Abstract
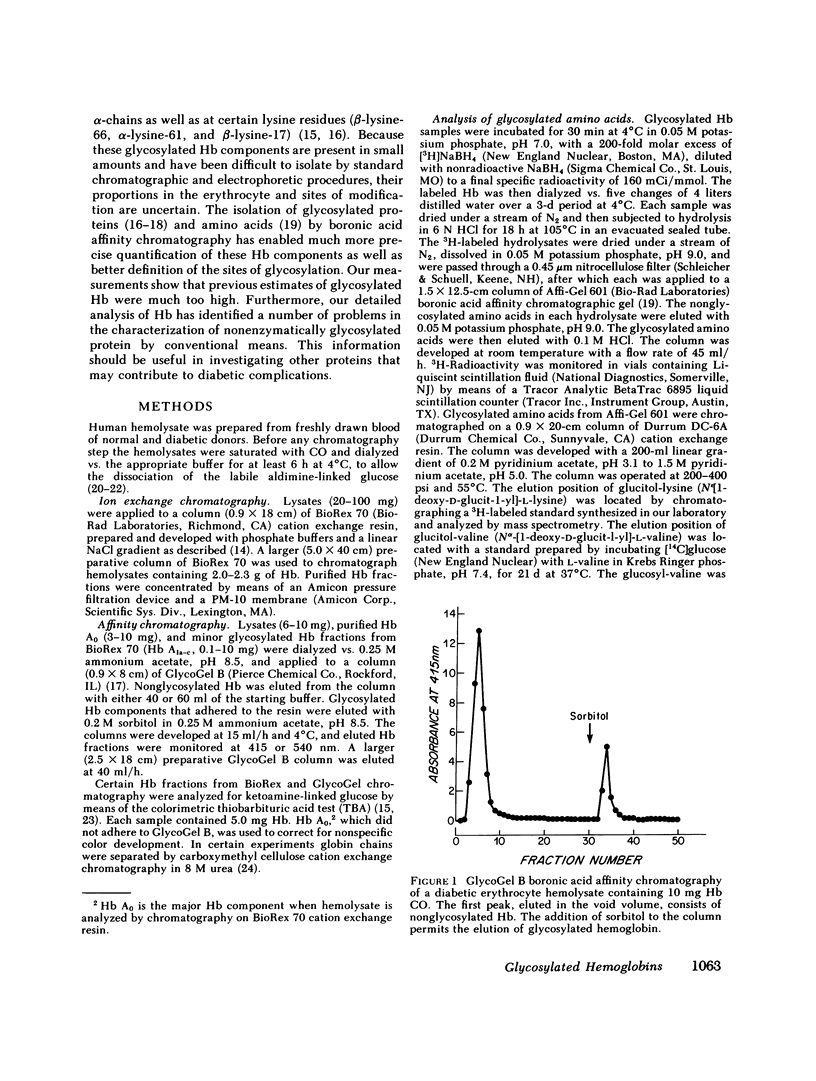
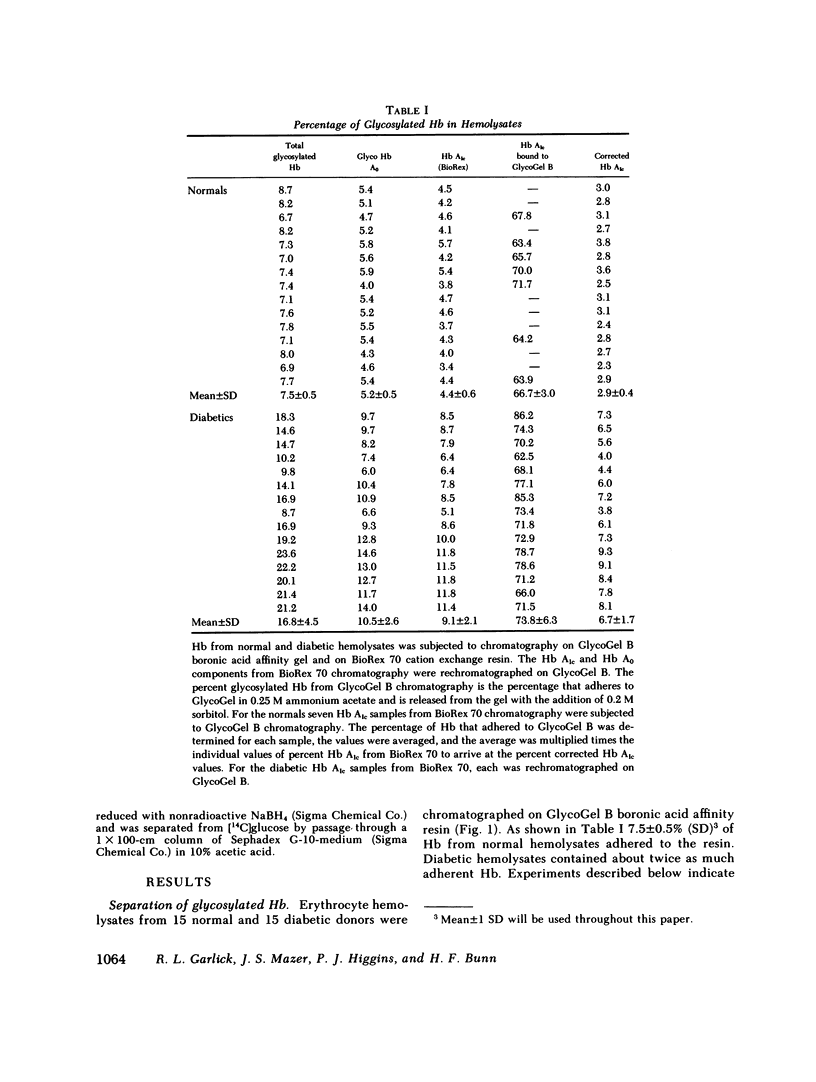
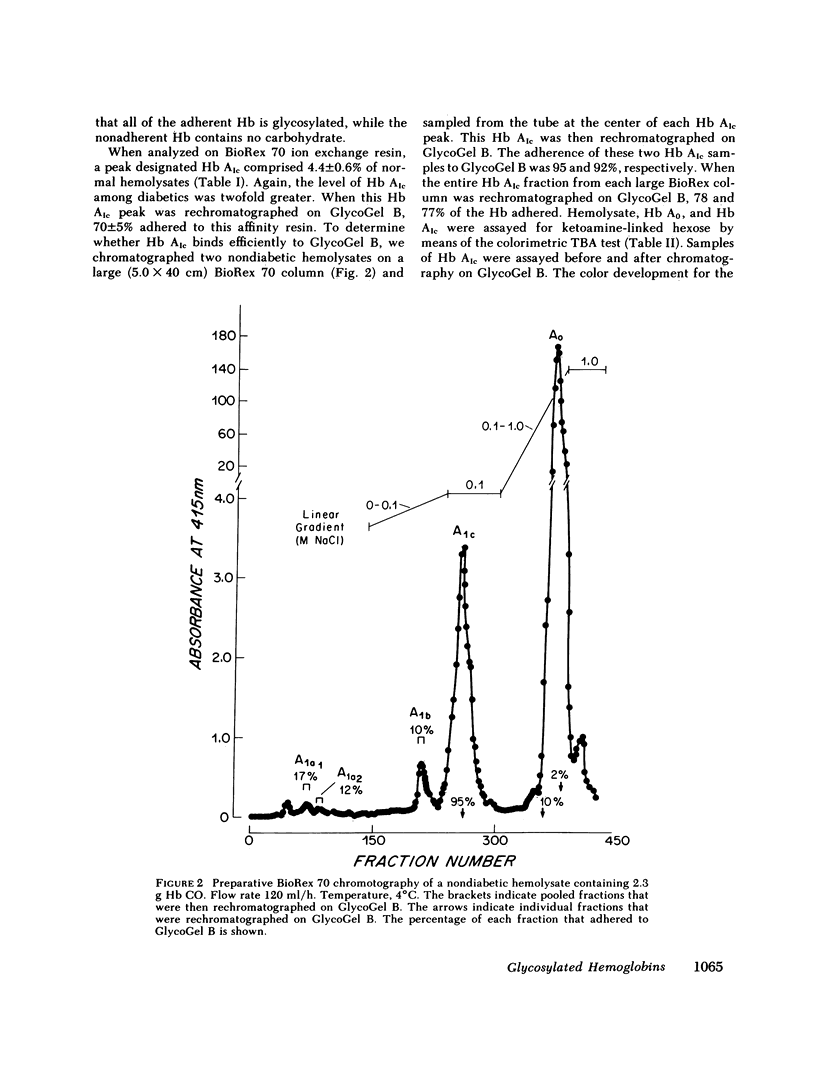
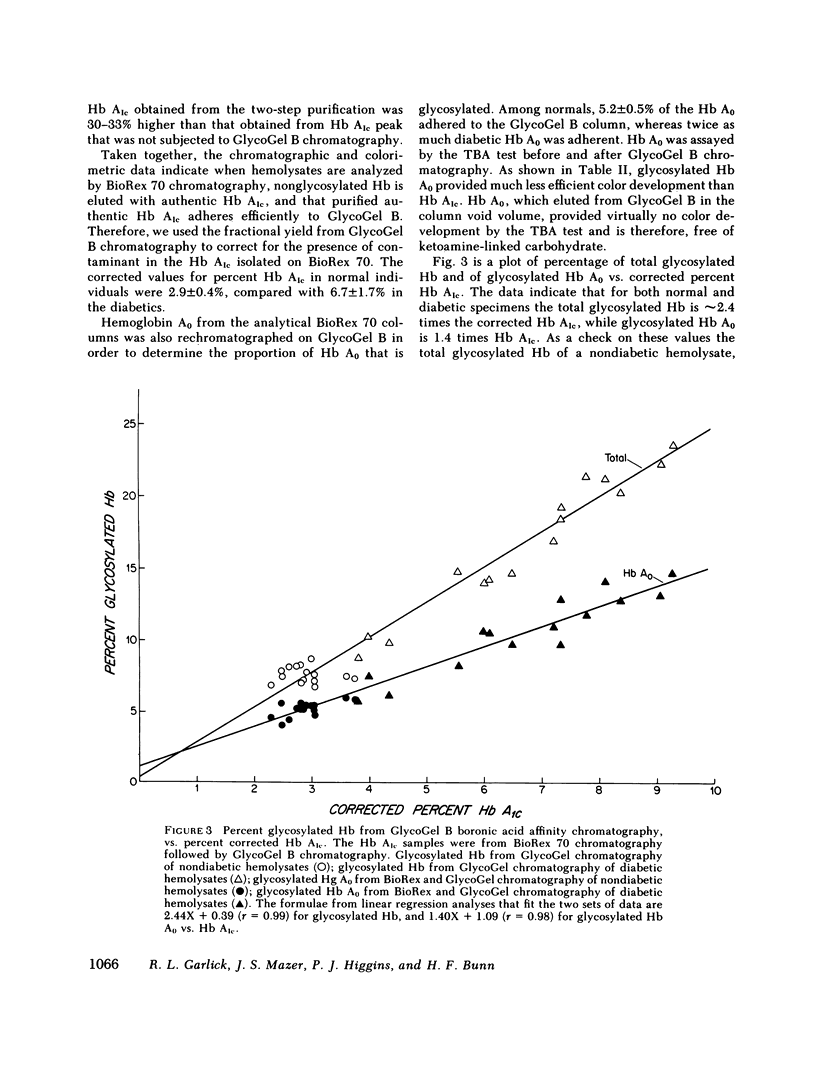
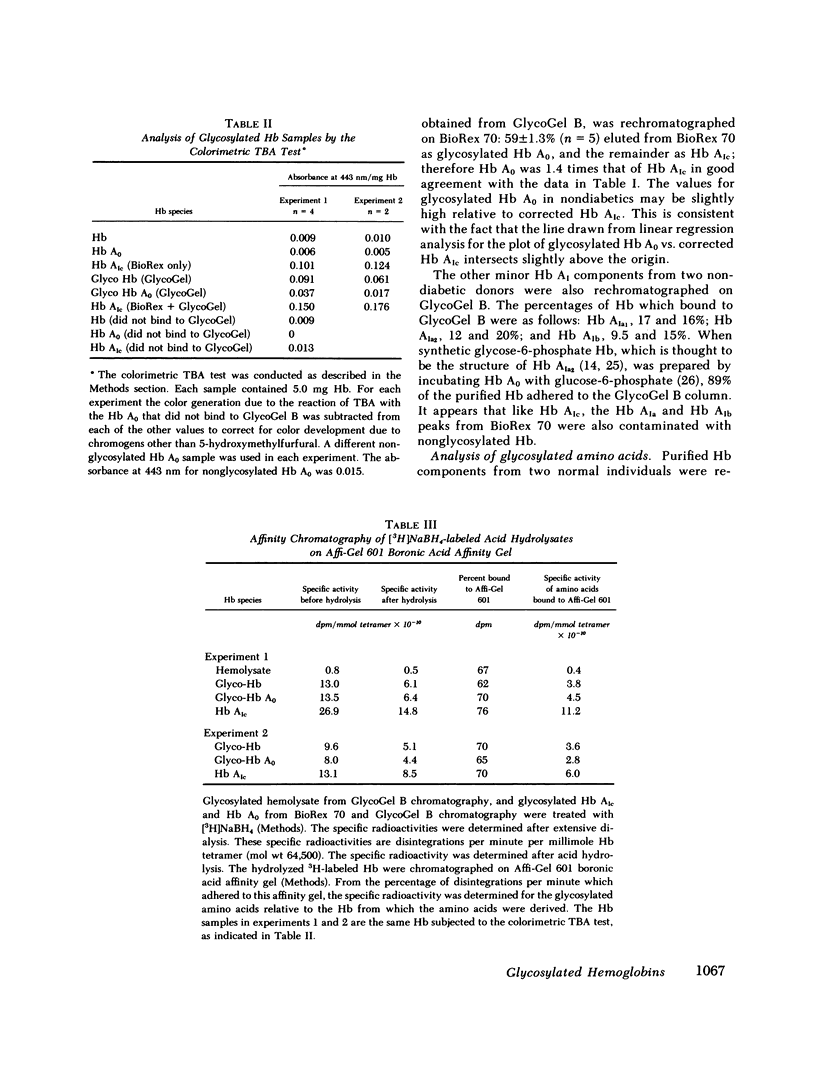
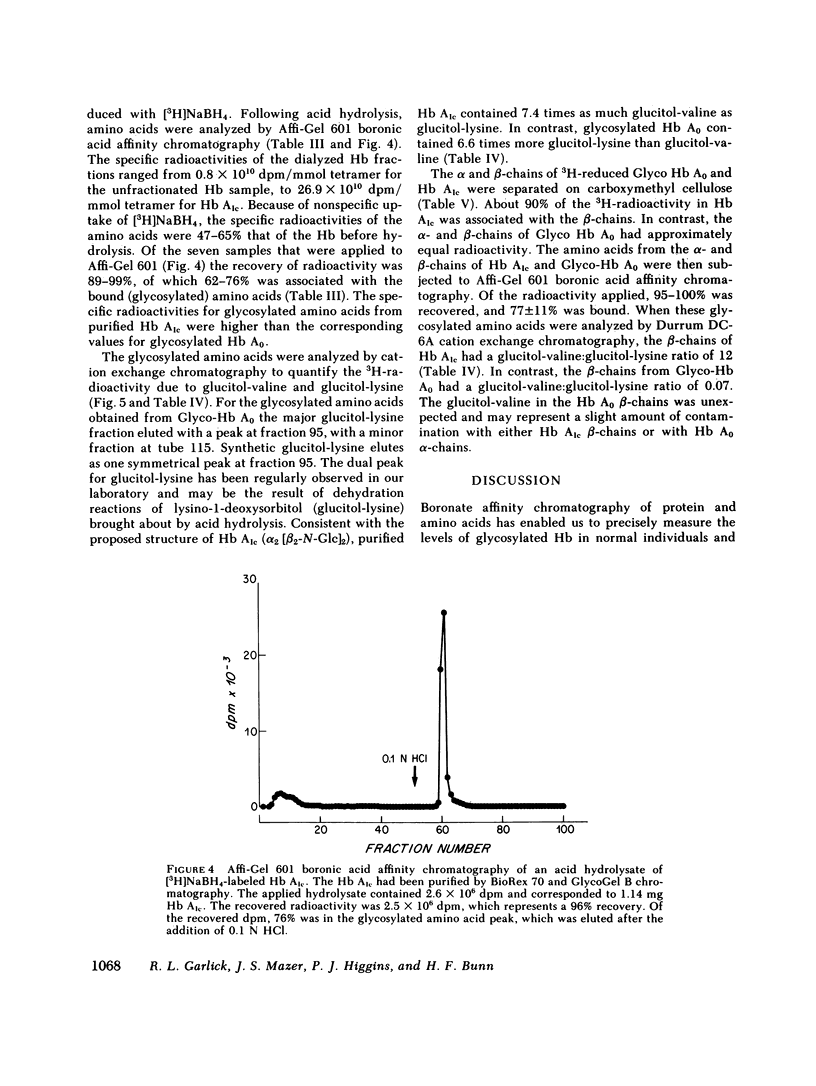
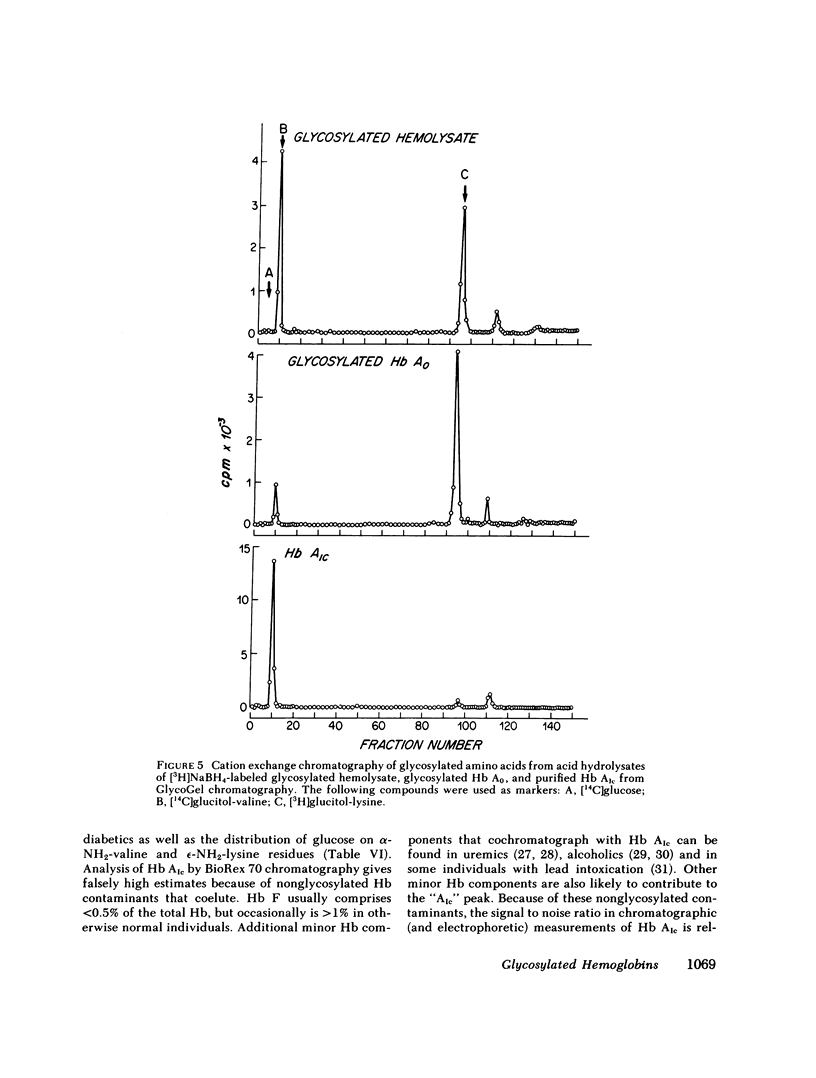
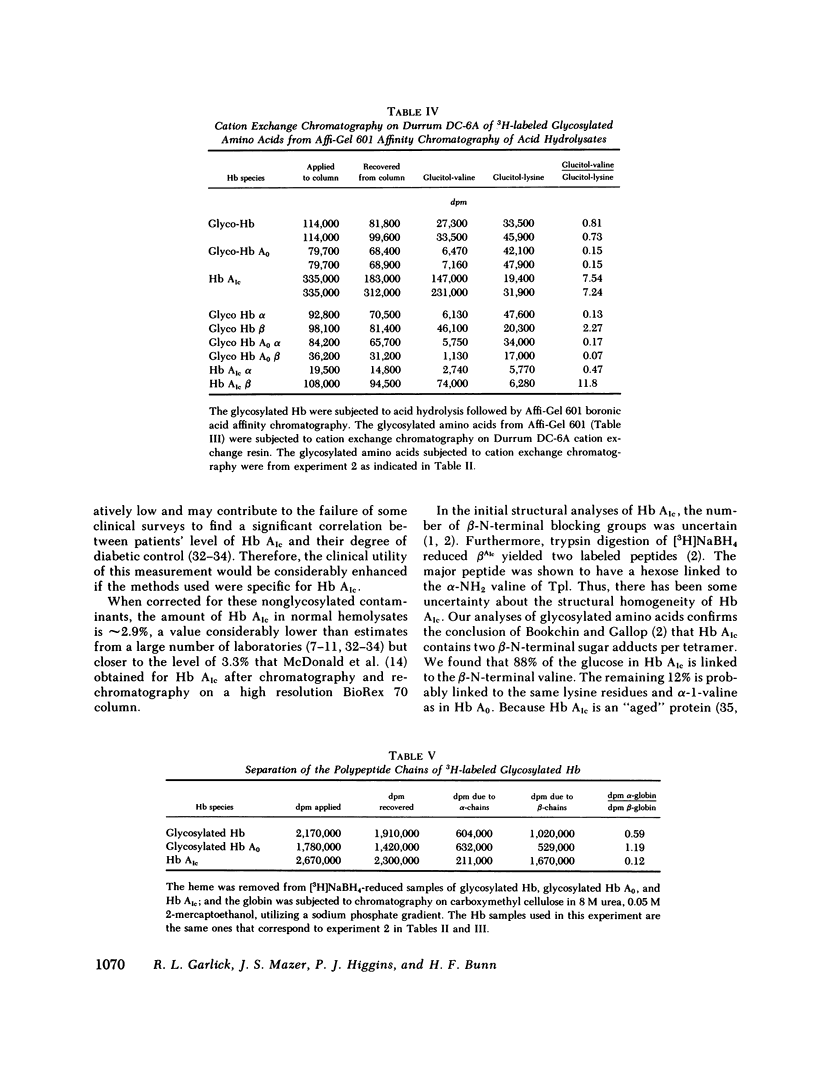
Boronate affinity chromatography and ion exchange chromatography were used to measure the levels of glycosylated hemoglobins in normal and diabetic hemolysates, as well as the distribution of glucose adducts on alpha-NH2-valine and epsilon-NH2-lysine residues. When analyzed by ion exchange chromatography on BioRex 70 resin, the Hb Alc peak comprised 4.4 +/- 0.6% of 15 normal hemolysates and 9.1 +/- 2.1% of 15 diabetic hemolysates. The "Hb Alc" was rechromatographed on GlycoGel B boronate affinity resin that binds vicinal hydroxyl groups of covalently linked sugars. Only 70 +/- 5% of the hemoglobin adhered to the resin. Analysis by the thiobarbituric acid colorimetric test confirmed that the affinity resin effectively separated glycosylated from nonglycosylated hemoglobin. When corrected for nonglycosylated contaminants, the mean level of Hb Alc in normal hemolysates was 2.9 +/- 0.4%, a value considerably lower than those previously reported. In addition to Hb Alc, 5.2 +/- 0.5% of the remaining hemoglobin (Hb Ao) was glycosylated. In diabetics, glycosylated Ao was increased in parallel with Hb Alc. After reduction with [3H]borohydride and acid hydrolysis, glycosylated amino acids were first purified on Affi-Gel boronate affinity resin and then analyzed by ion exchange chromatography. The glucose adducts on Hb Ao were distributed as follows: alpha-chain N-terminal valine, 14%; alpha-chain lysines, 40%; beta-chain lysines, 46%. This study has revealed several pitfalls in the analysis of nonenzymatically glycosylated proteins. Peaks isolated by ion exchange chromatography or electrophoresis are likely to be contaminated by nonglycosylated proteins. Furthermore, both the thiobarbituric acid test and [3H]borohydride reduction show variable reactivity depending upon the site of the ketoamine-linked glucose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. C., Huff T. A., Cope N. D., Wilson J. B., Jr, Bransome E. D., Jr, Huisman T. H. Determination of the glycosylated hemoglobins (HB AI) with a new microcolumn procedure. Suitability of the technique for assessing the clinical management of diabetes mellitus. Diabetes. 1978 Sep;27(9):931–937. doi: 10.2337/diab.27.9.931. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Gallop P. M. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968 Jul 11;32(1):86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A. The biochemistry of the complications of diabetes mellitus. Annu Rev Biochem. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Measurement of glycosylated amino acids and peptides from urine of diabetic patients using affinity chromatography. Diabetes. 1980 Dec;29(12):1044–1047. doi: 10.2337/diab.29.12.1044. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Gabbay K. H., Gallop P. M. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochem Biophys Res Commun. 1975 Nov 3;67(1):103–109. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Kamin S., Gabbay K. H., Gallop P. M. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J Clin Invest. 1976 Jun;57(6):1652–1659. doi: 10.1172/JCI108436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Shapiro R., McManus M., Garrick L., McDonald M. J., Gallop P. M., Gabbay K. H. Structural heterogeneity of human hemoglobin A due to nonenzymatic glycosylation. J Biol Chem. 1979 May 25;254(10):3892–3898. [PubMed] [Google Scholar]
- Charache S., Weatherall D. J. Fast hemoglobin in lead poisoning. Blood. 1966 Sep;28(3):377–386. [PubMed] [Google Scholar]
- Chiou S. H., Garrick L. M., McDonald M. J. Functional properties of human adult hemoglobin specifically modified at the alpha-amino groups of the beta chains with D-glucose 6-phosphate. Biochemistry. 1982 Jan 5;21(1):13–20. doi: 10.1021/bi00530a003. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Dolhofer R., Städele A., Wieland O. H. [Clinical and biochemical studies on the significance and formation of hemoglobins AIc and AIa+b in diabetes mellitus]. Klin Wochenschr. 1977 Oct 1;55(19):945–954. doi: 10.1007/BF01479226. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons J. F., Koler R. D., Jones R. T. Red cell age-related changes of hemoglobins AIa+b and AIc in normal and diabetic subjects. J Clin Invest. 1976 Oct;58(4):820–824. doi: 10.1172/JCI108534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flückiger R., Harmon W., Meier W., Loo S., Gabbay K. H. Hemoglobin carbamylation in uremia. N Engl J Med. 1981 Apr 2;304(14):823–827. doi: 10.1056/NEJM198104023041406. [DOI] [PubMed] [Google Scholar]
- Flückiger R., Winterhalter K. H. In vitro synthesis of hemoglobin AIc. FEBS Lett. 1976 Dec 1;71(2):356–360. doi: 10.1016/0014-5793(76)80969-6. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Hasty K., Breslow J. L., Ellison R. C., Bunn H. F., Gallop P. M. Glycosylated hemoglobins and long-term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab. 1977 May;44(5):859–864. doi: 10.1210/jcem-44-5-859. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Sosenko J. M., Banuchi G. A., Mininsohn M. J., Flückiger R. Glycosylated hemoglobins: increased glycosylation of hemoglobin A in diabetic patients. Diabetes. 1979 Apr;28(4):337–340. doi: 10.2337/diab.28.4.337. [DOI] [PubMed] [Google Scholar]
- Garrick L. M., McDonald M. J., Shapiro R., Bleichman M., McManus M., Bunn H. F. Structural analysis of the minor human hemoglobin components: Hb AIa1, Hb AIa2 and Hb AIb. Eur J Biochem. 1980 May;106(2):353–359. doi: 10.1111/j.1432-1033.1980.tb04581.x. [DOI] [PubMed] [Google Scholar]
- Goldstein D. E., Peth S. B., England J. D., Hess R. L., Da Costa J. Effects of acute changes in blood glucose on HbA1c. Diabetes. 1980 Aug;29(8):623–628. doi: 10.2337/diab.29.8.623. [DOI] [PubMed] [Google Scholar]
- Gonen B., Rubenstein A., Rochman H., Tanega S. P., Horwitz D. L. Haemoglobin A1: An indicator of the metabolic control of diabetic patients. Lancet. 1977 Oct 8;2(8041):734–737. doi: 10.1016/s0140-6736(77)90237-9. [DOI] [PubMed] [Google Scholar]
- Graf R. J., Halter J. B., Porte D., Jr Glycosylated hemoglobin in normal subjects and subjects with maturity-onset diabetes. Evidence for a saturable system in man. Diabetes. 1978 Aug;27(8):834–839. doi: 10.2337/diab.27.8.834. [DOI] [PubMed] [Google Scholar]
- Hoberman H. D., Chiodo S. M. Elevation of the hemoglobin A1 fraction in alcoholism. Alcohol Clin Exp Res. 1982 Spring;6(2):260–266. doi: 10.1111/j.1530-0277.1982.tb04972.x. [DOI] [PubMed] [Google Scholar]
- Holmquist W. R., Schroeder W. A. A new N-terminal blocking group involving a Schiff base in hemoglobin AIc. Biochemistry. 1966 Aug;5(8):2489–2503. doi: 10.1021/bi00872a002. [DOI] [PubMed] [Google Scholar]
- Inada M., Oishi M., Nishikawa M., Kurata S., Imura H. Clinical evaluation of measuring glycosylated hemoglobin levels for assessing the long-term blood glucose control in diabetics. Endocrinol Jpn. 1980 Aug;27(4):411–415. doi: 10.1507/endocrj1954.27.411. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Blobstein S. H., Cerami A. Structure of carbohydrate of hemoglobin AIc. J Biol Chem. 1977 May 10;252(9):2992–2997. [PubMed] [Google Scholar]
- Koenig R. J., Peterson C. M., Jones R. L., Saudek C., Lehrman M., Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976 Aug 19;295(8):417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- Lanoe R., Soria J., Thibult N., Soria C., Eschwege E., Tchobroutsky G. Glycosylated haemoglobin concentrations and clinitest results in insulin-dependent diabetes. Lancet. 1977 Dec 3;2(8049):1156–1157. doi: 10.1016/s0140-6736(77)91541-0. [DOI] [PubMed] [Google Scholar]
- McDonald J. M., Davis J. E. Glycosylated hemoglobins and diabetes mellitus. Hum Pathol. 1979 May;10(3):279–291. doi: 10.1016/s0046-8177(79)80025-8. [DOI] [PubMed] [Google Scholar]
- McDonald M. J., Shapiro R., Bleichman M., Solway J., Bunn H. F. Glycosylated minor components of human adult hemoglobin. Purification, identification, and partial structural analysis. J Biol Chem. 1978 Apr 10;253(7):2327–2332. [PubMed] [Google Scholar]
- Paulsen E. P., Koury M. Hemoglobin AIc levels in insulin-dependent and -independent diabetes mellitus. Diabetes. 1976;25(2 Suppl):890–896. [PubMed] [Google Scholar]
- Shapiro R., McManus M. J., Zalut C., Bunn H. F. Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem. 1980 Apr 10;255(7):3120–3127. [PubMed] [Google Scholar]
- Stevens V. J., Fantl W. J., Newman C. B., Sims R. V., Cerami A., Peterson C. M. Acetaldehyde adducts with hemoglobin. J Clin Invest. 1981 Feb;67(2):361–369. doi: 10.1172/JCI110043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen P. A., Christiansen J. S., Søegaard U., Welinder B. S., Nerup J. Rapid changes in chromatographically determined haemoglobin A1c induced by short-term changes in glucose concentration. Diabetologia. 1980 Aug;19(2):130–136. doi: 10.1007/BF00421859. [DOI] [PubMed] [Google Scholar]
- Widness J. A., Rogler-Brown T. L., McCormick K. L., Petzold K. S., Susa J. B., Schwartz H. C., Schwartz R. Rapid fluctuations in glycohemoglobin (hemoglobin Alc) related to acute changes in glucose. J Lab Clin Med. 1980 Mar;95(3):386–394. [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Church D. B., Turtle J. R. The measurement of glycosylated hemoglobin in man and animals by aminophenylboronic acid affinity chromatography. Diabetes. 1982 Aug;31(8 Pt 1):701–705. doi: 10.2337/diab.31.8.701. [DOI] [PubMed] [Google Scholar]
- de Boer M. J., Miedema K., Casparie A. F. Glycosylated haemoglobin in renal failure. Diabetologia. 1980 Jun;18(6):437–440. doi: 10.1007/BF00261697. [DOI] [PubMed] [Google Scholar]



