Abstract
Background
To assess the risk of cerebrovascular accidents (CVAs) and second brain tumors (SBTs) in patients with pituitary adenoma after surgery or radiotherapy.
Methods
A cohort of 143 people from Olmsted County, who were diagnosed with pituitary adenoma between 1933 and 2000, was studied. Only patients from Olmsted County were included because of the unique nature of medical care in Olmsted County, which allows the ascertainment of virtually all cases of pituitary adenoma for this community's residents and comparisons to the general population in the county. Surgical resection was performed in 76 patients, 29 patients underwent radiotherapy (with 21 undergoing both surgery and radiotherapy), 5 patients were reirradiated, and 59 patients were managed conservatively and observed.
Results
Median follow-up was 15.5 years. There was no difference in CVA-free survival between treatment groups. On univariate analysis age > 60 years (hazard ratio [HR], 11.93; 95% CI, 6.26–23.03; P < .001); male sex (HR, 3.67; 95% CI, 2.03–6.84; P < .001), and reirradiation (HR, 3.41; 95% CI, 1.05–9.68; P = .04) were associated with worse CVA-free survival. In multivariate analysis, only age > 60 years was associated with worse CVA-free survival. Compared with the general population, there was a 4-fold increase in the rate of CVAs in pituitary adenoma patients (HR, 4.2; 95% CI, 2.8–6.1). Two patients developed SBT (an irradiated patient and a surgically managed patient).
Conclusion
CVA is a significant risk for patients with pituitary tumors, but treatment does not seem to impact the risk. Even with long-term follow-up, SBTs are a rare event regardless of treatment modality.
Keywords: pituitary adenoma, radiotherapy, secondary malignancy, stroke, surgery
Radiotherapy is a well-established treatment modality for pituitary adenomas and is commonly utilized after subtotal resection of nonsecreting tumors to prevent progression of tumor or achieve hormonal control of secreting adenomas when it cannot be achieved with surgery or medical management. Radiotherapy has been found to be quite effective for decreasing the recurrence rate at 10 years after surgery from 51% down to 2%.1 However, even with these high rates of success, there are concerns regarding the side effects of radiotherapy, particularly late side effects because patients with pituitary adenomas have long expected survival times. Two late radiation side effects of particular concern are secondary brain tumors and cerebrovascular accidents (CVAs). However, there are conflicting reports on whether radiotherapy for pituitary adenomas increases the risk for secondary brain tumors2,3 or CVAs.4,5
When assessing the risk of CVA in patients with pituitary adenomas, it is important to compare a similar representative population that does not have a pituitary tumor to provide a baseline risk of CVA. Virtually all patients living in Olmsted County, Minnesota, are treated at Mayo Clinic or Olmsted Medical Center. In addition, virtually all Olmsted County patients diagnosed with a complex medical issue (eg, pituitary neoplasm) are evaluated at Mayo Clinic due to the geographic isolation of this community from other tertiary medical centers.6 The Rochester Epidemiology Project is a collaboration between health care providers in Olmsted County that captures all medical diagnoses. This population-based database allows the exact determination of CVA incidence in Olmsted County. Therefore, to investigate the risk of CVA and second brain tumors in patients with pituitary adenomas and allow population-based comparisons, we studied all patients diagnosed with a pituitary tumor in Olmsted County between 1933 and 2000.
Materials and Methods
Patients
With Mayo Clinic Institutional Review Board approval, the medical records for Olmsted County residents diagnosed with a pituitary tumor between 1933 and 2000 were reviewed. In accordance with Minnesota state statutes, living patients were required to consent to review of their medical records. No patients who met the eligibility criteria refused research authorization, and therefore no patients were excluded from this study.
Radiotherapy
All patients receiving radiotherapy were treated at Mayo Clinic with localized fields. The energy of the external-beam radiation devices changed over time, with 7 (24%) patients treated with orthovoltage (3 mm Cu to 4 mm Cu HVL), 4 (14%) with cobalt (80 cm teletherapy or Gamma Knife), and 18 (62%) with megavoltage (4 MV or 6 MV).
Follow-up and Endpoints
Demographic, patient and treatment characteristics, survival, CVA, and second brain tumor information were obtained by review of individual charts, Social Security Death Index, and death certificates. Radiation-induced secondary brain tumors are classically defined by Cahan et al. as being located in the previously irradiated field, having a latency period of several years, and demonstrating histological features different from those of the primary lesion.7 For the current study of patients previously treated with radiotherapy, any second cranial tumor, regardless of radiation beam path, was defined as being located in the previously irradiated field. In the time-to-event analyses, the date of diagnosis of pituitary tumor was utilized as the start point to allow comparisons with those patients who underwent conservative management and observation after their diagnosis of a pituitary tumor. Overall survival (OS) and CVA were determined from the last date of confirmed contact with a health care provider or death. Follow-up data were collected through May 2012.
Statistical Analysis
The 2-tailed Fisher exact test was used to assess for pretreatment and treatment variables potentially associated with the development of CVA and second brain tumors.8 Variables considered were age, sex, extent of initial surgery (gross vs radical subtotal resection vs subtotal resection), treatment, radiotherapy, repeat radiotherapy, and tumor size (microadenoma vs macroadenoma). The Kaplan-Meier method was used to estimate OS and development of CVA.9 Univariate analyses were conducted using the log-rank test to assess whether the pretreatment and treatment variables listed above were associated with OS, CVA, and second brain tumors.10
The incident CVA rate after diagnosis of pituitary tumor was compared with the incident stroke rate in the population of Rochester, Minnesota, using data from the Rochester Stroke Registry11 to calculate standardized morbidity ratios. Expected CVA rates were calculated by applying sex-, age-, and period-specific rates in the general population of Rochester to the person-time follow-up of those with a diagnosis of pituitary tumor. The standardized morbidity ratio was calculated by dividing the number of observed strokes by the expected number of strokes over the duration of follow-up. To be conservative, the maximum expected number of strokes from 1955–1965 was used to estimate the expected number of strokes in the time period before 1955, and the maximum number of strokes from 1980–1989 was used to estimate the expected number of strokes after 1990. In sensitivity analysis, the minimum values were also used, and the results were similar. Confidence intervals were calculated assuming a Poisson distribution. For all statistical tests, a P value <.05 was considered significant. Cox regression analysis was utilized for multivariate analysis.12 Statistical analysis was performed with JMP 10.0 and SAS Version 9.2 (SAS Institute).
Results
A total of 143 Olmsted County residents were diagnosed with a pituitary tumor between the years of 1933 and 2000 (Table 1). The median age was 36 years (range, 12–87 years). The majority of patients were female (62%) with macroadenoma (76%), and secreting tumors (67%). Surgical resection was performed in 76 patients (53%), and 29 patients (20%) underwent radiotherapy with 21 of them undergoing both surgery and radiotherapy. Fifty-nine patients (41%) underwent conservative management and were simply observed after their diagnosis. Patients with microadenomas were more likely to undergo conservative management than patients with macroadenomas (P < .001). Of the patients who received radiotherapy, 27 were treated with fractionated radiotherapy at a median dose of 45 Gy (range, 17.6–60 Gy) and median daily fraction size of 2 Gy, while 2 patients were treated with radiosurgery (25 Gy) to the 50% isodose line covering the tumor margin in a single fraction. Five patients received repeat fractionated radiotherapy (median dose, 11.3 Gy; range, 8.4–50 Gy). Median interval between the original radiation treatment and the repeat radiation treatment was 1.4 years (range, 1.1–8.4 years).
Table 1.
Patient demographics and clinical characteristics
| No. of Patients | |
|---|---|
| Sex | |
| Male | 55 |
| Female | 88 |
| Age at diagnosis | |
| Median | 36 years |
| Range | 12–87 years |
| Tumor size | |
| Macroadenoma | 108 |
| Microadenoma | 35 |
| Tumor type | |
| Nonsecreting | 47 |
| Secreting | 96 |
| Prolactin | 55 |
| GH | 11 |
| ACTH | 5 |
| TSH | 1 |
| Prolactin + GH | 5 |
| Mixed other | 19 |
| Treatment | |
| Conservative management | 59 |
| Surgery | 76 |
| Radiation | 29 |
| Surgery plus radiation | 21 |
| Repeat radiation | 5 |
| Extent of primary surgery | |
| Subtotal resection | 11 |
| Radical subtotal resection | 15 |
| Gross total resection | 50 |
| Dose of radiotherapy (cGy) | |
| <3 500 | 8* |
| 3 500–4 499 | 5 |
| 4 500–5 499 | 12 |
| 5 500–6 499 | 4 |
Abbreviations: ACTH, adrenocorticotropic hormone; GH, growth hormone; TSH, thyroid-stimulating hormone.
*2 received radiosurgery 25 Gy in single fraction to tumor margin.
The median follow-up after diagnosis of a pituitary tumor for the entire cohort was 15.5 years (range, 0.44–63 years). The median follow-up was longer for those patients who received radiotherapy (24.8 vs 14.4 years for those who did not receive radiotherapy; P < .001).
Overall Survival
For the entire cohort, the OS at 15 years was 74%. Median survival for conservative management was 13.9 years versus 16.5 years for those who underwent treatment (P = .04). Median survival was better for those treated with radiotherapy (24.8 vs 14.2 years; P = .001). Surgery did not have an impact on median survival (16.3 vs 13.9 years; P = .34).
Cerebrovascular Accidents
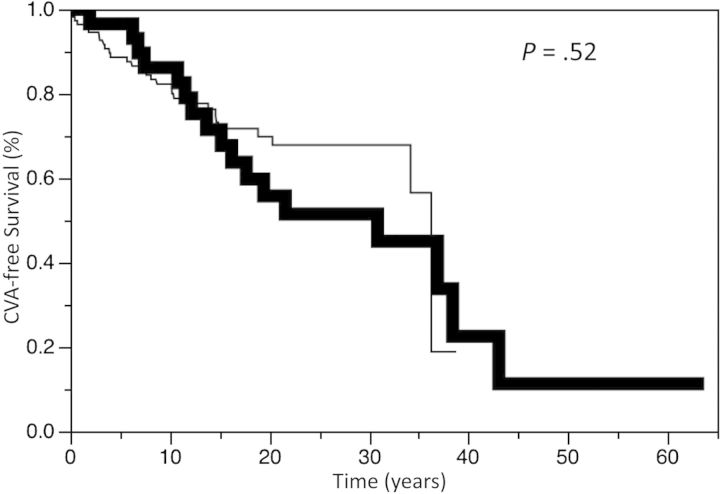
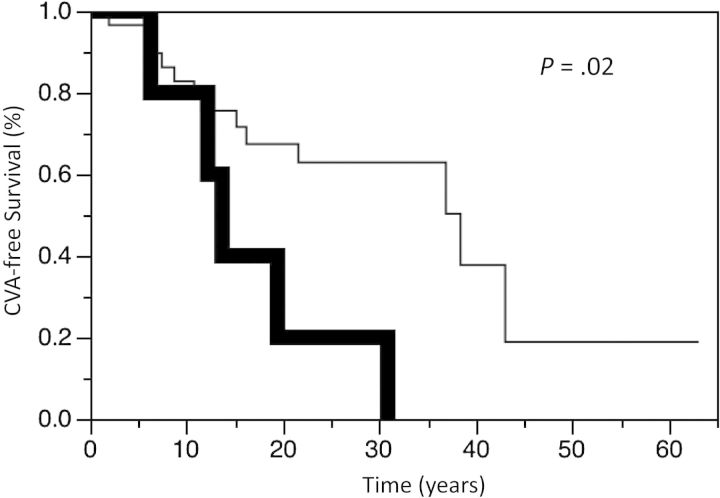
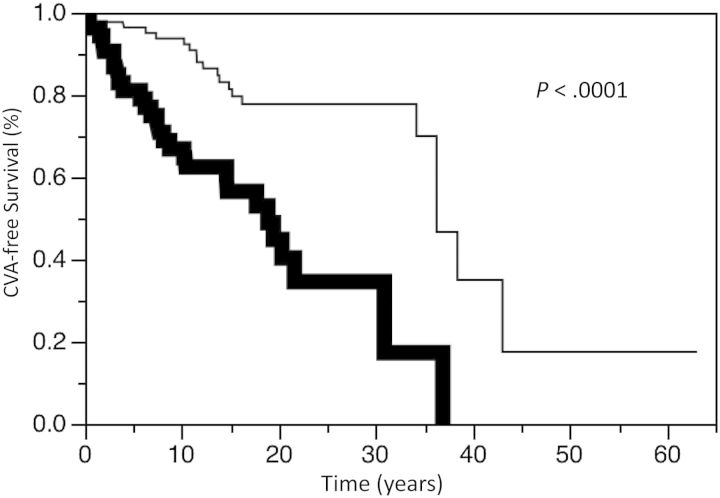
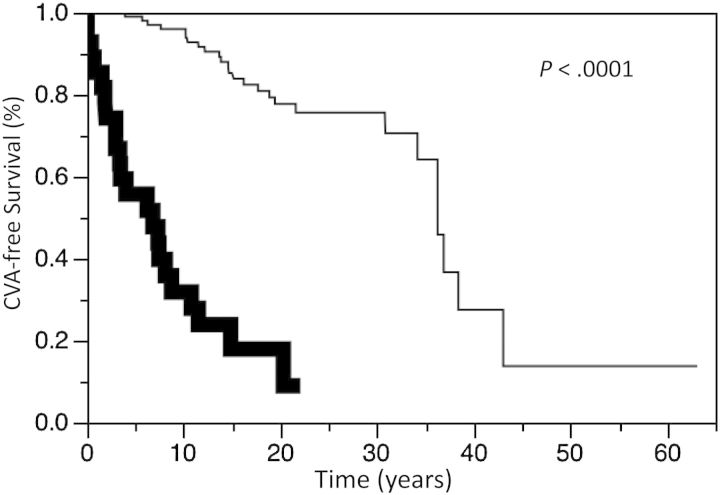
Twenty-seven patients had a CVA. Nine patients died of CVA, 6 of whom had no prior radiotherapy. The CVA-free survival for the entire cohort at 5, 10, and 15 years was 90.4%, 83.1%, and 71.7%, respectively. There was no difference in CVA-free survival between the treatment groups, including those conservatively managed compared with those actively treated (P = .42). The CVA-free survival at 5, 10, and 15 years was 96.6%, 86.2%, and 71.5%, respectively, for those patients treated with radiotherapy compared with 88.6%, 82.3%, and 71.7%, respectively, for those not receiving radiotherapy (P = .52, Fig. 1). The CVA-free survival at 5, 10, and 15 years was 95.7%, 88.1%, and 72.4%, respectively, for those patients who underwent surgery compared with 84.5%, 77.6%, and 70.8%, respectively, for the nonsurgery patients (P = .32). There was a significant increase in the incidence of CVA in those patients undergoing repeat radiotherapy. CVA-free survival at 5, 10, and 15 years was 100%, 80.0%, and 40.0%, respectively, for those patients treated with repeat radiotherapy compared with 96.6%, 82.8%, and 75.6%, respectively (P = .02; Fig. 2), for those patients never managed with reirradiation. Of note, the CVA events occurred after repeat radiotherapy in this cohort. There was no difference in CVA-free survival between tumor types, including comparisons of growth hormone-secreting tumors versus other tumors (hazard ratio [HR], 1.43; 95% CI, 0.61–2.92; P = .38). There was also no difference in CVA-free survival in different time periods (ie, 1933–1979 vs 1980–2000) of diagnosis (HR = 1.00; P > .99). There was worse CVA-free survival in males (P < .01; Fig. 3) and those older than aged 60 years old at time of diagnosis (P < .01; Fig. 4).
Fig. 1.
Kaplan-Meier estimates of cerebrovascular accident (CVA) survival in patients treated with radiotherapy (bold line) or without radiotherapy (thin line) for pituitary adenomas.
Fig. 2.
Kaplan-Meier estimates of cerebrovascular accident (CVA) survival in patients treated with repeat radiotherapy (bold line) or without repeat radiotherapy (thin line) for pituitary adenomas.
Fig. 3.
Kaplan-Meier estimates of cerebrovascular accident (CVA) survival in male (bold line) or female (thin line) patients with pituitary adenomas.
Fig. 4.
Kaplan-Meier estimates of cerebrovascular accident (CVA) survival in patients aged 60 years or older at diagnosis (bold line) or aged <60 years (thin line) with pituitary adenomas.
Univariate analysis (Table 2) showed that age >60 years and male sex had the greatest risk of CVA with HRs of 11.93 and 3.67, respectively. Amongst the various treatment groups, those undergoing repeat radiation therapy had an increased risk for CVA with HR of 3.41. In multivariate analysis, the only variable to show a worse CVA-free survival was age > 60 years, although there was a trend to significance with repeat radiotherapy (P = .07).
Table 2.
Univariate and multivariate Cox proportional-hazards regression models of cerebrovascular accidents in patients with pituitary adenoma
| Variables | No. of patients | HR* (95% CI, P value) | HR** (95% CI, P-value) |
|---|---|---|---|
| Age (years) | |||
| >60 | 30 | 11.93 (6.26–23.03 <.0001) | 152.58 (26.88–343.71, .0001) |
| <60 | 113 | Ref | Ref |
| Sex | |||
| Male | 55 | 3.67 (2.03–6.84, <.0001) | 1.43 (0.29–7.28, .66) |
| Female | 88 | Ref | Ref |
| Tumor size | |||
| Microadenoma | 35 | 0.54 (0.18–1.25, .16) | n/a |
| Macroadenoma | 108 | Ref | |
| Treatment | |||
| Conservative | 59 | 1.28 (0.70–2.30, .42) | n/a |
| Nonconservative | 84 | Ref | |
| Surgery | 76 | 0.75 (0.42–1.33, 0.32) | n/a |
| No surgery | 67 | Ref | |
| RT | 29 | 1.23 (0.64–2.27, .53) | 2.97 (0.41–63.43, .31) |
| No RT | 114 | Ref | Ref |
| Surgery plus RT | 21 | 1.17 (0.55–2.26, .67) | n/a |
| Not combination of surgery plus RT | 122 | Ref | |
| Repeat RT | 5 | 3.41 (1.05–9.68, .04) | 5.38 (0.84–37.49, .07) |
| No repeat RT | 138 | Ref | Ref |
| Less than GTR | 26 | 1.11 (0.44–2.64, .82) | 1.07 (0.15–9.13, .94) |
| GTR | 50 | Ref | Ref |
Abbreviations: GTR, gross total resection; HR, hazard ratio; Ref, reference variable; RT, radiotherapy.
*univariate analysis.
**multivariate analysis.
Cerebrovascular Accidents in Pituitary Adenoma Patients Compared with the General Population
The CVA rate in the pituitary tumor population was compared with that of the general population of Olmsted County after adjusting for time period, age, and sex. Compared with the expected stroke rate in the general population, there was a 4-fold increase for stroke in those with a pituitary tumor (standardized morbidity ratio, 4.2:1; 95% CI, 2.8–6.1). Since older age was associated with an increased risk of CVA in the pituitary tumor population, the subgroup of older pituitary patients was compared with the older general population of Olmsted County, and there was a 6-fold increase for stroke in those with a pituitary tumor (standardized morbidity ratio, 6.1:1; 95% CI 3.1–10.9).
Second Brain Tumors
Two patients developed an intracranial tumor after diagnosis of a pituitary adenoma, one in an irradiated patient and the other in a surgically managed patient. One patient developed a WHO grade 3 (of 4) fibrillary astrocytoma in the posterior limb of the internal capsule 35 years after surgery and radiotherapy. The patient underwent stereotactic biopsy for diagnosis. Following biopsy, the patient developed decreased responsiveness and progressive neurologic dysfunction attributed to postbiopsy hemorrhage and edema. Within 1 month of biopsy, the patient died at home, secondary to neurologic deterioration. The other patient developed a tentorial meningioma 18 years after surgical resection of a pituitary adenoma. The tentorial meningioma was asymptomatic and incidentally discovered on MRI as part of routine follow-up for the pituitary adenoma. The patient has been subsequently observed without further intervention to date.
Discussion
Although radiotherapy is an effective treatment modality for pituitary adenomas, there are concerns about the long-term side effects of radiation, particularly secondary brain tumors and CVAs. The current study found no difference in the rate of CVA between the treatment groups, including those who were managed conservatively. However, the risk of CVA in pituitary adenoma patients was more than 4 times higher than that in the general population in Olmsted County. A review of 331 pituitary adenoma patients treated with radiotherapy at the Royal Marsden Hospital found the actuarial incidence of CVA to be 11% at 10 years after radiotherapy.13 A follow-up study from the same center found 33 cerebrovascular deaths with a relative risk (RR) of 4.1 (95% CI, 2.8–5.8) compared with a normal reference population.5 Interestingly, there was an increased incidence of CVA in patients after debulking surgery (RR, 5.19; 95% CI, 3.50–7.42) compared with no surgery or biopsy only (RR, 1.33; 95% CI 0.27–3.88; P = .02). Other series have not found an association between pituitary irradiation and CVA.4 Investigators at the University Medical Center Groningen evaluated the incidence of stroke in a cohort of 462 irradiated and nonirradiated pituitary adenoma patients.14 Postoperative radiotherapy was administered in 236 patients, with most patients treated to 45 Gy in 25 fractions. They found no difference in incidence of stroke (5.5% vs 5.3%, respectively; P = .23), even though the follow-up was more than twice as long in the irradiated patients.
There was a trend towards a worse CVA-free survival with repeat radiotherapy in the current series. This is consistent with other series of reirradiation, such as head and neck cancer, with a well-established risk of large-vessel late toxicity.15–17 Small series with repeat radiotherapy to the sella have noted toxicities such as hypopituitarism, optic neuropathy, and temporal lobe necrosis but not CVA, which was possibly due to small patient numbers.18,19 Although there are no conclusive studies, a discussion of the risks of large-vessel late toxicity should be considered for patients planning to undergo reirradiation to the sella.
Some series have noted the incidence of second brain tumors after radiotherapy to be relatively high. A review of 426 pituitary adenoma patients who were treated with radiotherapy at the Royal Marsden Hospital after resection found a cumulative risk of second brain tumors of 2% at 10 years and 2.4% at 20 years with median follow-up of 12 years.20 In a study from Princess Margaret Hospital, 305 patients with pituitary adenoma who were treated with radiotherapy had a cumulative risk of glioma of 1.7% at 10 years with a median follow-up of 7.9 years.21 However, inherent study biases might play a role in these findings. It is quite plausible that many studies evaluating second malignancies are spurred by observations of second malignancies in the clinic. These observations provide motivation to retrospectively evaluate the risk of second malignancies after radiotherapy; however, the fact that these investigators observed the very rare event of a second malignancy significantly inflates the risk of second malignancies in their studied cohort. In fact, a review of 10 series and 1 388 pituitary adenoma patients treated with radiotherapy that focused on late side effects, but not second malignancies per se, revealed a second malignancy rate of 0.8% even though the Princess Margaret Hospital and Royal Marsden Hospital experiences were both included in the review.22
There is an interest in determining the RR of second malignancies after radiotherapy, as this clarifies the therapeutic ratio and aids in treatment decisions. In the previously reviewed Royal Marsden Hospital study, the RR of second brain tumor compared with a normal reference population was 10.5 (95% CI, 4.3–16.7).20 In the Princess Margaret Hospital study, the RR of malignant brain tumor was 16 times (95% CI, 4.4–41) greater than the general population.23 However, in estimating the RR of second brain tumors, comparisons with a general population are possibly flawed because the risk of second brain tumors may be higher in pituitary adenoma patients than in the general population. Several reports in the literature include co-occurrence of pituitary adenomas and other brain tumors, such as meningiomas, in nonirradiated pituitary tumor patients.24,25 In a review of the world literature that encompassed 22 years, 16 and 19 cases of meningiomas were noted in irradiated and nonirradiated pituitary patients, respectively, and 18 and 9 cases of gliomas were found in irradiated and nonirradiated pituitary patients, respectively.26 Also epidemiological studies have shown an association between pituitary adenomas and central nervous system tumors.27 Genetic factors may play a role in some associations between pituitary tumors and other cancers. Pituitary tumor-transforming gene is an oncogenic molecule that plays many roles in cell cycle regulation, and overexpression can lead to tumorigenesis.28 Pituitary tumor-transforming gene is overexpressed not only in pituitary tumors29,30 but also in a number of endocrine and nonendocrine-related tumors including gliomas.28,31 Therefore, a more ideal estimation of RR of second brain tumors after radiotherapy would be made by comparisons with a population of nonirradiated pituitary adenomas after surgery. In the current study, we were able to make such comparisons. We found second brain tumors to be quite rare, even with substantial follow-up, and there were no differences in second brain tumor rates between the irradiated and the nonirradiated patients despite imbalances in follow-up that favored the nonirradiated patients. Investigators at the University Medical Center Groningen compared 236 irradiated and 226 nonirradiated pituitary adenoma patients and found similar results.2 Again, they noted no statistically significant differences in second brain tumor rates between irradiated and nonirradiated patients, despite imbalances in follow-up that favored the nonirradiated patients. Their series identified one patient who developed a glioblastoma after surgery only for a pituitary adenoma. However, it is important to recognize the event rate for second brain tumors is extremely small, thereby considerably limiting the chance of any significant differences between groups. Nonetheless, if there is a difference in the development of second brain tumors between irradiated and nonirradiated pituitary adenoma patients, the absolute difference is certainly very small.
There are some limitations to our study. In order to ascertain sufficient follow-up data, patients were enrolled up to year 2000. Therefore, the majority of patients included in our study are represented by macroadenomas. In the imaging era after year 2000, it is quite likely that the majority of patients would have microadenomas, reflecting the increased prevalence of incidentalomas as sequelae of sensitive imaging technology. Ascertainment of CVA prior to the CT and MRI era may underestimate the total number of events. Prior to the CT era, the distinction between brain hemorrhage and ischemic stroke, which have different and distinctive risk factors, would not have been possible. In addition, stroke incidence has generally declined over the time frame because of advancements in therapeutics and prevention. Finally, due to the time frame of this study, it is not possible to ascertain the frequency of certain risk factors for stroke such as smoking history, hypertension, or even detailed evaluation of pituitary function, especially in the older patient charts. This is important because some studies have suggested that cerebrovascular disease after radiotherapy is more likely due to the resultant hypopituitarism rather than being directly related to the treatment itself.32 However, the rate of hypopituitarism in our study is almost certainly equal or higher in patients treated with radiotherapy33 compared with conservative management or surgery,34 especially since the majority of radiotherapy patients also had surgery. For example, in a prospective study of patients, treated contemporaneously with the current study, they found the development of deficiencies of adrenal, thyroid, and gonadal function respectively in only 13%, 13%, and 0% of patients treated with surgery alone, compared with 55%, 15%, and 50% , respectively, of patients treated with radiotherapy alone and 67%, 55%, and 67%, respectively, treated with surgery and radiotherapy.35 Nonetheless, a significant strength of the current study is the unique nature of health care in Olmsted County allowing a population-based trial such that we were able to assess all patients diagnosed with pituitary tumors, elucidate long-term risks of radiotherapy, and make comparisons with the general population.
In conclusion, this population-based study revealed a significant risk for CVA in patients with pituitary tumors, especially older patients diagnosed with pituitary adenomas. Except for repeat radiotherapy, treatment does not seem to impact the risk of CVA. In addition, second brain tumors are a very rare event regardless of treatment, even with long-term follow-up. This information should be considered when determining the therapeutic ratio of radiotherapy.
Funding
Study data were obtained from the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Conflict of interest statement: Dr. Erickson has served on an Advisory Board for Ipsen. Other Conflicts of Interest: None.
References
- 1.Park P, Chandler WF, Barkan AL, et al. The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery. 2004;55:100–106. doi: 10.1227/01.neu.0000126885.71242.d7. discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 2.Sattler MG, van Beek AP, Wolffenbuttel BH, et al. The incidence of second tumours and mortality in pituitary adenoma patients treated with postoperative radiotherapy versus surgery alone. Radiother Oncol. 2012;104:125–130. doi: 10.1016/j.radonc.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Erfurth EM, Bulow B, Mikoczy Z, et al. Is there an increase in second brain tumours after surgery and irradiation for a pituitary tumour? Clin Endocrinol. 2001;55:613–616. doi: 10.1046/j.1365-2265.2001.01385.x. [DOI] [PubMed] [Google Scholar]
- 4.Flickinger JC, Nelson PB, Taylor FH, et al. Incidence of cerebral infarction after radiotherapy for pituitary adenoma. Cancer. 1989;63:2404–2408. doi: 10.1002/1097-0142(19890615)63:12<2404::aid-cncr2820631205>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Brada M, Ashley S, Ford D, et al. Cerebrovascular mortality in patients with pituitary adenoma. Clin Endocrinol. 2002;57:713–717. doi: 10.1046/j.1365-2265.2002.01570.x. [DOI] [PubMed] [Google Scholar]
- 6.Petty GW, Brown RD, Jr., Whisnant JP, et al. Ischemic stroke subtypes : a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31(5):1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 7.Cahan WG, Woodard HQ, Higinbotham NL, et al. Sarcoma arising in irradiated bone: report of eleven cases. 1948. Cancer. 1998;82:8–34. doi: 10.1002/(sici)1097-0142(19980101)82:1<8::aid-cncr3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc. 1922;85:87–94. [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 11.Brown RD, Whisnant JP, Sicks JD, et al. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–380. [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life tables. J R Stat Soc [B]. 1972;34:187–220. [Google Scholar]
- 13.Brada M, Burchell L, Ashley S, et al. The incidence of cerebrovascular accidents in patients with pituitary adenoma. Int J Radiat Oncol Biol Phys. 1999;45:693–698. doi: 10.1016/s0360-3016(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 14.Sattler MGA, van Beek AP, Vroomen PC, et al. The incidence of stroke in pituitary adenoma patients treated with postoperative radiotherapy versus surgery alone. Int J Radiat Oncol Biol Phys. 2011;81:S37–S38. doi: 10.1016/j.ijrobp.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007;25:4800–4805. doi: 10.1200/JCO.2006.07.9194. [DOI] [PubMed] [Google Scholar]
- 16.McDonald MW, Moore MG, Johnstone PA. Risk of carotid blowout after reirradiation of the head and neck: a systematic review. Int J Radiat Oncol Biol Phys. 2012;82:1083–1089. doi: 10.1016/j.ijrobp.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Salama JK, Vokes EE, Chmura SJ, et al. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2006;64:382–391. doi: 10.1016/j.ijrobp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Schoenthaler R, Albright NW, Wara WM, et al. Re-irradiation of pituitary adenoma. Int J Radiat Oncol Biol Phys. 1992;24:307–314. doi: 10.1016/0360-3016(92)90686-c. [DOI] [PubMed] [Google Scholar]
- 19.Flickinger JC, Deutsch M, Lunsford LD. Repeat megavoltage irradiation of pituitary and suprasellar tumors. Int J Radiat Oncol Biol Phys. 1989;17:171–175. doi: 10.1016/0360-3016(89)90385-4. [DOI] [PubMed] [Google Scholar]
- 20.Minniti G, Traish D, Ashley S, et al. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab. 2005;90:800–804. doi: 10.1210/jc.2004-1152. [DOI] [PubMed] [Google Scholar]
- 21.Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239–244. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 22.Becker G, Kocher M, Kortmann R-D, et al. Radiation therapy in the multimodal treatment approach of pituitary adenoma. Strahlenther Onkol. 2002;178:173–186. doi: 10.1007/s00066-002-0826-x. [DOI] [PubMed] [Google Scholar]
- 23.Tsang RW, Laperriere NJ, Simpson WJ, et al. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer. 1993;72:2227–2233. doi: 10.1002/1097-0142(19931001)72:7<2227::aid-cncr2820720727>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Abs R, Parizel PM, Willems PJ, et al. The association of meningioma and pituitary adenoma: report of seven cases and review of the literature. Eur Neurol. 1993;33:416–422. doi: 10.1159/000116986. [DOI] [PubMed] [Google Scholar]
- 25.Jones A. Radiation oncogenesis in relation to the treatment of pituitary tumours. Clin Endocrinol. 1991;35:379–397. doi: 10.1111/j.1365-2265.1991.tb03554.x. [DOI] [PubMed] [Google Scholar]
- 26.Gittoes NJL. Radiotherapy for non-functioning pituitary tumors--when and under what circumstances? Pituitary. 2003;6:103–108. doi: 10.1023/b:pitu.0000004801.95086.e2. [DOI] [PubMed] [Google Scholar]
- 27.Hemminki K, Forsti A, Ji J. Incidence and familial risks in pituitary adenoma and associated tumors. Endocr Relat Cancer. 2007;14:103–109. doi: 10.1677/ERC-06-0008. [DOI] [PubMed] [Google Scholar]
- 28.Tfelt-Hansen J, Kanuparthi D, Chattopadhyay N. The emerging role of pituitary tumor transforming gene in tumorigenesis. Clin. Med. Res. 2006;4:130–137. doi: 10.3121/cmr.4.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filippella M, Galland F, Kujas M, et al. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol. 2006;65:536–543. doi: 10.1111/j.1365-2265.2006.02630.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Horwitz GA, Heaney AP, et al. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. 1999;84:761–767. doi: 10.1210/jcem.84.2.5432. [DOI] [PubMed] [Google Scholar]
- 31.Vlotides G, Eigler T, Melmed S. Pituitary tumor-transforming gene: physiology and implications for tumorigenesis. Endocr Rev. 2007;28:165–186. doi: 10.1210/er.2006-0042. [DOI] [PubMed] [Google Scholar]
- 32.Erfurth EM, Bulow B, Svahn-Tapper G, et al. Risk factors for cerebrovascular deaths in patients operated and irradiated for pituitary tumors. J Clin Endocrinol Metab. 2002;87:4892–4899. doi: 10.1210/jc.2002-020526. [DOI] [PubMed] [Google Scholar]
- 33.Littley MD, Shalet SM, Beardwell CG, et al. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989;70:145–160. [PubMed] [Google Scholar]
- 34.Freda PU, Wardlaw SL, Post KD. Long-term endocrinological follow-up evaluation in 115 patients who underwent transsphenoidal surgery for acromegaly. J Neurosurg. 1998;89:353–358. doi: 10.3171/jns.1998.89.3.0353. [DOI] [PubMed] [Google Scholar]
- 35.Snyder PJ, Fowble BF, Schatz NJ, et al. Hypopituitarism following radiation therapy of pituitary adenomas. Am J Med. 1986;81:457–462. doi: 10.1016/0002-9343(86)90299-8. [DOI] [PubMed] [Google Scholar]