+/−/ Lepob/ob and NPY−/−/ Lepob/ob male mice. (E-G) Leptin infusion for 12 days at 10 weeks of age: percent of initial body weight (E), daily food intake (F) and glycemia (G) in NPY+/−/Lepob/ob and NPY−/−/ Lepob/ob male mice. Errors bars are shown as SEM (n = 3-7/group). Statistical analyses were done using Student's t test. *p < 0.05 versus NPY+/−/Lepob/ob mice.
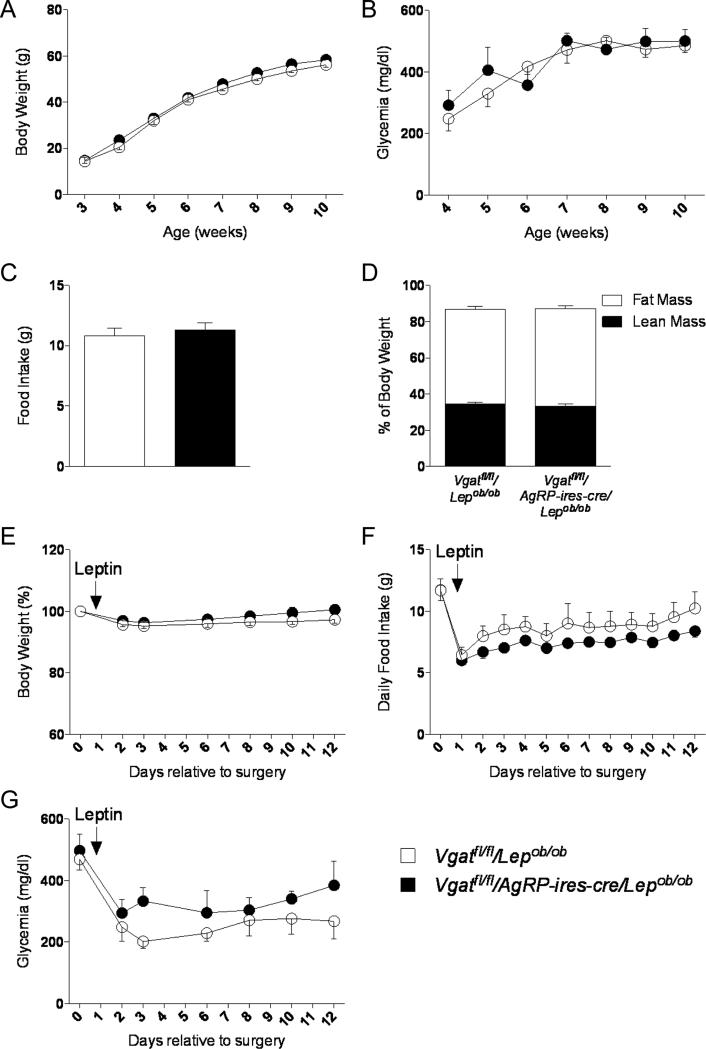
GABA released by AgRP neurons are not required for leptin's anti-diabetic actions
Finally, we investigated whether the neurotransmitter GABA, which is also released by AgRP neurons (Horvath et al., 1997; Vong et al., 2011), might influence glucose metabolism in Lepob/ob mice and play a role in mediating leptin's anti-diabetic actions in this model. For this purpose, we generated diabetic Lepob/ob with conditional knockout of the vesicular GABA transporter (VGAT), required for presynaptic release of GABA, in AgRP neurons (Vgatfl/fl/AgRP-ires-cre/Lepob/ob mice). Surprisingly, loss of GABA from AgRP neurons in Lepob/ob mice did not show any effect on body weight, food intake, glycemia or body composition compared to Vgatfl/fl/Lepob/ob control mice (Figures 5A-D). Using that same experimental design as above, we measured effects of low dose leptin (25ng/hr) on glucose metabolism. Leptin treatment did not affect body weight or food intake (Figure 5E and 5F). In addition, leptin improved blood glucose levels to a similar extent in both Vgatfl/fl/AgRP-ires-cre/Lepob/ob and control Vgatfl/fl/Lepob/ob mice (Figure 5G). Therefore, GABA produced by AgRP neurons does not play a role in control of circulating glucose levels at baseline in Lepob/ob mice nor is GABA derived from AgRP neurons required for the blood-glucose lowering effects by leptin.
Figure 5. GABA released by AgRP neurons is not required for leptin's anti-diabetic actions.
(A-D) Body weight (A), glycemia (B), food Intake (9 weeks of age) (C) and body composition (9-10 weeks of age) (D) in Vgatfl/fl/Lepob/ob and Vgatfl/fl/AgRP-ires-cre/Lepob/ob male mice. (E-G) Leptin infusion for 12 days at 10 weeks of age: percent of initial body weight (E), daily food intake (F) and glycemia (G) in Vgatfl/fl/Lepob/ob and Vgatfl/fl/AgRP-ires-cre/Lepob/ob male mice. Errors bars are shown as SEM (n = 3-8/group).
DISCUSSION
Leptin has potent effects on glucose metabolism though its actions in the hypothalamus. However, the neurochemical identity of the neurons responsible for this effect and the downstream mediators involved in these actions have remained unknown. Here, we applied genetics and pharmacology to investigate these issues in obese diabetic mouse models. We report that hypothalamic AgRP neurons, but not POMC or SF1 neurons, are both required and sufficient in mediating leptin's anti-diabetic actions. In addition, the ability of leptin to normalize hyperglycemia does not involve GABA derived from AgRP neurons or NPY, but instead requires an intact melanocortin-4 receptor system. Combined, these data suggest the existence and importance of a leptin-LepRb-AgRP-MC4R-glucose axis.
The beneficial effects of leptin on glucose balance have been reported in rodent models of type 1 and type 2 diabetes and in severely insulin resistant humans (Coppari and Bjorbaek, 2012). Studies in mice suggest that these effects are mediated by leptin's direct actions in the hypothalamus (Coppari et al., 2005; Fujikawa et al., 2013). It is further reported that LepRb signaling only in POMC neurons within the ARC of the hypothalamus is sufficient to rescue diabetes of Leprdb/db mice (Huo et al., 2009), indicating some role of POMC neurons. On the other hand, because selective loss of LepRb in POMC neurons does not lead to hyperglycemia (Balthasar et al., 2004), other neurons in addition of POMC neurons must exist that also play important roles in leptin's anti-diabetic actions. To identify such neurons, we considered hypothalamic AgRP neurons and SF1 neurons as likely candidates, due to their anatomical localization (ARC and VMH, respectively) and data suggesting roles of these nuclei in leptin's effect on peripheral glucose balance (Coppari et al., 2005; Meek et al., 2013; Minokoshi et al., 1999). We therefore first selectively re-expressed LepRb in AgRP or SF1 neurons of diabetic LepRb-deficient Leprdb/db mice to determine the sufficiency of those cells to correct hyperglycemia by leptin. Secondly, we selectively deleted LepRb in the same candidate neurons of diabetic leptin-deficient Lepob/ob mice to determine the requirement of those cells in a model of low-leptin-induced normalization of circulating glucose levels (Hedbacker et al., 2010). We found that LepRb in SF1/VMH neurons was neither sufficient nor required for leptin to reduce hyperglycemia. In stark contrast, LepRb expression in AgRP neurons was both required and sufficient to mediate leptin-dependent normalization of the severe diabetes in these mice. These effects via AgRP neurons were entirely independent of changes in body weight, adipose tissue mass and energy intake. We further show that LepRb in POMC neurons is not required for leptin's ability to correct hyperglycemia in Lepob/ob mice, despite the earlier studies showing the sufficiency of POMC-LepRb to prevent diabetes in LEPR-deficient mice (Berglund et al., 2012; Huo et al., 2009). We conclude that although both AgRP and POMC neurons are sufficient in mediating leptin's glucose-lowering actions in diabetic states, only AgRP neurons are fully required for these actions, altogether suggesting that AgRP neurons may serve a crucial superior role for leptin's ability to ameliorate diabetes in severe hyperglycemic states.
AgRP neurons co-produce and secrete AgRP, NPY and GABA. We therefore investigated the possible role of each these molecules in leptin's anti-diabetic actions via AgRP neurons by assessing the ability of recombinant leptin to correct hyperglycemia in Lepob/ob mice after systematic deletion of genes preventing the synthesis, release or action by each of the three molecules. We found that neither global deletion of the Npy gene nor selectively ablating GABA release by AGRP neurons via deletion of the vgat gene affected the potency of leptin to normalize hyperglycemia. However and notably, we show that leptin's glucose-lowering effects are completely blunted in Lepob/ob mice deficient in MC4Rs, demonstrating the requirement of the melanocortin-4 receptor system in this effect of leptin. Consistent with this finding, several studies have suggested that MC4Rs serve an important role in the control of glucose homeostasis (Rossi et al., 2011). In addition, the MC4R appears to be required for leptin's anti-diabetic actions in type-1 diabetic animal models (da Silva et al., 2009). Considering that AgRP neurons release the AgRP neuropeptide which acts predominantly as a melanocortin receptor antagonist (Corander et al., 2011) and our results showing that AgRP neurons are both required and sufficient in mediating leptin's anti-diabetic actions, it seems plausible that leptin exerts its effect on glucose balance in these diabetic mouse models, at least in part, by decreasing the synthesis/release of the AgRP neuropeptide from AgRP neurons.
GABA is the major neurotransmitter synthesized by AgRP neurons (Horvath et al., 1997; Vong et al., 2011) and recent evidences show an important role of GABA release by hypothalamic LepRb-expressing neurons in control of glucose homeostasis: mice with specific deletion of LepRb in GABAergic neurons are severely obese, hyperglycemic and hyperinsulinemic (Vong et al., 2011); also, LepR's in hypothalamic GABAergic neurons are sufficient to correct diabetes and to mediate life-saving actions of leptin in insulin-deficient type-1 diabetic animal models (Fujikawa et al., 2013). However, our data does not support a role of GABAergic neurotransmission by AgRP neurons in the development of diabetes in leptin-deficient animals or in the glucose-lowering effects of leptin treatment. Considering the studies mentioned above, our data implies that if in fact GABAergic LepRb-expressing neurons exert any impact on glucose metabolism in type 2 diabetic mouse models, these non-AgRP GABAergic neurons appear to serve a relatively lesser role in glucose control by leptin. Such neurons could be localized in the dorsomedial hypothalamus (DMH) or lateral hypothalamic area (LHA), both nuclei that contain neurons that co-express GABA and LEPRs (Vong et al., 2011).
It has been reported that animals lacking both leptin and NPY have reduced serum glucose levels compared to Lepob/ob control animals (Erickson et al., 1996a). However, we did not find any effect of NPY deletion on glycemia or in the glucose-lowering effects of leptin administration in NPY−/−/Lepob/ob mice compared to control mice. The reason for the discrepancy in baseline glycemia is unclear, but may be explained by the fact that glucose levels were measured by Erickson et al. at 16 weeks of age when double mutants were slightly leaner compared to control animals. Our measurements were done at 9 weeks of age when body weights were similar. Alternatively, differences in genetic background may also explain the divergent results on glycemia.
Besides our principal findings on glucose control by leptin, we also provide important new data on the role of specific neurons on the control of energy balance by leptin. First, we showed that re-expression of LepRb only in AgRP or SF1 neurons of Leprdb/db mice reduces body weight (BW) by ~15% (8 g, 12 wks of age), which accounts for ~30% of the difference between Leprdb/db and lean Lepr+/+ mice (~25 g). These data are roughly in line with the increased BW observed in animals with deletion of LepRb in AgRP or SF1 neurons (Dhillon et al., 2006; van de Wall et al., 2008) suggesting that both AgRP and SF1 neurons mediate a significant proportion of leptin's effect on BW. Interestingly, despite their massive obesity, we found that mice with re-expression of LepRb specifically in AgRP neurons (HA-LepRbflox/AgRP-ires-cre/Leprdb/db) had completely normalized food intake, suggesting a significant capability of AgRP neurons to mediate leptin-dependent inhibition of caloric intake, at least in the hyper-leptinemic Leprdb/db model. Interestingly, these results further suggest that reduced energy expenditure rather than hyperphagia is the main determinator of BW in Leprdb/db mice. Indeed, when we food restricted Leprdb/db to the caloric intake level of Lepr+/+ control mice over 12 weeks, the Leprdb/db animals remained severely obese. Leprdb/db mice expressing LepRb only in SF1 neurons had a similar caloric intake compared to Leprdb/db mice, but yet exhibited a BW loss. This suggests that LepRb in SF1 neurons plays a role in increasing energy expenditure, but not in reducing food intake by leptin. This was also concluded from analyses of mice with selective deletion of LepRb in SF1 neurons, which showed increased BW without a change in food intake (Dhillon et al., 2006).
We also report on the effects on energy homeostasis in leptin-deficient mice that lack NPY, MC4R and GABA release by AgRP neurons. Deletion of NPY and MC4R in Lepob/ob mice has been already described (Erickson et al., 1996a; Trevaskis and Butler, 2005). Supporting the findings of those studies, we did not observed any major effect of the deletion of MC4R or NPY on energy balance in our double mutant mice. We also investigate the role of GABA release from AgRP neurons in leptin-deficient states. However, based on studies reporting a role of GABAergic transmission from AgRP neurons on energy balance (Tong et al., 2008; Wu et al., 2009), it was somewhat surprising that Vgatfl/fl/AgRP-ires-cre/Lepob/ob mice lacked a phenotype on body weight and food intake. This may suggest that GABA release from AgRP neurons does not have a significant role in energy balance in leptin-deficient animals.
In conclusion, our results bring important advances in the understanding of a neurocircuitry involved in mediating beneficial anti-diabetic actions of leptin. We suggest that AgRP neurons serve a major role in the control of glucose metabolism by leptin in obese diabetic states. In addition, this beneficial glucose-lowering action of leptin does not involve NPY or GABA, but instead requires the melanocortin system, suggesting a specific role of the AgRP peptide. Combined, the data indicates the existence of a LepRb-AgRP-MC4R-glucose axis.
EXPERIMENTAL PROCEDURES
Animal Care
Animal care and procedures were approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center. Mice were single-housed at 22-24°C using a 14 hr light/10 hr dark cycle. Unless otherwise specified, mice were fed a standard chow diet with ad libitum access to food and water.
Generation of transgenic mice
HA-LepRbflox, POMC-cre, AgRP-ires-cre, SF1-cre, LepRbflox, MC4R+/−, NPY+/− and Vgatflox mice were generated as previously described (Balthasar et al., 2004; Balthasar et al., 2005; Cohen et al., 2001; Dhillon et al., 2006; Erickson et al., 1996b; Huo et al., 2009; Tong et al., 2008). Leprdb/+ (stock #001192) and Lepob/+ (stock #000696) mice were purchased from Jackson Laboratory (Bar Harbor, ME). To generate the HA-LepRbflox/AgRP-ires-cre/Leprdb/db or HA-LepRbflox/SF1-cre/Leprdb/db mice, HA-LepRbflox mice were mated with AgRP-ires-cre or SF1-cre mice. HA-LepRbflox/AgRP-ires-cre or HA-LepRbflox/SF1-cre mice were then mated with Leprdb/+ animals. HA-LepRbflox/AgRP-ires-cre/Leprdb/db or HA-LepRbflox/SF1-cre/Leprdb/db mice were finally obtained by mating HA-LepRbflox/AgRP-ires-cre/Leprdb/+ or HA-LepRbflox/SF1-cre/Leprdb/+ mice with HA-LepRbflox/Leprdb/+ mice. To generate the LepRbfl/fl/AgRP-ires-cre/Lepob/ob, LepRbfl/fl/POMC-cre/Lepob/ob or LepRbfl/fl/SF1-cre/Lepob/ob mice, LepRbfl/fl mice were first mated with AgRP-ires-cre, POMC-cre or SF1-cre mice. LepRbfl/+/AgRP-ires-cre, LepRbfl/+/POMC-cre or LepRbfl/+/SF1-cre mice were then mated with Lepob/+ mice. LepRbfl/fl/AgRP-ires-cre/Lepob/ob, LepRbfl/fl/POMC-cre/Lepob/ob or LepRbfl/fl/SF1-cre/Lepob/ob mice were obtained by mating LepRbfl/+/AgRP-ires-cre/Lepob/+, LepRbfl/+/POMC-cre/Lepob/+ or LepRbfl/+/SF1-cre/Lepob/+ mice with LepRbfl/+/Lepob/+ mice. To generate the MC4R−/−/Lepob/ob or NPY−/−/ Lepob/ob mice, MC4R−/+ or NPY−/+ mice were first mated with Lepob/+ mice.MC4R−/− Lepob/ob or NPY−/−/Lepob/ob mice were obtained by intermating MC4R−/+/Lepob/+ or NPY−/+/Lepob/+ mice. To generate the Vgatfl/fl/AgRP-ires-cre/Lepob/ob mice, Vgatfl/+ mice were first mated with AgRP-ires-cre mice. Vgatfl/+/AgRP-ires-cre mice were then mated with Lepob/+ mice. Finally, Vgatfl/+/AgRP-ires-cre/Lepob/+ mice were mated with Vgatfl/+/Lepob/+ mice. Littermates were used in the studies.
Body Composition
Mice at 10-12 weeks of age were subjected to magnetic resonance imaging (MRI) using EchoMRI (Echo Medical Systems, Houston, TX) to obtain the body composition.
Leptin administration via mini-osmotic pumps
Mice at 8-10 weeks of age were anesthetized intraperitoneally using ketamine/xylazine. Alzet 2002 mini-osmotic pumps (Palo Alto, CA) filled with indicated concentrations of leptin (Dr. E. Parlow, NIDDK; Torrance, CA) or PBS (placebo) were implanted subcutaneously on day 0. At day 8-13, mice were sacrificed and blood was collected.
Blood Composition
Tail vein blood was collected at 8 a.m. ± 2hr from ad libitum-fed mice. Blood glucose was assayed with OneTouch Ultra Blood Glucose Monitoring System (Fischer Scientific; Morrison Plains, NJ). ELISAs were used to measure serum insulin and leptin (Alpco; Salem, NH).
Immunohistochemistry
Twenty five micrometer coronal brain sections were obtained as previously described (Munzberg et al., 2003). P-STAT3 IHC was performed as described earlier (Huo et al., 2006).
Statistical analyses
Data are presented as means ± SEM, and significant level was set at p ≤ 0.05. Analyses were done using one-way ANOVA or two-way ANOVA (Bonferroni post-hoc analyses).
Supplementary Material
RESEARCH HIGHLIGHTS.
We show that leptin receptor signaling through AgRP neurons prevents diabetes in mice.
The AgRP neuropeptide, but not NPY nor GABA, is required for this action.
The data reveals the existence of a novel Leptin-LepRb-AgRP-MC4R-glucose axis
ACKNOWLEDGEMENTS
This work was supported by grants from the American Diabetes Association (7-09-BS-17 and 7-12-BS-010), the National Institutes of Health (DK094040 and DK060673) (all to C.B.) and the National Council for Scientific and Technological Development (CNPq - Brazil) (to G.H.M.G). The work was also supported by the Boston Obesity Nutrition Research Center (DK46200). We are particularly grateful to Dr. Brad Lowell (BIDMC, Boston) for providing us with some animals. Finally, we thank Kristen R. Vella (BIDMC, Boston), Felix D. Ye (BIDMC, Boston), Linh Vong (BIDMC, Boston) and Michael J. Krashes (BIDMC, Boston) for also providing some animals for our breeding purposes.
REFERENCES
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Vianna CR, Donato J, Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Coppari R, Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11:692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corander MP, Rimmington D, Challis BG, O'Rahilly S, Coll AP. Loss of agouti-related peptide does not significantly impact the phenotype of murine POMC deficiency. Endocrinology. 2011;152:1819–1828. doi: 10.1210/en.2010-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- da Silva AA, do Carmo JM, Freeman JN, Tallam LS, Hall JE. A functional melanocortin system may be required for chronic CNS-mediated antidiabetic and cardiovascular actions of leptin. Diabetes. 2009;58:1749–1756. doi: 10.2337/db08-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;19:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Berglund ED, Patel VR, Ramadori G, Vianna CR, Vong L, Thorel F, Chera S, Herrera PL, Lowell BB, Elmquist JK, Baldi P, Coppari R. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18:431–444. doi: 10.1016/j.cmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- Huang H, Lee SH, Ye C, Lima IS, Oh BC, Lowell BB, Zabolotny JM, Kim YB. ROCK1 in AgRP Neurons Regulates Energy Expenditure and Locomotor Activity in Male Mice. Endocrinology. 2013;154:3660–3670. doi: 10.1210/en.2013-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Grill HJ, Bjørbæk C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjørbæk C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus Delayed Stimulation of Feeding by the Endogenously Released AgRP Neuron Mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Meek TH, Matsen ME, Dorfman MD, Guyenet SJ, Damian V, Nguyen HT, Taborsky GJ, Jr, Morton GJ. Leptin action in the ventromedial hypothalamic nucleus is sufficient, but not necessary, to normalize diabetic hyperglycemia. Endocrinology. 2013;154:3067–3076. doi: 10.1210/en.2013-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbæk C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr, Woods SC, Seeley RJ, Weigle DS. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis JL, Butler AA. Double leptin and melanocortin-4 receptor gene mutations have an additive effect on fat mass and are associated with reduced effects of leptin on weight loss and food intake. Endocrinology. 2005;146:4257–4265. doi: 10.1210/en.2005-0492. [DOI] [PubMed] [Google Scholar]
- van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–34. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.