Abstract
Background
Between 80%–85 percent of all adult brain tumors are high-grade gliomas (HGGs). Despite aggressive treatment with surgical resection, radiotherapy and chemotherapy, the survival of patients with HGG is limited. Brain tumor patients develop unique symptoms and needs throughout their disease trajectory, and the majority lose the ability to communicate during the end-of-life phase. Palliative care (PC) is a proactive and systematic approach to manage issues that are important to patients and families affected by serious illness. The goal is to improve quality of life and symptom control and thereby reduce suffering. Most PC interventions take place during the end-of-life phase; however, newer data suggest that early PC interventions might improve symptom control and quality of life.
Methods
A literature review focusing on PC, hospice care, and end-of-life care was performed with the aim to describe the integration of PC into neuro-oncology practice.
Results
Recently there has been increased interest in the effects of PC and brain tumor patients. The origins, methodology, and conceptual models of delivering PC and how it might be applied to the field of neuro-oncology were reviewed. Patterns of referral and utilization in neuro-oncology are described based on the findings of a recent survey.
Conclusions
Despite a very high symptom burden, many HGG patients do not receive the same level of PC and have fewer interactions with PC services than other cancer populations. Early PC interventions and structured advance-care planning might improve symptom control and quality of life for brain tumor patients.
Keywords: end-of-life, glioblastoma, glioma, palliative care, supportive care
Palliative care (PC) is a proactive and systematic approach to managing issues that are important to patients and families affected by serious illness. The goal is to improve quality of life and control symptoms and thereby reduce suffering. Modern PC medicine has undergone tremendous growth and change since the 1960s. While the early focus was primarily on controlling symptoms caused by cancer and near the end of life, today's subspecialty training in PC medicine prepares physicians and other providers to work in a multidisciplinary team to support patients and their families affected by cancer and other serious illnesses.1
PC is defined by the World Health Organization as “… an approach that improves the quality of life of patients and their families, facing the problem associated with life threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual.”2
PC is strictly multidisciplinary and is delivered by a team of physicians, nurses, and other specialists who work together with a patient's other doctors to help with symptom management in patients facing a life-threatening disease. In the beginning, PC and hospice medicine were focused on patients with cancer, but modern PC encompasses symptom management with the goal of improving the quality of life for any patient affected by a serious illnesses. PC is appropriate at any stage in a serious illness and can be provided together with curative treatment.
In recent years, there have been discussions about changing the term PC into “supportive care” because there is concern that the term “palliative care” carries negative connotations for patients and referring physicians and might impose a barrier when accessing PC services.3–5 Although controversies remain about the exact definition,6 the terms “palliative care” and “supportive care” will be used synonymously for the purpose of this article.
In the United States, there is a clear separation between PC medicine and hospice based on reimbursement rules established by Medicare. Hospice services and PC medicine provide symptom relief and pain management; however, PC services can be offered concurrently to any patient without restriction to prognosis, while hospice care requires that a patient have a life expectancy <6 months under the Medicare Hospice Benefit.
PC originated in the hospice movement but is now often practiced separately from traditional hospice care. The first modern hospice, St. Christopher's Hospice, was founded by Dame Cicely Saunders in the United Kingdom in 1967.1 Since then, PC and hospice medicine have developed rapidly. PC was recognized by the American Board of Medical Specialties as a specialty in 2007, and the number of programs has expanded rapidly ever since. While PC originated in Great Britain, one of the first reported inpatient PC units in North America was opened in Montreal in 1976,9 and these services are now widely available.8 Ninety-eight percent of all National Cancer Institute-designated cancer centers and 85% of all large hospitals are reported to have PC programs.7,8
In the beginning of the PC movement, attention was focused strictly on the end-of-life care needs of patients with advanced cancer. The interest in symptom control and development of modern PC medicine as a separate service apart from hospice care started in the 1970s. PC services focused on improving the physical, social, psychological, and spiritual support of patients with life-limiting illness by utilizing a multidisciplinary team.1 In addition to providing inpatient services only, PC has expanded into outpatient assessment and treatment as well.
Glioblastomas (GBMs) and the majority of anaplastic gliomas are treated with maximal surgical resection, followed by radiotherapy with concurrent and adjuvant chemotherapy to the residual tumor and surrounding brain tissue.10,11 Despite this aggressive approach, the median survival for anaplastic glioma is estimated to be 2–5 years, and median survival is only 15 months for patients with GBM.10,12,13 For most patients with high-grade glioma, long-term survival remains elusive. Therefore, quality of life has become an increasingly important outcome assessed in brain tumor clinical trials as well as in standard of care evaluations in brain tumor centers.14,15
While it is the goal of neuro-oncologists to extend survival, PC focuses on the well-being and symptom control of patients and families. Aspects defining most people's lives include physical, emotional, spiritual, sexual, social, and financial domains. PC services can be utilized at any point along the disease trajectory, independent of the goal achieving a cure or merely extending survival to address these concerns (Table 1).16
Table 1.
Roles and Goals of Palliative Care
|
The aim of this topic review is to understand the different conceptual models of PC and the timing of PC involvement and how these models apply to neuro-oncology because the integration of PC into the field of neuro-oncology might improve the care and quality of life of brain tumor patients and their families.
Methods
For this topic review, a literature search was conducted in PubMed and Cochrane Library on November 19, 2013. The following search string was used:
(glioma* OR glioblastoma* OR brain neoplasm* OR brain tumor* OR brain cancer* OR gbm AND (palliative care OR supportive care OR hospice)).
This search produced 884 citations. When the articles were limited to English (n = 732) and “human,” the results were narrowed to 696 articles. After focusing on adult patients only (aged ≥19 years), a total of 420 titles were retained for further review. The article titles and, if needed the abstracts, were reviewed and retained if they related to: (i) adult patients with the diagnosis of primary brain tumor; (ii) end-of-life care; (iii) end-of-life decision-making, and (iv) palliative care, supportive care or related needs.
Twenty-five articles were retained for final review. In addition, references and the authors' personal collections were reviewed, and an additional 21 articles that were not identified earlier were added. (Please see Supplementary Data for complete listing.) The literature review described above and the results of a recent survey of the neuro-oncology community, as part of the Society of Neuro-Oncology Quality of life day 2012,17 are used as the basis for this topical review.
Results
Delivery of Palliative Care Service
Traditional nonhospice PC has been based on inpatient consultation service. PC has been focused on the acute setting of the hospital ward, the intensive care unit, or the emergency room centering on symptom control and care transition to hospice. Outpatient PC clinics have become more common over recent years. Outpatient PC services can be more proactive and can focus on preventing symptoms and creating continuity of care parallel to active neoplastic treatment.8
At least 3 different conceptual models have evolved for integrating palliative and supportive care in the outpatient setting.18 Traditionally, in the so called “solo practice model,” a single oncologist manages the primary disease and addresses any PC needs of the patient. This model has the advantage that the patient receives all care from one clinician without any additional clinic visits. However, in times of subspecialization, oncologists might lack the appropriate training in PC and might not be able to assess and address multiple physical, psychological, and social concerns in one visit.
In the “Congress practice model,” the oncologist refers to expert subspecialty care-based on patient-specific needs. While the patient has access to specialty care, coordination and integration of recommendations for additional clinic visits can become challenging. This model requires a high level of coordination, communication, and collaboration between different specialists and the primary oncologist.
In the integrated-care model, the patient's needs are met by an integrated team of oncologists and PC specialists. The patient receives comprehensive care by a coordinated team without incurring additional visits. Complex issues such as pain management, delirium and , as well as end-of-life issues and spiritual distress, can be addressed in one setting, thereby reducing visits and costs.19
Timing of Palliative Care Involvement
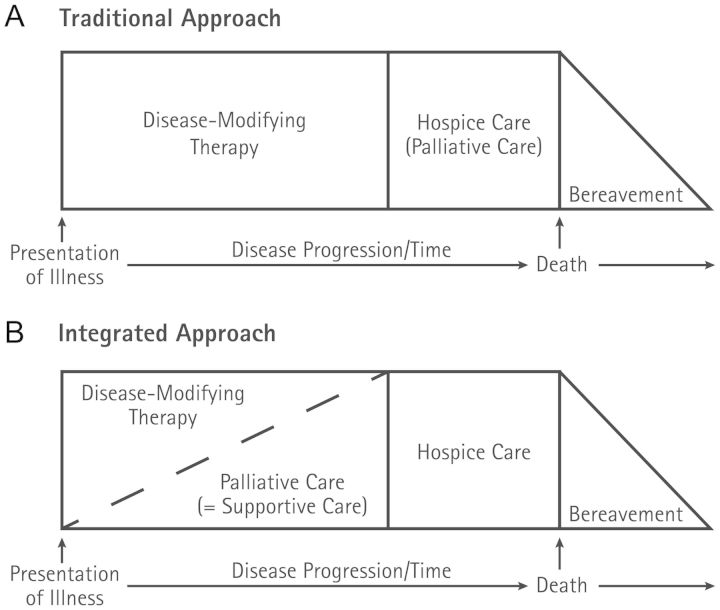
Currently, the standard practice in most programs is to involve hospice and PC providers only after investigative and standard care options have failed. At this point, curative and disease-focused treatments have usually failed, and the focus is on delivering end-of-life care. This traditional care model is based on the “first cure, then comfort” paradigm and has been reinforced in the United States by the Medicare Hospice Benefit, which pays for the majority of all hospice care in the United States (Fig. 1A). This model requires that the patient have a life expectancy of <6 months, and the hospice provider receives a fixed daily reimbursement rate, which effectively prohibits the use of antineoplastic therapy.
Fig. 1.
Traditional and early introduction of palliative care
Over the last years, there has been increasing evidence that early referral to PC might help with timely detection and treatment of symptoms and might improve longitudinal psychosocial support and end-of-life planning (Fig. 1B).20 The referral of brain tumor patients to PC occurs typically late in the trajectory of the disease at a median of 28–70 days before death.21–23 While there is no data on how early referral to PC might affect end-of-life symptoms and care in brain tumor patients, a recent randomized clinical trial demonstrated a range of benefits for patients with metastatic non-small cell lung cancer. This single-center, nonblinded, randomized controlled trial by Temel et al24 compared the effects of early integrated PC with a standard oncological approach to patients with metastatic lung cancer in the outpatient setting. Patients in the intervention arm visited with a PC team within 3 weeks of enrollment and monthly thereafter, with additional visits with the team at the discretion of the patient, primary oncologist, or PC provider. In the standard arm, patients were not directly referred to the PC team, but could be referred to PC as needed. The study demonstrated meaningful improvements in quality of life, symptom burden, and mood. In addition, the findings suggested that early integrated PC results in improved end-of-life care and prolonged survival in patients with metastatic non-small cell lung cancer. While it could be argued that this trial is not a true “early” intervention, as the 1-year survival for patients with stage 4 metastatic non-small cell lung cancer is ∼30%–35%, it might serve as a good example for patients with GBM facing a median survival of ∼15 months.10
Another study by Fraser et al25 investigated if the referral of pediatric cancer patients to a specialized pediatric PC service might reduce the number of planned hospital admissions before death. Children with central nervous system tumors in particular showed significant decreases for all planned and emergency admissions compared with all other diagnostic groups in this particular study.
The question remains if these findings could be replicated with adult patients affected by brain tumors.
End-of-life Symptoms in Brain Tumor Patients
Only limited data exist on symptoms of brain tumor patients during the end-of-life phase. However, based on several retrospective studies, it becomes clear that patients suffer from a consistently high symptom burden.21–23,26–30 Prevalent symptoms include drowsiness (85%–90%), poor communication (64%–90%), focal neurological deficits (51%–62%), seizures (30%–56%), dysphagia (7%–85%), and headaches (4%–62%).31 These symptoms differ significantly from the terminal symptoms seen in the general oncology population and especially the metastatic lung cancer population (Table 2).32 Sizoo et al33 reported that 52% of the patients in their study were considered to be incompetent by their treating physicians in the final weeks of their lives. This number increases to 85% in the last week of life. This observation is consistent with several other studies that showed a steep increase in symptom burden and decrease in ability to participate in end-of-life decision-making within the last 4 weeks of life and specifically the last days prior to death.23,26,33 Even if patients document their end-of-life wishes, there is no guarantee that these preferences will be carried out: one study showed that the treating physician was unaware of an existing advanced directive 40% of the time.33 The high prevalence of impaired consciousness and inability to communicate in brain tumor patients raises concern over the extent they are still able to participate in end-of-life decision-making in their final weeks of life.
Table 2.
Frequency of symptoms in neuro-oncology and cancer in the end-of-life phase
| Symptoms | High-grade Glioma31 | General Cancer – Last 2 Weeks of Life32 |
|---|---|---|
| Fatigue | NR | 88% |
| Weight loss | NR | 86% |
| Appetite loss | NR | 56% |
| Neurological symptoms | 32% | |
| Drowsiness | 44%–90% | 38% |
| Poor communication | 64%–90% | NR |
| Focal deficits | 51%–62% | NR |
| Poor mobility | 77% | NR |
| Weakness | 17%–25% | 74% |
| Seizures | 10%–56% | NR |
| Speech difficulties | 29%–64% | NR |
| Pain | ||
| Headache | 23%–62% | NR |
| Bodily pain | 13%–25% | 45% |
Abbreviation: NR, not reported
Discussion
Given the above-described high symptom burden, it is surprising that only a minority of high-grade glioma patients seem to have interactions with PC, and one questions whether this might be caused by referral patterns of the primary oncologist. A study by Gofton et al22 identified the PC needs of brain tumor patients and described their contact with palliative medicine service in a major comprehensive cancer center. In this retrospective data analysis, only 12% of patients were seen by a PC team in their end-of-life phase.
To determine whether the findings in this report represent a general pattern in the United States, we created a 22-question survey to assess the utilization patterns of PC in neuro-oncology.17 This survey was conducted as part of the 2012 Society of Neuro-Oncology (SNO) “Quality of Life Day.” The questions were sent to members of SNO and participants of that year's annual SNO meeting. A total of 239 evaluable responses were received. Fifty-seven percent of providers referred patients to PC at the time of symptoms requiring treatment, and 18% referred them near the end of life. Fifty-one percent of practitioners felt comfortable or very comfortable dealing with end-of-life issues and symptoms, while 33% indicated that they felt uncomfortable with the situation. Fifty-one percent of respondents preferred a service titled “supportive care” rather than “palliative care” (MDs > other providers, P< .001). One-third of respondents felt that patient expectations for ongoing therapy hindered their ability to make PC referrals. Female gender, formal training in neuro-oncology, and medical versus surgical neuro-oncology training were significantly (P <.05) associated with hospice referral, personal comfort dealing with end-of-life issues, and ease of access to PC services.17
Brain tumor patients have a high symptom burden during the end-of-life phase, and communication and decision-making capacity are highly impaired.31 While PC traditionally focused on symptom management during the terminal phase of illness, a new paradigm has evolved of integrating PC early in the disease trajectory. This approach has been evaluated in metastatic lung cancer patients in a randomized controlled trial and showed improvement not only in symptom scores and quality of life but also in overall survival.24 It is unclear if such an approach would yield similar success in brain tumor patients. Given the high neurocognitive symptom burden, the often impaired ability to communicate, and the rapid deterioration of decision-making capacity in brain tumor patients, an early and structured intervention to facilitate end-of-life planning might be helpful. Early involvement of PC services on a routine basis could be helpful to discuss end-of-life issues and to facilitate end-of-life planning. Hesitation to involve PC might be decreased by educating providers and the public about the differences between PC and hospice care. A name change to “supportive care” has been shown to result in increased and earlier PC consultations3 and was supported by 51% of respondents in our recent survey.
Supplementary Material
Funding
Department of Neurosurgery and the Hermelin Brain Tumor Center, Henry Ford Health System.
Conflict of interest statement. None declared.
References
- 1.Clark D. From margins to centre: a review of the history of palliative care in cancer. Lancet Oncol. 2007;8(5):430–438. doi: 10.1016/S1470-2045(07)70138-9. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization: Programmes and Projects: Cancer Control. Policies and Managerial Guidelines. http://www.who.int/cancer/palliative/definition/en/s . Accessed 10/12/2013.
- 3.Dalal S, Palla S, Hui D, et al. Association between a name change from palliative to supportive care and the timing of patient referrals at a comprehensive cancer center. Oncologist. 2011;16(1):105–111. doi: 10.1634/theoncologist.2010-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: what's in a name?: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer. 2009;115(9):2013–2021. doi: 10.1002/cncr.24206. [DOI] [PubMed] [Google Scholar]
- 5.Maciasz RM, Arnold RM, Chu E, et al. Does it matter what you call it? A randomized trial of language used to describe palliative care services. Support Care Cancer. 2013;21(12):3411–3419. doi: 10.1007/s00520-013-1919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallon M, Smyth J. Terminology: the historical perspective, evolution and current usage–room for confusion? Eur J Cancer. 2008;44(8):1069–1071. doi: 10.1016/j.ejca.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith B, Dietrich J, Du Q, et al. Variability in access to hospital palliative care in the United States. J Palliat Med. 2008;11(8):1094–1102. doi: 10.1089/jpm.2008.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui D, Elsayem A, De la Cruz M, et al. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303(11):1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. Can Med Assoc J. 1976;115(2):119–121. [PMC free article] [PubMed] [Google Scholar]
- 10.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 11.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 12.Prados MD, Seiferheld W, Sandler HM, et al. Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int J Radiat Oncol Biol Phys. 2004;58(4):1147–1152. doi: 10.1016/j.ijrobp.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 13.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24(18):2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 14.Minniti G, Scaringi C, Baldoni A, et al. Health-related quality of life in elderly patients with newly diagnosed glioblastoma treated with short-course radiation therapy plus concomitant and adjuvant temozolomide. Int J Radiat Oncol Biol Phys. 2013;86(27):285–291. doi: 10.1016/j.ijrobp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong TS, Wefel JS, Wang M, et al. Net clinical benefit analysis of radiation therapy oncology group 0525: A phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. doi: 10.1200/JCO.2013.49.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier DE, Brawley OW. Palliative care and the quality of life. J Clin Oncol. 2011;29(20):2750–2752. doi: 10.1200/JCO.2011.35.9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walbert T, Glantz M, Schultz L, et al. Utilization patterns of palliative care in neuro-oncology - results of an international provider survey, Poster #0359. Paper presented at: 4th Quadrennial Meeting of the World Federation of Neuro-Oncology held in conjunction with the 2013 Scientific Meeting and Education Day of the Society for Neuro-Oncology; San Francisco, CA: 2013. [Google Scholar]
- 18.Bruera E, Hui D. Conceptual models for integrating palliative care at cancer centers. J Palliat Med. 2012;15(11):1261–1269. doi: 10.1089/jpm.2012.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruera E, Hui D. Integrating supportive and palliative care in the trajectory of cancer: establishing goals and models of care. J Clin Oncol. 2010;28(25):4013–4017. doi: 10.1200/JCO.2010.29.5618. [DOI] [PubMed] [Google Scholar]
- 20.Greer JA, Jackson VA, Meier DE, et al. Early integration of palliative care services with standard oncology care for patients with advanced cancer. Cancer J Clinic. 2013;63(5):349–363. doi: 10.3322/caac.21192. [DOI] [PubMed] [Google Scholar]
- 21.Arber A, Faithfull S, Plaskota M, et al. A study of patients with a primary malignant brain tumour and their carers: symptoms and access to services. Int J Palliat Nurs. 2010;16(1):24–30. doi: 10.12968/ijpn.2010.16.1.46180. [DOI] [PubMed] [Google Scholar]
- 22.Gofton TE, Graber J, Carver A. Identifying the palliative care needs of patients living with cerebral tumors and metastases: a retrospective analysis. J Neurooncol. 2012;108(3):527–534. doi: 10.1007/s11060-012-0855-y. [DOI] [PubMed] [Google Scholar]
- 23.Oberndorfer S, Lindeck-Pozza E, Lahrmann H, et al. The end-of-life hospital setting in patients with glioblastoma. J Palliat Med. 2008;11(1):26–30. doi: 10.1089/jpm.2007.0137. [DOI] [PubMed] [Google Scholar]
- 24.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 25.Fraser LK, van Laar M, Miller M, et al. Does referral to specialist paediatric palliative care services reduce hospital admissions in oncology patients at the end of life? Br J Cancer. 2013;108(6):1273–1279. doi: 10.1038/bjc.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bausewein C, Hau P, Borasio GDI, et al. How do patients with primary brain tumours die? Palliat Med. 2003;17(6):558–559. doi: 10.1177/026921630301700615. [DOI] [PubMed] [Google Scholar]
- 27.Faithfull S, Cook K, Lucas C. Palliative care of patients with a primary malignant brain tumour: case review of service use and support provided. Palliat Med. 2005;19(7):545–550. doi: 10.1191/0269216305pm1068oa. [DOI] [PubMed] [Google Scholar]
- 28.Pace A, Di Lorenzo C, Lorenzo CD, et al. End of life issues in brain tumor patients. J neurooncol. 2009;91(1):39–43. doi: 10.1007/s11060-008-9670-x. [DOI] [PubMed] [Google Scholar]
- 29.Pace A, Villani V, Di Lorenzo C, et al. Epilepsy in the end-of-life phase in patients with high-grade gliomas. J Neurooncol. 2013;111(1):83–86. doi: 10.1007/s11060-012-0993-2. [DOI] [PubMed] [Google Scholar]
- 30.Sizoo EM, Braam L, Postma TJ, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010;12(11):1162–1166. doi: 10.1093/neuonc/nop045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walbert T, Khan M. End-of-life symptoms and care in patients with primary malignant brain tumors: A systematic literature review. J Neuro Oncol. 2014 doi: 10.1007/s11060-014-1393-6. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34(1):94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Sizoo EM, Pasman HR, Buttolo J, et al. Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer. 2012;48(2):226–232. doi: 10.1016/j.ejca.2011.11.010. [DOI] [PubMed] [Google Scholar]