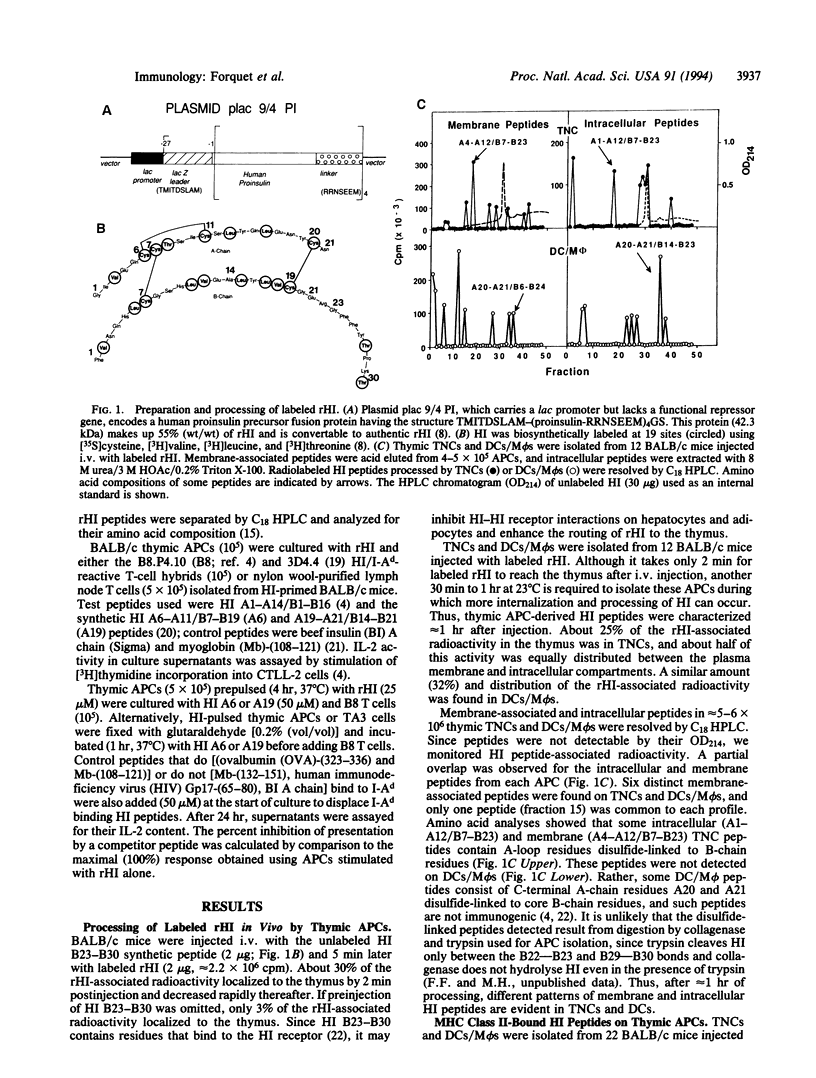
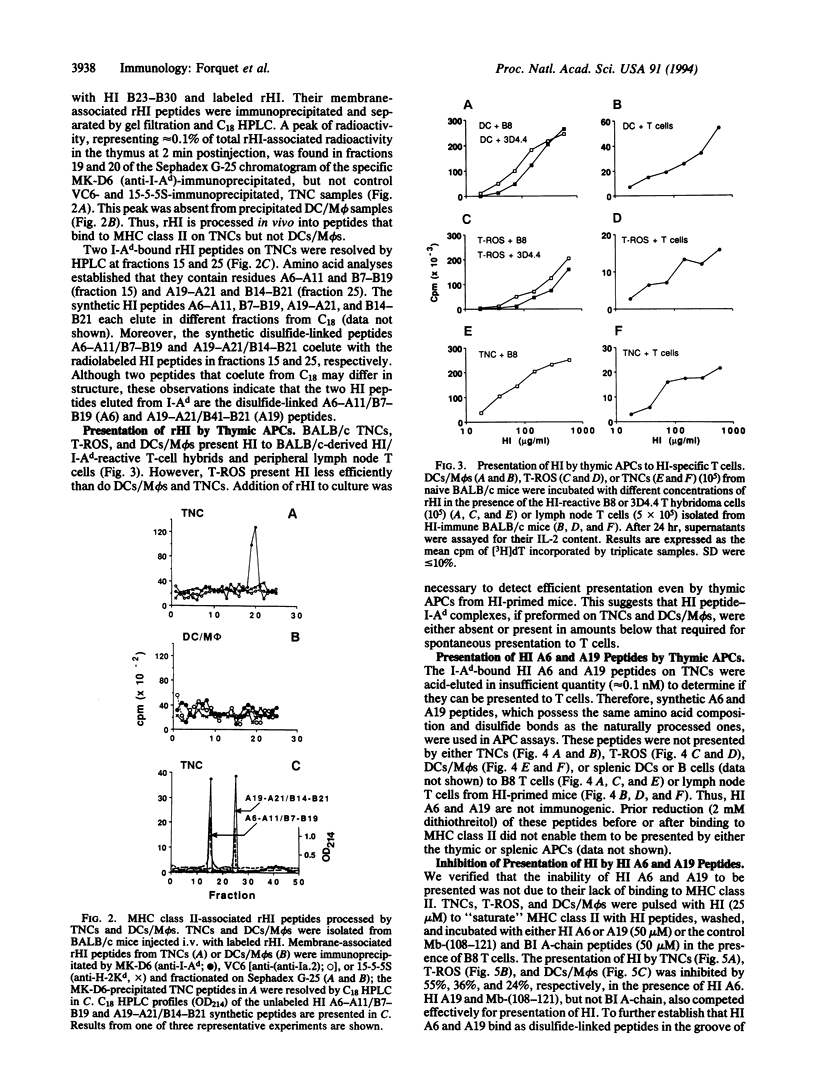
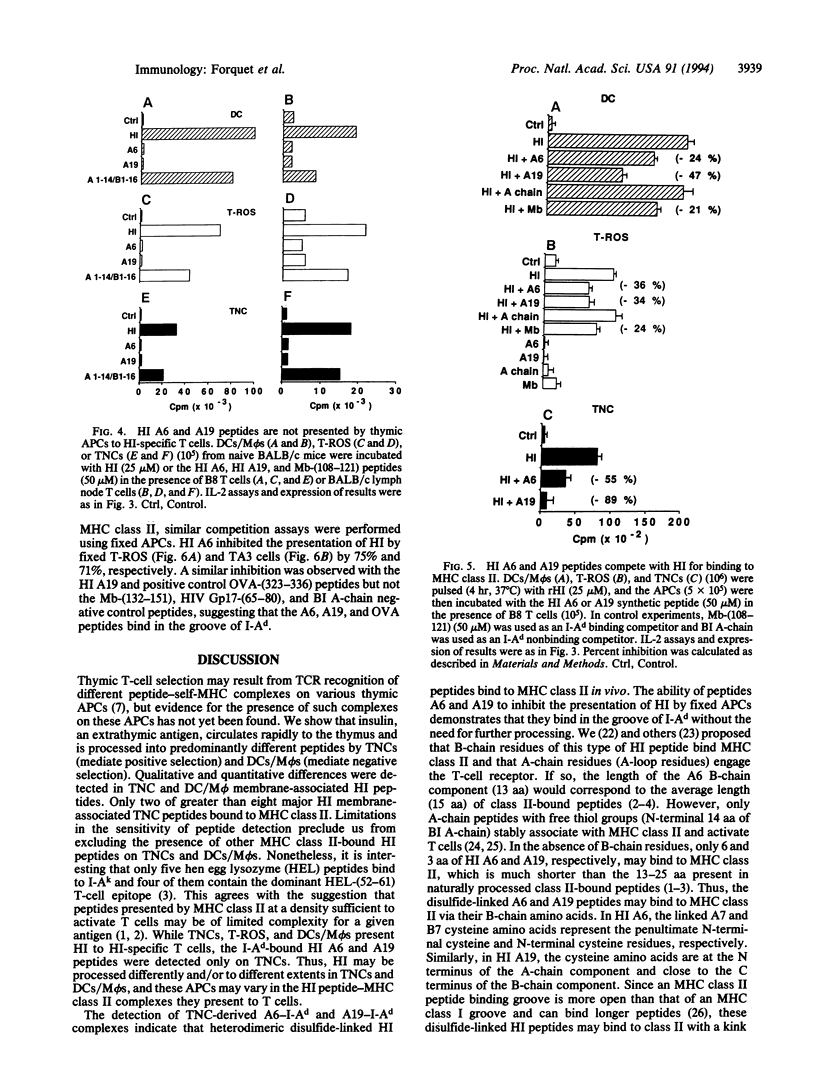
Abstract
We determined whether disulfide-linked insulin peptides that are immunogenic in vitro for CD4+ T cells bind to major histocompatibility complex class II in vivo. Radiolabeled recombinant human insulin (rHI) was injected into BALB/c mice, and processed rHI peptides bound to I-Ad molecules on different thymic antigen-presenting cells were characterized. The A6-A11/B7-B19 and A19-A21/B14-B21 disulfide-linked I-Ad-bound rHI peptides were isolated from thymic epithelial cells but not dendritic cells. While both thymic epithelial cells and dendritic cells present rHI to HI/I-Ad-specific T cells, these antigen-presenting cells do not present the reduced or nonreduced forms of the disulfide-linked rHI peptides. Thus, a naturally processed disulfide-linked peptide can bind to major histocompatibility complex class II in vivo. The potential role of these peptides in immunological tolerance is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackman M., Kappler J., Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990 Jun 15;248(4961):1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Cease K. B., Berkower I., York-Jolley J., Berzofsky J. A. T cell clones specific for an amphipathic alpha-helical region of sperm whale myoglobin show differing fine specificities for synthetic peptides. A multiview/single structure interpretation of immunodominance. J Exp Med. 1986 Nov 1;164(5):1779–1784. doi: 10.1084/jem.164.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Lane W. S., Gorga J. C., Stern L. J., Vignali D. A., Strominger J. L. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992 Aug 27;358(6389):764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- Crowley M., Inaba K., Witmer-Pack M., Steinman R. M. The cell surface of mouse dendritic cells: FACS analyses of dendritic cells from different tissues including thymus. Cell Immunol. 1989 Jan;118(1):108–125. doi: 10.1016/0008-8749(89)90361-4. [DOI] [PubMed] [Google Scholar]
- Delovitch T. L., Lazarus A. H., Phillips M. L., Semple J. W. Antigen binding and processing by B-cell antigen-presenting cells: influence on T- and B-cell activation. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):333–343. doi: 10.1101/sqb.1989.054.01.041. [DOI] [PubMed] [Google Scholar]
- Delovitch T. L., Semple J. W., Naquet P., Bernard N. F., Ellis J., Champagne P., Phillips M. L. Pathways of processing of insulin by antigen-presenting cells. Immunol Rev. 1988 Dec;106:195–222. doi: 10.1111/j.1600-065x.1988.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Glimcher L. H., Hamano T., Asofsky R., Sachs D. H., Pierres M., Samelson L. E., Sharrow S. O., Paul W. E. IA mutant functional antigen-presenting cell lines. J Immunol. 1983 May;130(5):2287–2294. [PubMed] [Google Scholar]
- Hadzija M., Semple J. W., Delovitch T. L. Influence of antigen processing on thymic T-cell selection. Res Immunol. 1991 Jun-Aug;142(5-6):421–424. doi: 10.1016/0923-2494(91)90041-g. [DOI] [PubMed] [Google Scholar]
- Hampl J., Gradehandt G., Kalbacher H., Rüde E. In vitro processing of insulin for recognition by murine T cells results in the generation of A chains with free CysSH. J Immunol. 1992 May 1;148(9):2664–2671. [PubMed] [Google Scholar]
- Jensen P. E. Reduction of disulfide bonds during antigen processing: evidence from a thiol-dependent insulin determinant. J Exp Med. 1991 Nov 1;174(5):1121–1130. doi: 10.1084/jem.174.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyewski B. A., Momburg F., Schirrmacher V. Phenotype of stromal cell-associated thymocytes in situ is compatible with selection of the T cell repertoire at an "immature" stage of thymic T cell differentiation. Eur J Immunol. 1987 Jul;17(7):961–967. doi: 10.1002/eji.1830170711. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. G., Allen P. M. Thymic cortical epithelial cells can present self-antigens in vivo. Nature. 1989 Feb 9;337(6207):560–562. doi: 10.1038/337560a0. [DOI] [PubMed] [Google Scholar]
- Miller G. G., Hoy J. F., Thomas J. W. Insulin B chain functions as an effective competitor of antigen presentation via peptide homologies present in the thymus. J Exp Med. 1989 Jun 1;169(6):2251–2256. doi: 10.1084/jem.169.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naquet P., Ellis J., Singh B., Hodges R. S., Delovitch T. L. Processing and presentation of insulin. I. Analysis of immunogenic peptides and processing requirements for insulin A loop-specific T cells. J Immunol. 1987 Dec 15;139(12):3955–3963. [PubMed] [Google Scholar]
- Nelson C. A., Roof R. W., McCourt D. W., Unanue E. R. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7380–7383. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Phillips M. L., Harris J. F., Delovitch T. L. Idiotypic analysis of anti-I-Ak monoclonal antibodies. I. Production and characterization of syngeneic anti-idiotypic mAb against an anti-I-Ak mAb. J Immunol. 1984 Nov;133(5):2587–2594. [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Semple J. W., Cockle S. A., Delovitch T. L. Purification and characterization of radiolabelled biosynthetic human insulin from Escherichia coli. Kinetics of processing by antigen presenting cells. Mol Immunol. 1988 Dec;25(12):1291–1298. doi: 10.1016/0161-5890(88)90044-2. [DOI] [PubMed] [Google Scholar]
- Semple J. W., Ellis J., Delovitch T. L. Processing and presentation of insulin. II. Evidence for intracellular, plasma membrane-associated and extracellular degradation of human insulin by antigen-presenting B cells. J Immunol. 1989 Jun 15;142(12):4184–4193. [PubMed] [Google Scholar]
- Semple J. W., Lang Y., Speck E. R., Delovitch T. L. Processing and presentation of insulin. III. Insulin degrading enzyme: a neutral metalloendoproteinase that is non-homologous to classical endoproteinases mediates the processing of insulin epitopes for helper T cells. Int Immunol. 1992 Oct;4(10):1161–1167. doi: 10.1093/intimm/4.10.1161. [DOI] [PubMed] [Google Scholar]
- Weiner H. L. Treatment of autoimmune diseases by oral tolerance to autoantigens. Autoimmunity. 1993;15 (Suppl):6–7. doi: 10.3109/08916939309008850. [DOI] [PubMed] [Google Scholar]
- Whiteley P. J., Lake J. P., Selden R. F., Kapp J. A. Tolerance induced by physiological levels of secreted proteins in transgenic mice expressing human insulin. J Clin Invest. 1989 Nov;84(5):1550–1554. doi: 10.1172/JCI114331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. J., Davidson L., Eisenbarth G., Weiner H. L. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10252–10256. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]



