Abstract
Objective
We hypothesized that elevated Galectin-3 (Gal-3) levels would identify patients with more advanced heart failure (HF) with preserved ejection fraction (HFpEF) as assessed by key pathophysiologic domains.
Background
Gal-3 is implicated in the pathogenesis of cardiac fibrosis but is also increased with normal aging and renal dysfunction. Cardiac fibrosis may contribute to cardiac dysfunction, exercise intolerance and congestion in HFpEF.
Methods
208 patients from the RELAX trial of sildenafil in HFpEF had Gal-3 measured at enrollment. Pathophysiologic domains assessed included biomarkers of neurohumoral activation, fibrosis, inflammation and myocardial necrosis, congestion severity and quality of life, cardiac structure and function, and exercise performance. Analysis adjusted for age, sex and/or cystatin-C levels. Potential interaction between baseline Gal-3 and treatment (sildenafil) effect on the RELAX primary endpoint (change in peak oxygen consumption) was tested.
Results
Gal-3 levels were associated with age and severity of renal dysfunction. Adjusting for age, sex and/or cystatin-C, Gal-3 was not associated with biomarkers of neurohumoral activation, fibrosis, inflammation or myocardial necrosis, congestion or quality of life impairment, cardiac remodeling or dysfunction or exercise intolerance. Gal-3 did not identify patients who responded to PDE-5 inhibitors (interaction p = 0.53).
Conclusion
In overt HFpEF, Gal-3 was related to severity of renal dysfunction and accounting for this, was not independently associated with severity of pathophysiologic derangements or response PDE-5 inhibition. These findings underscore the need to adjust for renal function when interpreting Gal-3 levels, and call into question the value of Gal-3 to quantify disease severity in overt HFpEF.
Keywords: Galectin-3, Heart Failure, Diastole, Biomarkers
Introduction
Approximately 50% of patients with chronic heart failure (HF) have preserved ejection fraction (HFpEF) (1). Coronary microvascular endothelial and myocardial inflammation may play a role in the genesis of cardiac fibrosis in HFpEF (2). Galectin-3 (Gal-3) is secreted by activated macrophages and has been implicated in the regulation of pro-inflammatory and pro-fibrotic pathways in the heart (3–6). In rodent models, myocardial Gal-3 expression predicts future HF (7) exogenous Gal-3 administration promotes fibrosis and HF (4) and genetic or pharmacologic inhibition of Gal-3 attenuates fibrosis and cardiac dysfunction in response to pro-fibrotic stimuli (6,8).
Given the role of myocardial inflammation and fibrosis in the pathogenesis of HFpEF, Gal-3 may serve as a novel biomarker of HFpEF severity which is incremental to established biomarkers and readily available clinical information such as renal function (9,10). In this regard, studies of persons without HF (11,12) and of patients with HF with reduced ejection fraction (HFrEF) (13–17), have shown that Gal-3 levels are associated with the severity of renal dysfunction.
While circulating levels of Gal-3 have been shown to be associated with outcomes (13–15,17–21), exercise intolerance (13,21) and treatment effect of statins (22) and angiotensin receptor blockers (17) in HFrEF, data regarding the relationship of Gal-3 levels to renal function, markers of HF severity or treatment response in HFpEF are lacking.
The RELAX (Phosphdiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure) trial tested the effect of sildenafil on exercise capacity in 216 well-characterized patients with HFpEF. We hypothesized that higher levels of Gal-3 would be associated with worse HF as evidenced by more severe derangements in biomarkers of neurohumoral activation, fibrosis, inflammation and myocardial necrosis, cardiac structure and function, exercise capacity, congestion and quality of life. Finally, while the RELAX trial showed no effect of sildenafil on exercise capacity in HFpEF, we hypothesized that higher Gal-3 levels might identify patients with more advanced myocardial derangements, pulmonary hypertension and right ventricular (RV) dysfunction, a subset postulated to be sensitive to sildenafil (23). Thus, we investigated the potential for interaction between Gal-3 levels and treatment effect of sildenafil.
Methods
The RELAX trial was conducted by the Heart Failure Clinical Research Network (HFN) and funded by the National Heart, Lung, and Blood Institute (24). All patients provided written informed consent and the trial was approved by the institutional review board at each participating site.
The design, entry criteria and results of the RELAX trial have been reported previously (24,25). Briefly, RELAX enrolled 216 outpatients who had ejection fraction (EF) ≥50% and objective evidence of HF. Additionally, patients were required to have elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP, ≥400 pg/mL) or elevated invasively measured filling pressures and reduced exercise capacity (≤60% age, sex and body size specific predicted peak oxygen consumption (VO2)). Patients with an estimated glomerular filtration rate (GFR; Modification of Diet in Renal Disease (MDRD) equation) < 20 ml/min/1.73m2 were ineligible.
Participants underwent baseline studies which included a history and physical examination, echocardiography, cardiac magnetic resonance imaging (CMRI) if in sinus rhythm (n=115), cardiopulmonary exercise test (CPXT), six-minute walk test, Minnesota Living with Heart Failure Questionnaire (MLHFQ), and phlebotomy for biomarkers (25).
Comprehensive Doppler echocardiography and CMRI were performed according to study protocols (26) with measurements performed at the HFN echocardiography (Mayo Clinic, Rochester, MN) and CMRI (Duke University, Durham, NC) core laboratories. CPXT was performed according to a RELAX specific protocol and interpreted by the HFN CPXT core laboratory (Massachusetts General Hospital, Boston, MA) as previously reported (25). Plasma biomarker measurements were performed by the HFN biomarker core laboratory (University of Vermont, Burlington, VT) as previously described (24,25) and included markers of neurohumoral activation (NT-proBNP, aldosterone, endothelin-1), renal function (cystatin-C, creatinine, uric acid), fibrosis (Gal-3, Pro-collagen III N-terminal peptide, C-telopeptide for type I collagen, CITP), and myocardial injury or inflammation (high-sensitivity Troponin-I and high-sensitivity C-reactive protein).
Gal-3 levels were measured by ELISA (R&D systems, Minneapolis, MN; cat #DGAL30). The manufacturer healthy reference listed a mean of 6.44 ng/ml, with a range of 2.03–15.5 ng/ml and a standard deviation of 2.1 ng/ml. This assay compared well to another widely used assay (BG Medicine ELISA, Waltham, MA) when tested in 70 RELAX patients (Gal-3BG =1.08 * Gal-3R&D + 3.61; Pearson R = 0.92).
Statistical analysis
Data are presented as medians (intra-quartile ranges) or proportions across Gal-3 tertiles. Differences across Gal-3 tertiles were tested with Kruskal Wallis, Chi-square, or Fisher’s exact, tests as appropriate. Multivariable least squares linear regression was used to adjust for age, sex and cystatin-C and for cystatin-C alone. These variables were chosen given their previously reported association with Gal-3 levels in persons without HF (11,12). Association between Gal-3 levels and variables of interest were analyzed with Gal-3 as a categorical variable (tertiles) and in a sensitivity analysis, as a continuous (log transformed) variable. Relationships of cystatin-C and GFR with Gal-3 are shown in scatterplots with regression lines and Pearson correlation coefficients. A general linear model adjusting for treatment group, baseline peak VO2 and Gal-3 levels was used to examine interaction between treatment group and Gal-3 levels on change in peak VO2 from baseline to 24 weeks. With our sample size, we had 80% and 90% power to detect correlations of 0.195 and 0.225 respectively, between Gal-3 and other continuous measures. Analyses were performed by the HFN data coordinating center using SAS version 9.2. A P<0.05 (2-sided) was considered statistically significant.
Results
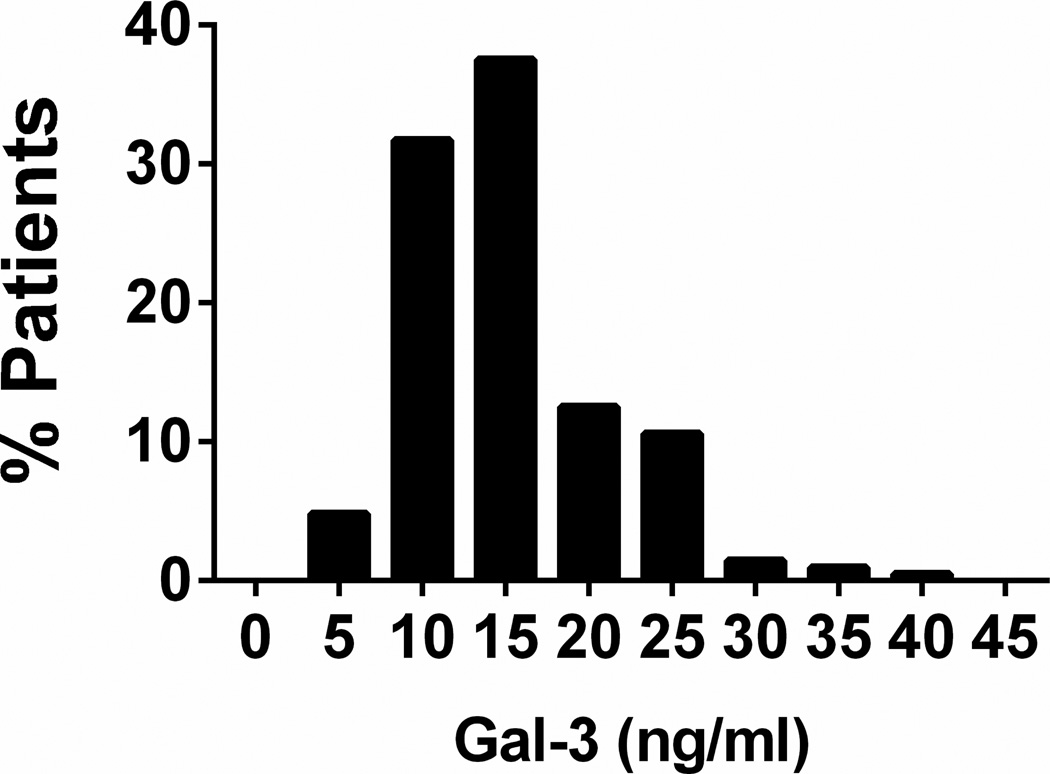
Of the 216 participants enrolled in RELAX, 208 had Gal-3 level data at baseline and comprise the study group. As previously reported, overall, patients in RELAX had a median age of 69 years, 52% were men and comorbidities (hypertension, diabetes, atrial fibrillation, coronary artery disease) were common (25). The median ejection fraction was 60% and participants were commonly treated with cardiovascular medications with 71% on angiotensin converting enzyme inhibitors and 11% on aldosterone antagonists. Median GFR was 64 mL/min/1.73m2. The median Gal-3 level was 13.8 ng/mL with a range from 4.2 to 41.6 ng/ml (Figure 1).
Figure 1. Frequency distribution of Galectin-3 levels in HFpEF.
Caption: The distribution of baseline Galectin-3 levels in the RELAX cohort (n=208). Median (IQR) Galectin-3 level: 13.8 (11.1–17.9) ng/mL
Clinical characteristics and Gal-3 levels in HFpEF
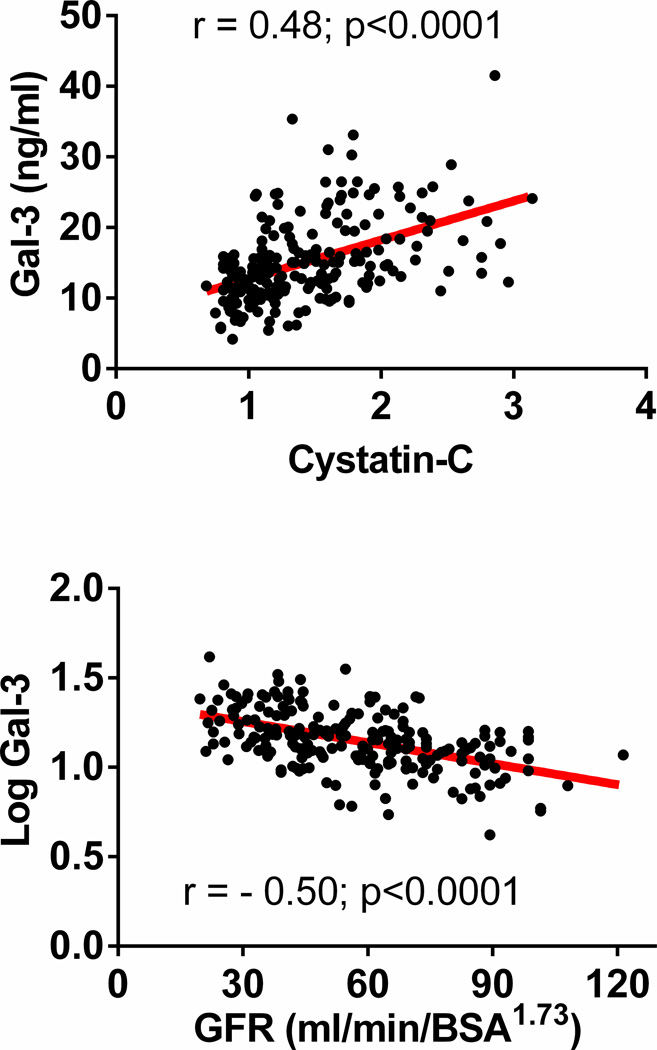
Participants with higher Gal-3 levels were older, had lower BSA and were more likely to have diabetes and be treated with mineralocorticoid receptor antagonists and diuretics (Table 1). Patients with higher Gal-3 levels had worse renal function (Table 1 and Figure 2) as assessed by creatinine, estimated GFR or cystatin-C and expected sequela of renal dysfunction including lower hemoglobin and higher uric acid. Age, sex and cystatin-C levels explained 24% of the variability in Gal-3 levels in HFpEF. Cystatin-C alone explained 23% of the variability in Gal-3 levels. Adjusting for age, sex and/or cystatin-C, patients with higher Gal-3 levels had lower BSA but there were no other statistically significant associations with clinical characteristics, medication use or likelihood of previous hospitalization.
Table 1.
Baseline patient characteristics by tertiles of baseline galectin-3 levels
| Low Gal-3 Tertile (N=69) |
Mid Gal-3 Tertile (N=70) |
High Gal-3 Tertile (N=69) |
P-value | P-value† | P-value‡ | |
|---|---|---|---|---|---|---|
| Gal-3 range, ng/ml | < 12.17 | 12.17 – 15.90 | > 15.90 | - | - | - |
| Gal-3, ng/mL | 10.0 (8.2 – 11.1) | 13.8 (13.1 – 15.1) | 21.0 (18.1 – 24.7) | - | - | - |
| Age, y | 67 (59 – 75) | 67 (62 – 77) | 71 (65 – 79) | 0.049 | - | 0.44 |
| Male | 39 (57) | 32 (46) | 37 (54) | 0.42 | - | 0.21 |
| Body Mass Index, Kg/m2 | 32.9 (28.7 – 37.5) | 33.2 (29.2 – 39.4) | 32.8 (28.1 – 39.6) | 0.61 | 0.48 | 0.32 |
| Body Surface Area, m2 | 2.17 (1.99 – 2.38) | 2.15 (1.89 – 2.36) | 2.07 (1.92 – 2.20) | 0.025 | 0.003 | 0.003 |
| Hypertension | 55 (80) | 59 (84) | 63 (91) | 0.16 | 0.59 | 0.60 |
| Ischemic heart disease | 24 (35) | 28 (40) | 28 (41) | 0.74 | 0.65 | 0.89 |
| Atrial fibrillation | 32 (46) | 33 (47) | 38 (55) | 0.53 | 0.57 | 0.44 |
| COPD | 12 (17) | 17 (24) | 13 (19) | 0.57 | 0.40 | 0.45 |
| Diabetes mellitus | 22 (32) | 29 (41) | 39 (57) | 0.013 | 0.24 | 0.58 |
| HF hospitalization | 21 (30) | 24 (34) | 31 (45) | 0.19 | 0.89 | 0.94 |
| Medications | ||||||
| ACE inhibitor or ARB | 47 (68) | 49 (70) | 52 (75) | 0.62 | 0.49 | 0.47 |
| Aldosterone antagonist | 7 (10) | 3 (4) | 12 (17) | 0.042 | 0.10 | 0.12 |
| Beta blocker | 49 (71) | 55 (79) | 55 (80) | 0.42 | 0.74 | 0.81 |
| Calcium channel blocker | 19 (28) | 22 (31) | 21 (30) | 0.87 | 0.72 | 0.76 |
| Loop diuretic | 45 (65) | 55 (79) | 58 (84) | 0.028 | 0.59 | 0.61 |
| Any diuretic | 53 (77) | 61 (87) | 64 (93) | 0.026 | 0.73 | 0.74 |
| Laboratories | ||||||
| Creatinine, mg/dL * | 0.9 (0.8 – 1.2) | 1.2 (0.8 – 1.3) | 1.3 (1.0 – 1.7) | <0.0001 | 0.90 | 0.48 |
| Cystatin-C, mg/L | 1.09 (0.91 – 1.30) | 1.24 (1.04 – 1.66) | 1.70 (1.33 – 2.04) | <0.0001 | - | - |
| GFR, mL/min/1.73 m2 * | 74 (60 – 92) | 63 (50 – 77) | 51 (35 – 68) | <0.0001 | 0.86 | 0.61 |
| Hemoglobin, mg/dl (n=198) | 13.4 (12.0 – 14.3) | 13.2 (12.2 – 14.1) | 12.4 (11.4 – 13.3) | 0.001 | 0.13 | 0.17 |
| Uric acid, mg/dL* | 6.6 (5.7 – 8.1) | 7.2 (5.9 – 8.3) | 8.0 (6.4 – 9.6) | 0.004 | 0.82 | 0.87 |
| NT pro BNP, pg/mL* | 524 (132 – 946) | 727 (232 – 1296) | 1187 (397 – 2205) | 0.003 | 1.00 | 0.88 |
| Endothelin-1, pg/ml* | 2.1 (1.8 – 2.7) | 2.4 (2.1 – 3.2) | 2.4 (2.1 – 3.5) | 0.004 | 0.051 | 0.073 |
| Aldosterone , pg/mL | 182 (113 – 272) | 183 (130 – 270) | 201 (116 – 312) | 0.83 | 0.97 | 0.96 |
| Pro-collagen III NTP, ug/L | 7.6 (5.3 – 9.1) | 7.0 (5.6 – 8.8) | 8.3 (6.7 – 11.5) | 0.023 | 0.57 | 0.55 |
| CITP I, ug/L | 5.2 (4.2 – 6.7) | 5.8 (4.3 – 9.0) | 8.0 (6.0 – 11.9) | <0.000 1 | 0.68 | 0.83 |
| hs CRP, mg/L | 3.1 (1.9 – 7.3) | 4.2 (1.9 – 8.5) | 3.8 (1.7 – 8.5) | 0.64 | 0.88 | 0.82 |
| hs Troponin I, pg/ml | 7.3 (4.4, 15.3) | 9.5 (5.5, 17.3) | 10.2 (6.7, 20.9) | 0.091 | 0.94 | 0.95 |
Data are median (IQR) or n (%);
Adjusted for Age, Sex, cystatin-C;
n=207;
Adjusted for cystatin-C alone
Abbreviations: ACE, Angiotensin Converting Enzyme; ARB, Angiotensin Receptor Blocker; CITP, C-telopeptide for Type I Collagen; COPD, Chronic Obstructive Pulmonary Disease; GFR, Glomerular Filtration Rate; HF, Heart Failure; hsCRP, High-sensitivity C-reactive Protein; NT Pro-BNP, N-terminal pro-brain natriuretic peptide
Figure 2. The relationship between Galectin-3 and renal function in HFpEF.
Raw Gal-3 levels increase with cystatin-C levels (A) and log10-transformed Gal-3 levels increase with decline in estimated glomerular filtration rate* (B). *Estimated from cystatin-C levels as previously described.(33)
Biomarkers and Gal-3 levels in HFpEF
NT-proBNP and endothelin-1 but not aldosterone levels were higher in patients with higher Gal-3 levels in bivariate analysis but not after adjusting for age, sex and/or cystatin-C (Table 1). Similarly, Gal-3 levels were associated with fibrosis biomarkers (Pro-collagen III N-terminal peptide and CITP) but not after adjustment for age, sex and/or cystatin-C. Gal-3 was not correlated with high-sensitivity C-reactive protein. Subjects with high Gal-3 levels tended to have higher high-sensitivity Troponin I levels, but not after adjusting for age, sex and/or cystatin-C.
Symptoms and Congestion and Gal-3 levels in HFpEF
Gal-3 levels were not associated with symptom severity as assessed by NYHA functional class or MLHFQ score (Table 2). Gal-3 appeared to be modestly associated with congestion as patients with higher Gal-3 levels had higher NT-proBNP levels (Table 1), a higher prevalence of orthopnea and a trend towards more peripheral edema (Table 2), but not after adjustment for age, sex and/or cystatin-C. There was no association between Gal-3 and jugular venous pressure elevation or rales on physical examination.
Table 2.
Congestion and quality of life by tertiles of baseline galectin-3 levels
| Low Gal-3 Tertile (N=69) |
Mid Gal-3 Tertile (N=70) |
High Gal-3 Tertile (N=69) |
p-value | p-value† | p-value‡ | |
|---|---|---|---|---|---|---|
| NYHA class II | 37 (54) | 31 (44) | 29 (42) | 0.35 | 0.77 | 0.74 |
| MLHFQ Score (n=200) | 44 (31 – 66) | 46 (32 – 61) | 40 (25 – 57) | 0.30 | 0.80 | 0.66 |
| Elevated JVP (n=202) | 28 (42) | 30 (44) | 33 (49) | 0.69 | 0.80 | 0.91 |
| Rales present | 2 (3) | 5 (7) | 7 (10) | 0.23 | 0.53 | 0.60 |
| S3 present | 3 (4) | 3 (4) | 2 (3) | 1.00 | 0.94 | 0.95 |
| ≥ Moderate edema | 6 (9) | 15 (21) | 20 (29) | 0.01 | 0.12 | 0.12 |
| Orthopnea (n=194) | 34 (53) | 41 (64) | 47 (71) | 0.10 | 0.35 | 0.43 |
Data are median (IQR) or n (%); Data available in all 208 participants except as noted
Adjusted for Age, Sex, cystatin-C;
Adjusted for cystatin-C alone
Abbreviations: JVP, Jugular Venous Pressure; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; S3; S3 Gallop
Cardiovascular structure and function and Gal-3 levels in HFpEF
Subjects with higher Gal-3 levels had greater relative wall thickness by echocardiography but not after adjusting for age, sex and/or cystatin-C (Table 3). There was no similar trend in the CMRI cohort (LV mass / LV volume ratio). Neither body size indexed LV diastolic dimension or mass, EF, LV diastolic function parameters nor pulmonary artery systolic pressure differed across Gal-3 tertiles by echo or CMRI in unadjusted analysis or when adjusting for age, sex and cystatin-C.
Table 3.
Baseline cardiac structure and function by tertiles of baseline galectin-3 levels
| n‡ | Low Gal-3 Tertile (N=69) |
Mid Gal-3 Tertile (N=70) |
High Gal-3 Tertile (N=69) |
p-value | p-value w† | p-value‡ | |
|---|---|---|---|---|---|---|---|
| Echocardiography | |||||||
| LVEDd/BSA, cm/ m2 | 157 | 2.2 (2.1 – 2.5) | 2.2 (2.0 – 2.4) | 2.2 (2.0 – 2.4) | 0.96 | 0.96 | 0.98 |
| Relative wall thickness | 151 | 0.38 (0.35 – 0.44) | 0.40 (0.34 – 0.51) | 0.45 (0.40 – 0.52) | 0.006 | 0.71 | 0.73 |
| LV mass/BSA, g/m2 | 151 | 75 (63 – 88) | 77 (63 – 91) | 80 (60 – 102) | 0.78 | 0.97 | 0.93 |
| LV mass / height1.7, g/m1.7 | 151 | 63 (52 – 76) | 67 (54 – 84) | 66 (54 – 88) | 0.64 | 0.91 | 0.85 |
| Ejection fraction, % | 205 | 61 (57 – 65) | 60 (55 – 67) | 60 (56 – 65) | 0.47 | 0.66 | 0.66 |
| E/A ratio | 137 | 1.2 (1.0 – 2.0) | 1.5 (1.0 – 2.0) | 1.3 (0.9 – 2.0) | 0.55 | 0.23 | 0.34 |
| Medial e', m/sec | 190 | 0.06 (0.05 – 0.07) | 0.06 (0.05 – 0.09) | 0.06 (0.04 – 0.08) | 0.22 0.17 | 0.13 0.99 | 0.20 |
| Medial E/e' | 181 | 14.6 (10.0 – 20.0) | 15.7 (12.0 – 22.3) | 17.5 (13.3 – 25.0) | 0.94 | ||
| Deceleration time, ms | 186 | 181 (155 – 219) | 190 (158 – 215) | 183 (153 – 220) | 0.95 0.48 | 0.94 0.59 | 0.96 |
| LA volume/BSA, mL/m2 | 144 | 43 (33 – 59) | 42 (34 – 58) | 46 (38 – 59) | 0.84 | ||
| PASP, mmHg | 131 | 41 (35 – 54) | 37 (30 – 49) | 45.(35 – 51) | 0.19 | 0.24 | 0.26 |
| CMRI | |||||||
| LVEDV / BSA, mL/ m2 | 115 | 55 (46 – 64) | 56 (44 – 67) | 59 (51 – 71) | 0.46 0.17 | 0.69 0.62 | 0.70 |
| LV mass/LV volume, g/ml | 115 | 1.19 (1.00 – 1.47) | 1.05 (0.86 – 1.40) | 1.05 (0.98 – 1.46) | 0.17 | ||
| Ejection fraction, % | 115 | 68 (57 – 71) | 66 (64 – 70) | 0.70 0.33 | 0.25 0.54 | 0.22 | |
| LV mass/BSA, g/m2 | 115 | 65 (57 – 69) | 60 (52 – 72) | 65 (55 – 77) | 0.090 | ||
| LV mass / height1.7, g/m1.7 | 115 | 64 (54 – 83) | 51 (45 – 61) | 59 (46 – 71) | 0.26 | 0.56 | 0.056 |
| Vascular Function | 57 (46 – 73) | ||||||
| Systolic BP, mmHg | 208 | 126 (116 – 137) | 128 (112 – 142) | 124 (112 – 138) | 0.81 | 0.81 | 0.73 |
| Diastolic BP, mmHg | 208 | 70 (65 – 80) | 72 (64 – 78) | 67 (60 – 74) | 0.020 | 0.46 | 0.37 |
| Ao distensability, 10−3 mm Hg−1 | 85 | 1.22 (0.70 – 1.64) | 1.20 (0.71 – 2.97) | 1.00 (0.58 – 1.76) | 0.63 | 0.042 | 0.59 |
Data are median (IQR); ‡ total n with data
Adjusted for Age, Sex, cystatin-C;
Adjusted for cystatin-C alone
Abbreviations: Ao, Aortic; CMRI, Cardiac Magnetic Resonance Imaging; LA, Left Atrium; LVEDd, Left Ventricular End Diastolic Dimension; LVEDV, Left Ventricular End Diastolic Volume; PASP, Pulmonary Artery Systolic Pressure
There was no association between systolic blood pressure and Gal-3 levels. Subjects with higher Gal-3 levels displayed lower diastolic blood pressure, but not after adjusting for age, sex and/or cystatin-C (Table 3). In the CMRI subset, adjusting for age, sex and cystatin-C, aortic distensibility was lower in subjects with higher Gal-3 levels,but this measurement was only available in a small subset of patients.
Exercise Performance and Gal-3 levels in HFpEF
Patients with higher Gal-3 levels had lower peak VO2 despite similar effort (respiratory exchange ratio) and peak exercise systolic blood pressure (Table 4). Patients with higher Gal-3 levels also had lower peak heart rate and chronotropic index. Six-minute walk distance was lower in patients with higher Gal-3 levels. However, when adjusted for age, sex and/or cystatin-C, associations between Gal-3 levels and exercise performance and chronotropic reserve were no longer significant. There was also no association between Gal-3 levels and peak VO2 when adjusting for known modifiers of peak VO2 (age, sex, BMI, hemoglobin and chronotropic index; p=0.42). There was no association between Gal-3 levels and ventilatory efficiency (VE/VCO2 slope).
Table 4.
Exercise performance by tertile of baseline galectin-3 levels
| Low Gal-3 Tertile (N=69) |
Mid Gal-3 Tertile (N=70) |
High Gal-3 Tertile (N=69) |
p-value | p-value† | p-value‡ | |
|---|---|---|---|---|---|---|
| Peak VO2, mL/kg/min | 13.3 (10.8 – 16.2)) | 11.7 (10.9 – 13.7) | 11.1 (9.5 – 13.7) | 0.004 | 0.68 | 0.24 |
| Respiratory exchange ratio | 1.11 (1.03 – 1.18) | 1.08 (1.02 – 1.14) | 1.09 (1.04 – 1.15) | 0.40 | 0.50 | 0.48 |
| Peak Systolic BP, mmHg | 158 (140 – 174) | 157 (133 – 173) | 148 (129 – 166) | 0.19 | 0.67 | 0.61 |
| Rest HR, bpm | 70.0 (61.5 – 77.0) | 70 (60 – 80) | 65 (59 – 75) | 0.17 | 0.46 | 0.40 |
| Peak HR, bpm | 114 (101 – 133) | 111 (93 – 128) | 97 (86 – 118) | 0.003 | 0.55 | 0.37 |
| Chronotropic index | 0.57 (0.36 – 0.69) | 0.53 (0.32 – 0.71) | 0.37 (0.25 – 0.61) | 0.034 | 0.74 | 0.72 |
| VE/VCO2 slope | 32.4 (28.2 – 37.1) | 31.7 (28.5 – 36.7) | 34.1 (28.9 – 37.9) | 0.69 | 0.92 | 0.99 |
| 6-Minute walk distance, meters | 310 (251 – 400) | 325 (244 – 384) | 290 (202 – 349) | 0.027 | 0.46 | 0.49 |
Total n with data (201–208);
Adjusted for Age, Sex, cystatin-C;
Adjusted for cystatin-C alone.
Abbreviations BP, Blood Pressure; HR, Heart Rate
Sensitivity analysis
Findings were similar when analyses were performed with Gal-3 as a continuous, log-transformed variable (data not shown).
Gal-3 levels as a biomarker of response to sildenafil
There was no interaction between baseline Gal-3 and treatment group (sildenafil vs placebo) on the RELAX primary endpoint of change in peak VO2 after 6 months of therapy (P-value for interaction = 0.53).
Discussion
In keeping with recent recommendations (9,10), this robust analysis assessed the performance of Gal-3 as an independent biomarker of HFpEF severity adjusting for readily available information. In this comprehensively phenotyped cohort of HFpEF patients, Gal-3 levels were associated with age, smaller body size and severity of renal dysfunction. Adjusting for age, sex and cystatin-C or cystatin-C alone, Gal-3 levels were not associated with clinical characteristics or comorbidities, symptomatic status or congestion, severity of LV remodeling or dysfunction or exercise performance. Patients with higher Gal-3 levels had more neurohumoral activation and higher levels of fibrosis biomarkers but no evidence of increased inflammation and these associations were largely eliminated after adjustment for age, sex and/or cystatin-C. The absence of treatment effect (sildenafil versus placebo) on exercise capacity in RELAX was consistent among patients regardless of Gal-3 levels. Our negative findings are additive to the scant available literature concerning Gal-3 in HFpEF and do not suggest that Gal-3 levels reflect disease severity in overt HFpEF.
Gal-3 as a biomarker in HFpEF
The median level of Gal-3 in the ambulatory HFpEF cohort enrolled in RELAX was over twice the assay specific normal level and similar to (13,15) or slightly lower (17,27) than mean levels reported in various HFrEF cohorts. Many previous studies used the BG assay. Given the relationship between the two assays, Gal-3 levels would be 3–5 ng/ml higher in RELAX had the BG assay been used. Thus, plasma Gal-3 levels in RELAX was similar to or higher than that observed in HFrEF cohorts.
Association of Gal-3 levels with renal function
As observed here in HFpEF, community cross-sectional studies indicate that Gal-3 levels increase with age and renal dysfunction and also show that Gal-3 levels are higher in women than in men (11,12). Our study did not include an age, sex and cystatin-C matched non-HF cohort so the increase in Gal-3 conferred by the HFpEF state (vs renal dysfunction) cannot be assessed. Gopal et al studied acutely decompensated or stable HFrEF or HFpEF patients, chronic kidney disease patients without HF and normal controls and reported that Gal-3 levels increase exponentially with the severity of renal dysfunction and that this relationship is similar regardless of presence, type or severity of HF (16). In RELAX, the relationship between renal dysfunction and Gal-3 levels was linear and while strong, weaker than that reported in the Gopal study which included patients with GFR<20 and noted an exponential relationship driven by extremely high Gal-3 levels in patients with GFR<20 (excluded in RELAX).
The mechanism underlying the association between renal impairment and Gal-3 levels is unclear and could include renal production or clearance of Gal-3 or covariance mediated by conditions common to or causative of renal impairment and HF (16). Of note, myocardial Gal-3 levels were up-regulated in cardiac tissue from patients with aortic stenosis associated with systolic dysfunction (4) but not in myocardial specimens from patients with HFrEF despite elevated plasma Gal-3 levels (28,29), and Gal-3 levels do not decrease post total artificial heart implantation (30). Thus, the source of elevated Gal-3 levels in HF is unclear and may be predominantly non-cardiac.
Association of Gal-3 levels with cardiac structure and function
While an association between Gal-3 and ventricular dysfunction was demonstrated in animal studies (6), a relationship between circulating Gal-3 levels and the severity of cardiac remodeling or dysfunction in human studies is less clear (11,15,31). In a cross-sectional volunteer cohort, Gal-3 levels were modestly associated with LV mass but this analysis did not adjust for renal function (11). In a diverse cohort of patients evaluated in an emergency department for dyspnea and subsequently found to have HFrEF, HFpEF or non-cardiac dyspnea (31), Gal-3 levels were higher in HF than non-cardiac dyspnea. In analysis including patients with and without HF, Gal-3 levels were associated with echocardiographic indices of LV diastolic dysfunction, RV systolic dysfunction and pulmonary artery pressures, but this analysis did not adjust for age, sex or renal function or examine the association of Gal-3 and cardiac parameters within HF cohorts. In a study of HFrEF patients, Gal-3 levels were not associated with echocardiographic parameters or invasively measured hemodynamic indices (15). In RELAX, concentric remodeling was more severe in patients with higher Gal-3 levels but there was no association between Gal-3 levels and the severity of LV hypertrophy, systolic or diastolic dysfunction or pulmonary artery systolic pressure and the modest association with concentric remodeling was no longer apparent after adjusting for age, sex and/or cystatin-C.
Association of Gal-3 levels with functional capacity
In HFpEF patients, we show that higher Gal-3 levels were associated with lower peak VO2 and six-minute walk distance but that this association was lost after adjusting for age, sex and/or cystatin-C. In chronic HFrEF patients, higher Gal-3 levels were associated with poorer functional capacity as assessed by peak VO2, 6 minute walk distance or NYHA class (13,21), but these studies did not adjust for renal function.
Gal-3 as a predictor of treatment response
We had hypothesized that higher Gal-3 levels might identify patients with more advanced myocardial derangements, pulmonary hypertension and RV dysfunction, a subset postulated to be sensitive to sildenafil (23). However, no such associations were apparent and we saw no interaction between treatment effect and Gal-3 levels.
In contrast to our findings in HFpEF, post-hoc analysis of clinical trials in HFrEF suggest that Gal-3 levels may identify patients responding to certain therapies. Although there was no overall benefit of rosuvasatin on outcomes in the controlled rosuvastatin multinational trial in HFrEF (CORONA), a post-hoc subgroup analysis showed those with lower Gal-3 levels appeared to benefit from statin therapy (22). A larger sub-group analysis from CORONA showed that patients with lower NT-proBNP levels also benefited from statins (32). Those with lower Gal-3 or NT-proBNP had milder HF suggesting that both Gal-3 and NT-proBNP identify patients with milder HF who may survive to experience a vascular rather than HF event. In a post-hoc analysis from the Val-HeFT trial, the incidence of HF hospitalization was lower with valsartan than placebo therapy among patients with lower Gal-3 levels, but no interaction between Gal-3 levels and treatment effect was noted for the trial’s primary endpoint of mortality or first morbid event (17). Milting et al (30) showed that Gal-3 levels at ventricular assist device implantation were significantly higher in patients who did not survive mechanical support due to multi-organ failure but did not adjust for baseline renal function, a known risk factor for poor outcomes after left ventricular assist device. While Gal-3 levels did not identify a group of HFpEF patients who responded to sildenafil, analysis of interaction between treatment effect and Gal-3 levels in clinical trials of other agents in HFpEF, including mineralocorticoid receptor antagonists will be of interest.
Limitations
The specific Gal-3 assay used in this study (R&D) differs from previous studies but is well correlated with the BG assay. The RELAX cohort had relatively advanced HFpEF and this may limit correlations although the range of Gal-3 levels were fairly broad. The association of Gal-3 levels and outcomes was not assessed in RELAX given the short duration of follow up and modest sample size. These data do not exclude the possibility that transient myocardial Gal-3 activation occurs in HF and contributes to myocardial fibrosis.
Conclusion
In HFpEF, Gal-3 levels were associated with higher age and worse renal function, but adjusting for age and renal function, were not independently associated with severity of pathophysiologic derangements in HFpEF and did not identify patients responding to PDE-5 inhibition. These findings underscore the need to adjust for renal function when interpreting Gal-3 levels and call into question the independent value of Gal-3 to quantify disease severity in overt HFpEF.
Acknowledgments
GMF consults for Singulex, Roche Diagnostics, and receives grant support from NIH and Roche Diagnostics. HHC reports that he and Mayo Clinic have patented and licensed designer natriuretic peptides to Anexon Inc and Capricor Therapeutics. Dr Chen is the co-founder of Zumbro Discovery. EB receives grant support to his institution from Duke University for his role as Chair of the NHLBI sponsored Heart Failure Network.
Sources of Funding: This study was supported by grants from the NHLBI: U01HL084904 (for the data coordinating center), and U01HL084861, U01HL084875, U01HL084877, U01HL084889, U01HL084890, U01HL084891, U01HL084899, U01HL084907, and U01HL084931 (for the clinical centers). This study was not supported by industry.
Abbreviations
- BSA
Body Surface Area
- CITP
C-telopeptide for Type I Collagen
- CPXT
Cardiopulmonary Exercise Test
- Gal-3
Galectin-3
- GFR
Glomerular Filtration Rate
- HF
Heart Failure
- HFN
Heart Failure Clinical Research Network
- HFpEF
Heart Failure with Preserved Ejection Fraction
- HFrEF
Heart Failure with Reduced Ejection Fraction
- V02
Oxygen Consumption
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All others report no disclosures relevant to this manuscript.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 3.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. European Journal of Heart Failure. 2009;11(9):811–817. doi: 10.1093/eurjhf/hfp097. [DOI] [PubMed] [Google Scholar]
- 4.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110(19):3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 5.Liu YH, D'Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, et al. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009;296(2):H404–H412. doi: 10.1152/ajpheart.00747.2008. PMCID: 2643891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6(1):107–117. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 7.Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, et al. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95(5):515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 8.Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33(1):67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 9.Januzzi JL, Jr, Felker GM. Surfing the biomarker tsunami at JACC: heart failure. JACC Heart Fail. 2013;1(3):213–215. doi: 10.1016/j.jchf.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad T, Fiuzat M, Pencina MJ, Geller NL, Zannad F, Cleland JG, et al. Charting a Roadmap for Heart Failure Biomarker Studies. JACC Heart Fail. 2014 doi: 10.1016/j.jchf.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60(14):1249–1256. doi: 10.1016/j.jacc.2012.04.053. PMCID: 3512095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. 2012;272(1):55–64. doi: 10.1111/j.1365-2796.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- 13.Felker GM, Fiuzat M, Shaw LK, Clare R, Whellan DJ, Bettari L, et al. Galectin-3 in ambulatory patients with heart failure: results from the HF-ACTION study. Circ Heart Fail. 2012;5(1):72–78. doi: 10.1161/CIRCHEARTFAILURE.111.963637. PMCID: 3261343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lok D, Bruggink P, van der Meer P. Prognostic value of galectin-3, a novel marker of fibrosis. Clin Res Cardiol. 2010;99(8):529. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang WH, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD, et al. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol. 2011;108(3):385–390. doi: 10.1016/j.amjcard.2011.03.056. PMCID: 3137764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopal DM, Kommineni M, Ayalon N, Koelbl C, Ayalon R, Biolo A, et al. Relationship of plasma galectin-3 to renal function in patients with heart failure: effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J Am Heart Assoc. 2012;1(5):e000760. doi: 10.1161/JAHA.112.000760. PMCID: 3541630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN. Baseline and serial measurements of galectin-3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur J Heart Fail. 2013;15(5):511–518. doi: 10.1093/eurjhf/hfs205. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Andrès N, Rossignol P, Iraqi W, Fay R, Nuée J, Ghio S, et al. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. European Journal of Heart Failure. 2012;14(1):74–81. doi: 10.1093/eurjhf/hfr151. [DOI] [PubMed] [Google Scholar]
- 19.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43(1):60–68. doi: 10.3109/07853890.2010.538080. PMCID: 3028573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48(6):1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 21.Ueland T, Aukrust P, Broch K, et al. Galectin-3 in heart failure: high levels are associated with all-cause mortality. Int J Cardiol. 2011;150(3):361–364. doi: 10.1016/j.ijcard.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 22.Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, et al. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) Eur Heart J. 2012;33(18):2290–2296. doi: 10.1093/eurheartj/ehs077. [DOI] [PubMed] [Google Scholar]
- 23.Forfia PR, Borlaug BA. Letter by Forfia and Borlaug regarding article, "Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study". Circulation. 2012;125(8):e408. doi: 10.1161/CIRCULATIONAHA.111.064584. author reply e9–10. [DOI] [PubMed] [Google Scholar]
- 24.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2013;309(12):1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, et al. PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale and design. Circulation Heart failure. 2012;5(5):653–659. doi: 10.1161/CIRCHEARTFAILURE.112.969071. PMCID: 3530955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 27.van der Velde AR, Gullestad L, Ueland T, Aukrust P, Guo Y, Adourian A, et al. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail. 2013;6(2):219–226. doi: 10.1161/CIRCHEARTFAILURE.112.000129. [DOI] [PubMed] [Google Scholar]
- 28.Erkilet G, Ozpeker C, Bothig D, Kramer F, Rofe D, Bohms B, et al. The biomarker plasma galectin-3 in advanced heart failure and survival with mechanical circulatory support devices. J Heart Lung Transplant. 2013;32(2):221–230. doi: 10.1016/j.healun.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Beiras-Fernandez A, Weis F, Rothkopf J, Kaczmarek I, Ledderose C, Dick A, et al. Local expression of myocardial galectin-3 does not correlate with its serum levels in patients undergoing heart transplantation. Ann Transplant. 2013;18:643–650. doi: 10.12659/AOT.889396. [DOI] [PubMed] [Google Scholar]
- 30.Milting H, Ellinghaus P, Seewald M, Cakar H, Bohms B, Kassner A, et al. Plasma biomarkers of myocardial fibrosis and remodeling in terminal heart failure patients supported by mechanical circulatory support devices. J Heart Lung Transplant. 2008;27(6):589–596. doi: 10.1016/j.healun.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12(8):826–832. doi: 10.1093/eurjhf/hfq091. PMCID: 2913048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cleland JG, McMurray JJ, Kjekshus J, Cornel JH, Dunselman P, Fonseca C, et al. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) J Am Coll Cardiol. 2009;54(20):1850–1859. doi: 10.1016/j.jacc.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 33.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. PMCID: 2390827. [DOI] [PMC free article] [PubMed] [Google Scholar]