Abstract
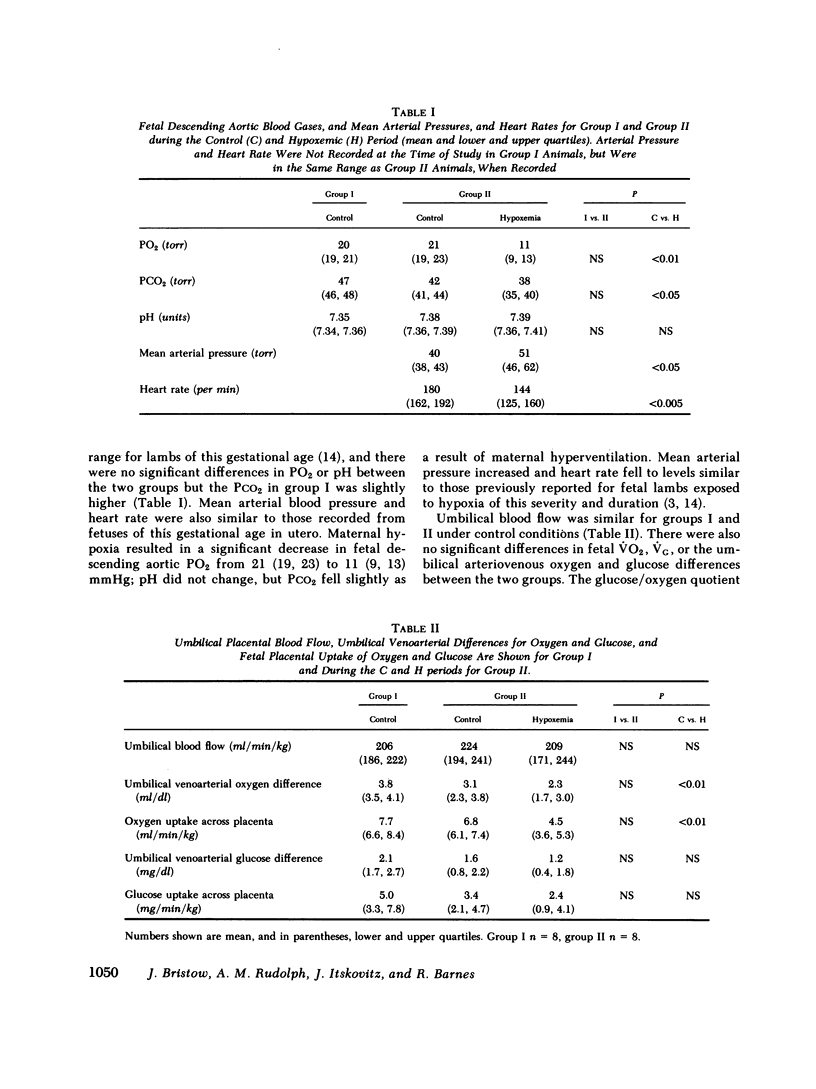
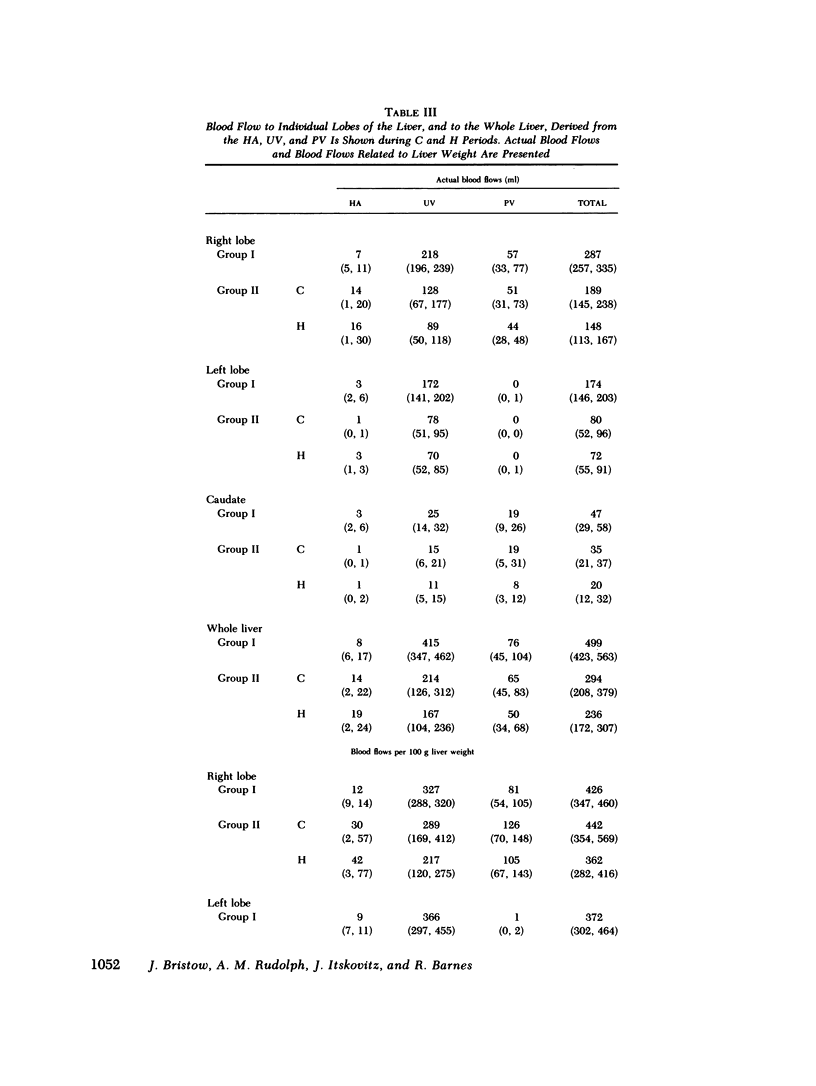
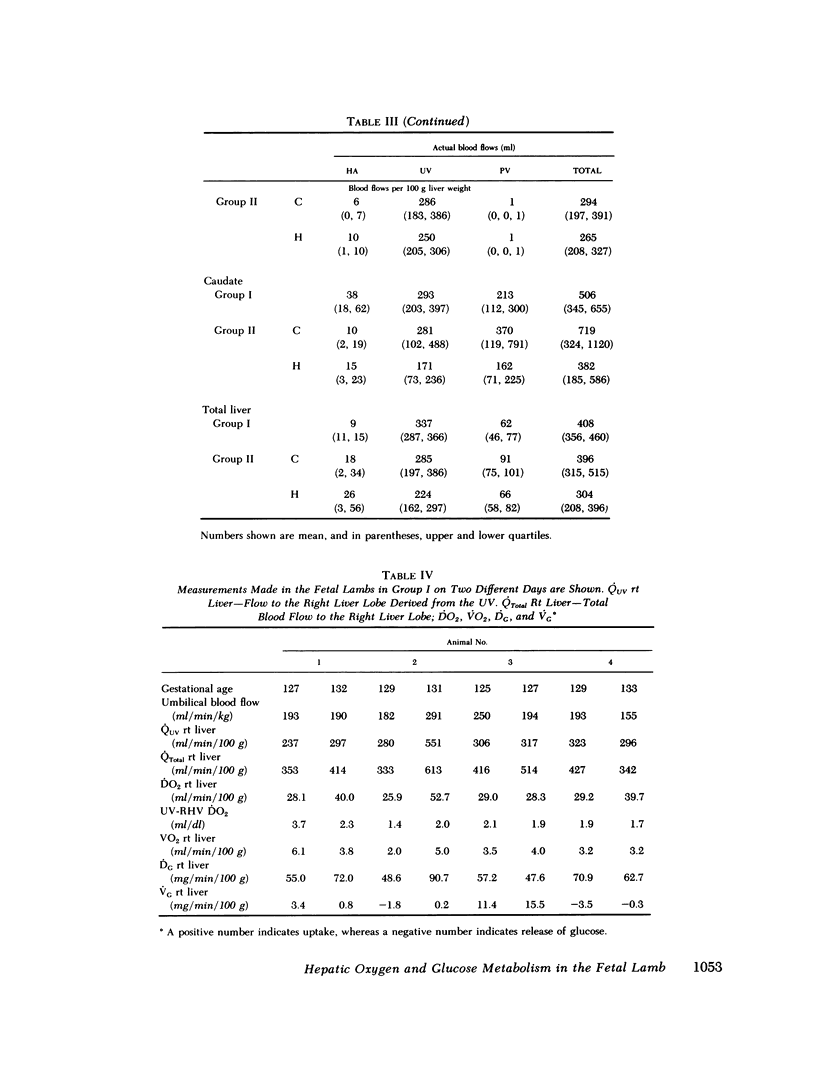
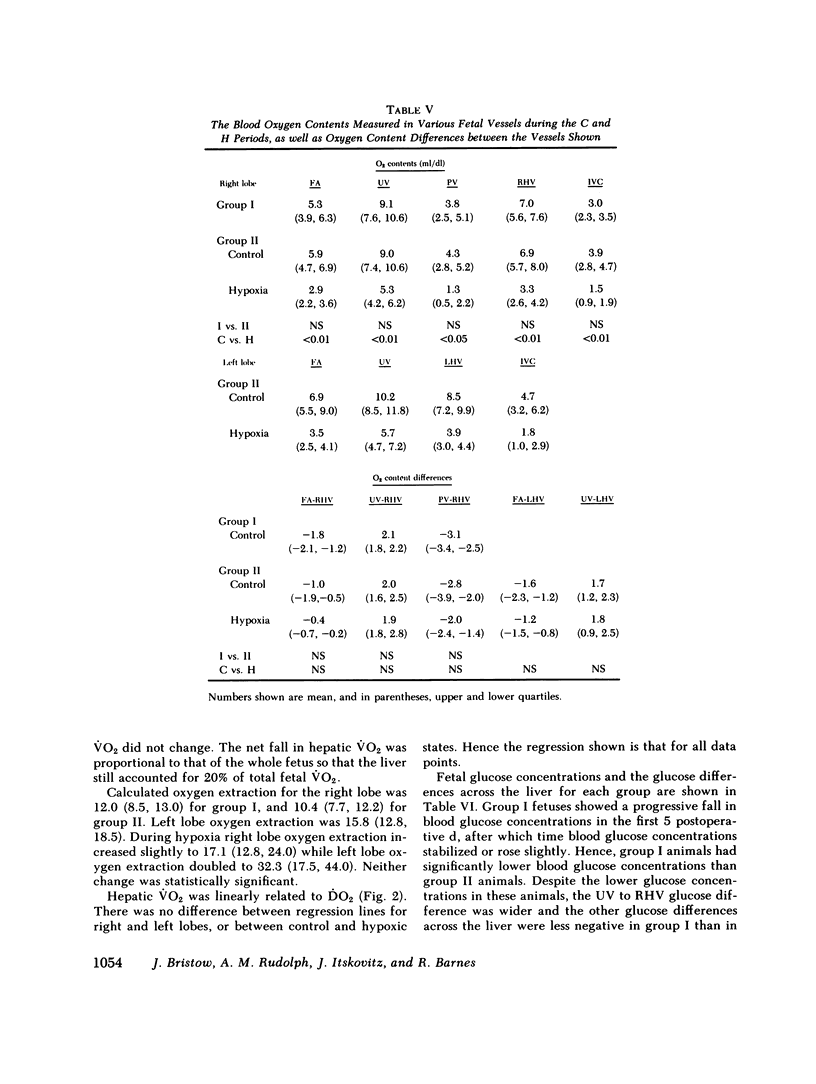
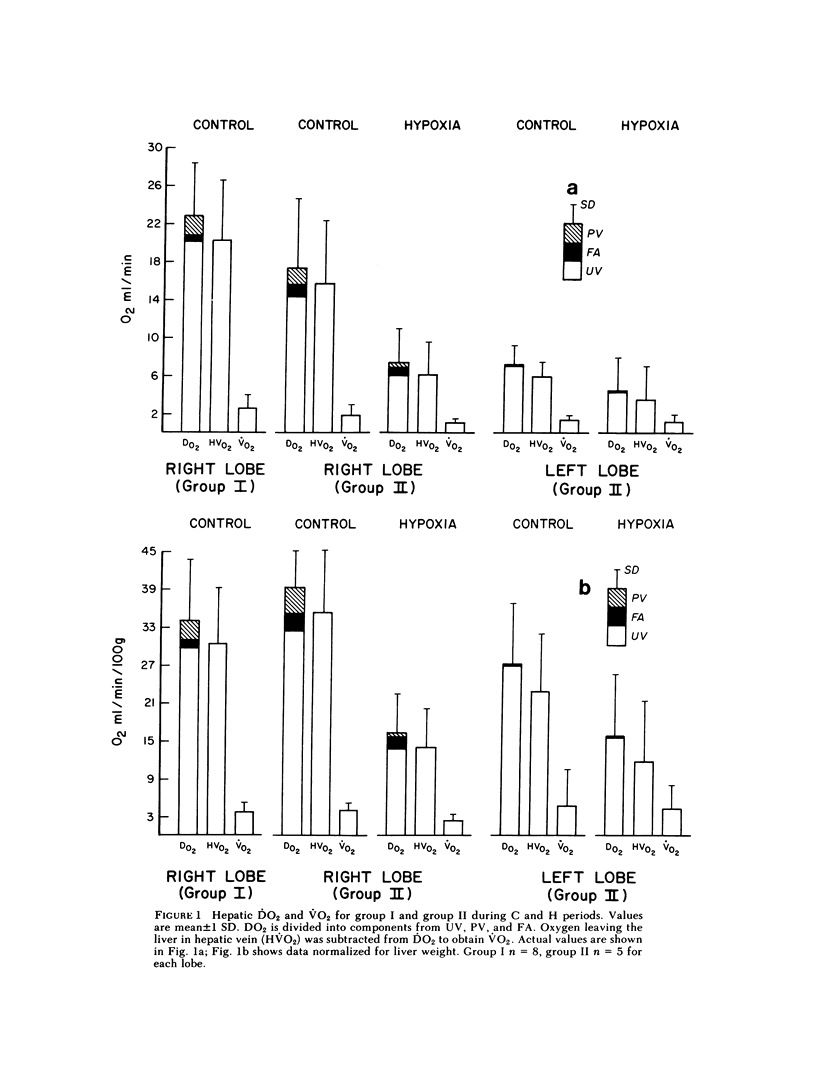
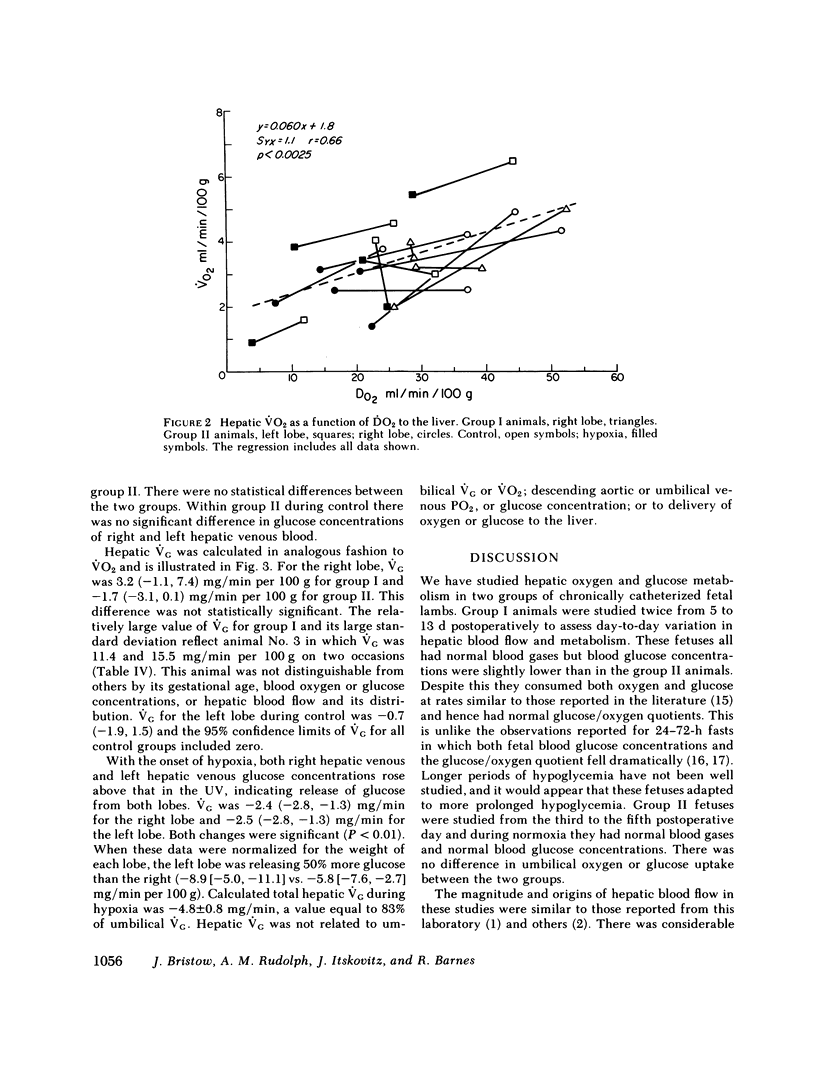
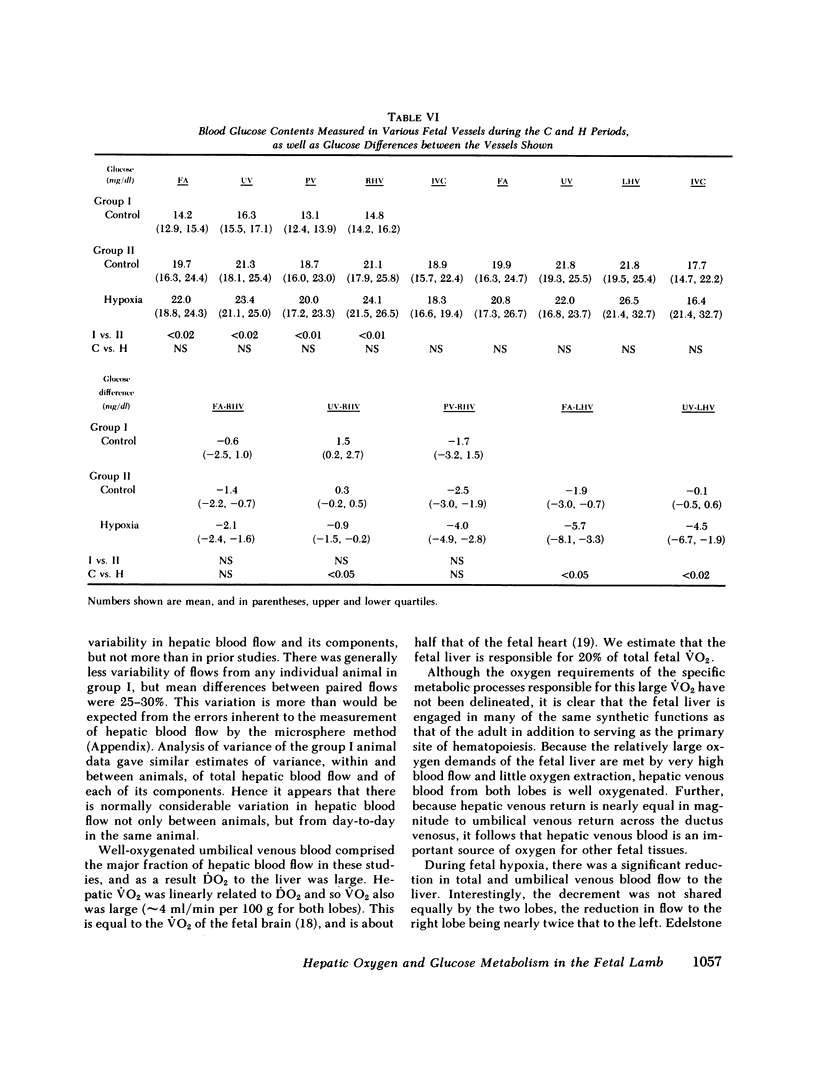
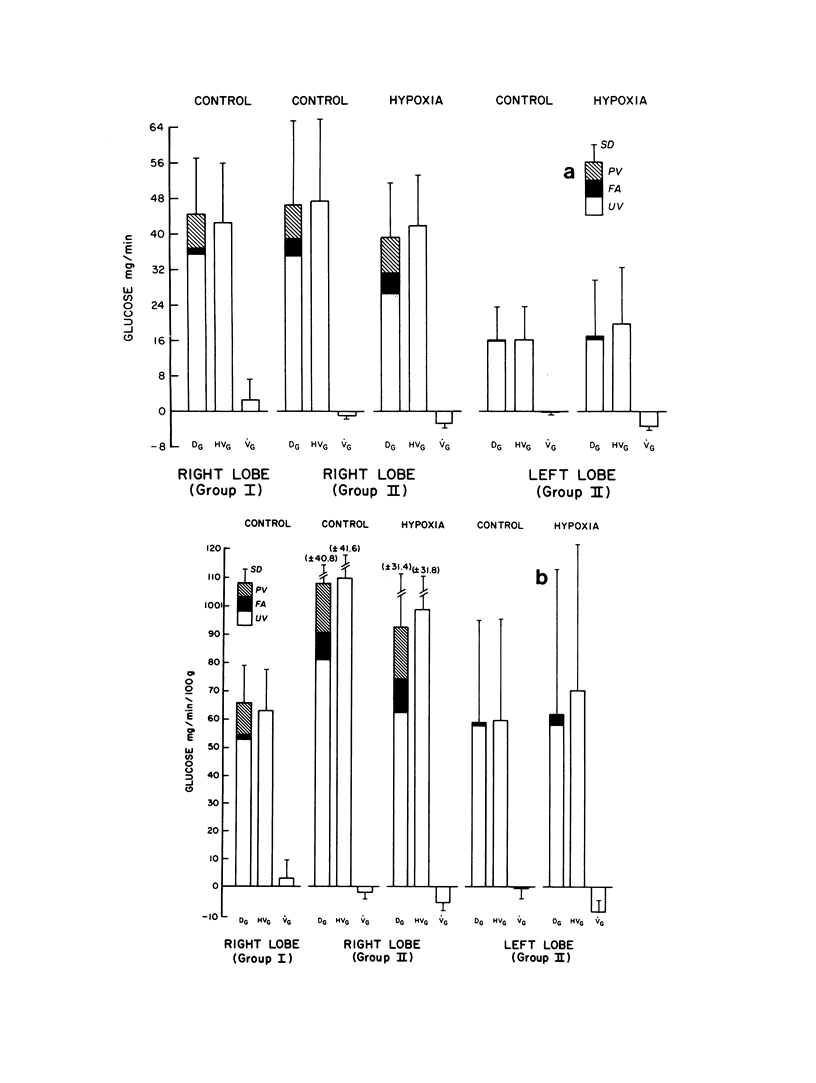
Although the fetal liver is an active metabolic organ, its oxygen and glucose requirements have not previously been described. We measured hepatic blood flows and the oxygen and glucose differences across the liver in 12 late gestation fetal lambs in utero. Four animals were studied at least 1 wk postsurgically and again 2-5 d later to assess daily variations in hepatic blood flow and metabolism (group I). A second group of eight animals was studied 3-5 d postsurgically during a control period and during acute fetal hypoxia (group II). Under control conditions total hepatic blood flow averaged 400 ml/min per 100 g in both groups, and 75-80% was of umbilical origin. Liver blood flow and oxygen consumption were usually similar during repeated measurements, but in one animal varied considerably. During periods of normoxia, oxygen consumption for both the right and left lobes of liver was 4 ml/min per 100 g. Oxygen consumption of the whole liver accounted for 20% of total fetal oxygen consumption. This was achieved with oxygen extraction of 10-15%, so that hepatic venous blood was well oxygenated and provided an important source of oxygen for other fetal tissues. Under control conditions we could demonstrate no net hepatic uptake or release of glucose suggesting that the liver ultimately utilizes another carbon source to support its oxidative metabolism. During acute hypoxia total liver blood flow and its umbilical venous contribution both fell by 20%. Blood flow to the right lobe of the liver fell twice as much as that to the left lobe. Hepatic oxygen consumption was linearly related to oxygen delivery during the control and hypoxic periods. Consequently, right hepatic oxygen uptake fell by 45% whereas left hepatic oxygen uptake was unchanged, suggesting a functional difference between the lobes. During hypoxia glucose was released from both liver lobes; 6 mg/min per 100 g for the right lobe and 9 mg/min per 100 g for the left lobe. Total hepatic release of glucose was estimated to nearly equal umbilical uptake, so that 45% of the glucose available to fetal tissues was of hepatic origin. We conclude that the fetal liver responds to acute hypoxia by reducing its own oxygen consumption and releasing glucose to facilitate anaerobic metabolism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. P., Forsling M. L., Martin M. J., Nixon D. A., Ratcliffe J. G., Redstone D., Tunbridge D. The effect of maternal hypoxia on fetal pituitary hormone release in the sheep. Biol Neonate. 1972;21(3):219–228. doi: 10.1159/000240510. [DOI] [PubMed] [Google Scholar]
- Barnes R. J., Comline R. S., Silver M. Effect of cortisol on liver glycogen concentrations in hypophysectomized, adrenalectomized and normal foetal lambs during late or prolonged gestation. J Physiol. 1978 Feb;275:567–579. doi: 10.1113/jphysiol.1978.sp012209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia F. C., Meschia G. Principal substrates of fetal metabolism. Physiol Rev. 1978 Apr;58(2):499–527. doi: 10.1152/physrev.1978.58.2.499. [DOI] [PubMed] [Google Scholar]
- Boyd R. D., Morriss F. H., Jr, Meschia G., Makowski E. L., Battaglia F. C. Growth of glucose and oxygen uptakes by fetuses of fed and starved ewes. Am J Physiol. 1973 Oct;225(4):897–902. doi: 10.1152/ajplegacy.1973.225.4.897. [DOI] [PubMed] [Google Scholar]
- Bristow J., Rudolph A. M., Itskovitz J. A preparation for studying liver blood flow, oxygen consumption, and metabolism in the fetal lamb in utero. J Dev Physiol. 1981 Aug;3(4):255–266. [PubMed] [Google Scholar]
- Burd L. I., Jones M. D., Jr, Simmons M. A., Makowski E. L., Meschia G., Battaglia F. C. Placental production and foetal utilisation of lactate and pyruvate. Nature. 1975 Apr 24;254(5502):710–711. doi: 10.1038/254710a0. [DOI] [PubMed] [Google Scholar]
- Char V. C., Creasy R. K. Lactate and pyruvate as fetal metabolic substrates. Pediatr Res. 1976 Apr;10(4):231–234. doi: 10.1203/00006450-197604000-00006. [DOI] [PubMed] [Google Scholar]
- Cohn H. E., Sacks E. J., Heymann M. A., Rudolph A. M. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974 Nov 15;120(6):817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- DAWES G. S., MOTT J. C., SHELLEY H. J. The importance of cardiac glycogen for the maintenance of life in foetal lambs and newborn animals during anoxia. J Physiol. 1959 Jun 11;146(3):516–538. doi: 10.1113/jphysiol.1959.sp006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos P., Hers H. G. Glycogen metabolism in the liver of the foetal rat. Biochem J. 1974 May;140(2):331–340. doi: 10.1042/bj1400331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERY J. L. FUNCTIONAL ASYMMETRY OF THE LIVER. Ann N Y Acad Sci. 1963 Dec 30;111:37–44. doi: 10.1111/j.1749-6632.1963.tb36947.x. [DOI] [PubMed] [Google Scholar]
- Edelstone D. I., Rudolph A. M., Heymann M. A. Effects of hypoxemia and decreasing umbilical flow liver and ductus venosus blood flows in fetal lambs. Am J Physiol. 1980 May;238(5):H656–H663. doi: 10.1152/ajpheart.1980.238.5.H656. [DOI] [PubMed] [Google Scholar]
- Edelstone D. I., Rudolph A. M., Heymann M. A. Liver and ductus venosus blood flows in fetal lambs in utero. Circ Res. 1978 Mar;42(3):426–433. doi: 10.1161/01.res.42.3.426. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Harper S. C. Role of cyclic AMP in the actions of catecholamines on hepatic carbohydrate metabolism. Adv Cyclic Nucleotide Res. 1975;5:519–532. [PubMed] [Google Scholar]
- Fisher D. J., Heymann M. A., Rudolph A. M. Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am J Physiol. 1980 Mar;238(3):H399–H405. doi: 10.1152/ajpheart.1980.238.3.H399. [DOI] [PubMed] [Google Scholar]
- Gresham E. L., James E. J., Raye J. R., Battaglia F. C., Makowski E. L., Meschia G. Production and excretion of urea by the fetal lamb. Pediatrics. 1972 Sep;50(3):372–379. [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Control of hepatic glycogenolysis. Physiol Rev. 1980 Jan;60(1):1–50. doi: 10.1152/physrev.1980.60.1.1. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Jones C. T. The development of some metabolic responses to hypoxia in the foetal sheep. J Physiol. 1977 Mar;265(3):743–762. doi: 10.1113/jphysiol.1977.sp011741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. D., Jr, Burd L. I., Makowski E. L., Meschia G., Battaglia F. C. Cerebral metabolism in sheep: a comparative study of the adult, the lamb, and the fetus. Am J Physiol. 1975 Jul;229(1):235–239. doi: 10.1152/ajplegacy.1975.229.1.235. [DOI] [PubMed] [Google Scholar]
- Lemons J. A., Adcock E. W., 3rd, Jones M. D., Jr, Naughton M. A., Meschia G., Battaglia F. C. Umbilical uptake of amino acids in the unstressed fetal lamb. J Clin Invest. 1976 Dec;58(6):1428–1434. doi: 10.1172/JCI108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski E. L., Schneider J. M., Tsoulos N. G., Colwill J. R., Battaglia F. C., Meschia G. Cerebral blood flow, oxygen consumption, and glucose utilization of fetal lambs in utero. Am J Obstet Gynecol. 1972 Oct 1;114(3):292–303. doi: 10.1016/0002-9378(72)90606-0. [DOI] [PubMed] [Google Scholar]
- Peeters L. L., Sheldon R. E., Jones M. D., Jr, Makowski E. L., Meschia G. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979 Nov 1;135(5):637–646. doi: 10.1016/s0002-9378(16)32989-1. [DOI] [PubMed] [Google Scholar]
- Reuss M. L., Rudolph A. M. Distribution and recirculation of umbilical and systemic venous blood flow in fetal lambs during hypoxia. J Dev Physiol. 1980 Feb-Apr;2(1-2):71–84. [PubMed] [Google Scholar]
- Simmons M. A., Meschia G., Makowski E. L., Battaglia F. C. Fetal metabolic response to maternal starvation. Pediatr Res. 1974 Oct;8(10):830–836. doi: 10.1203/00006450-197410000-00003. [DOI] [PubMed] [Google Scholar]
- Sperling M. A., Christensen R. A., Ganguli S., Anand R. Adrenergic modulation of pancreatic hormone secretion in utero: studies in fetal sheep. Pediatr Res. 1980 Mar;14(3):203–208. doi: 10.1203/00006450-198003000-00005. [DOI] [PubMed] [Google Scholar]
- VILLEE C. A., HAGERMAN D. D., HOLMBERG N., LIND J., VILEE D. B. The effects of anoxia on the metabolism of human fetal tissues. Pediatrics. 1958 Nov;22(5):953–971. [PubMed] [Google Scholar]
- Zink J., van Petten G. R. The effect of norepinephrine on blood flow through the fetal liver and ductus venosus. Am J Obstet Gynecol. 1980 May 1;137(1):71–77. doi: 10.1016/0002-9378(80)90388-9. [DOI] [PubMed] [Google Scholar]


