Abstract
Background
Brainstem metastases (BSMs) represent a significant treatment challenge. Stereotactic radiosurgery (SRS) is often used to treat BSM. We report our experience in the treatment of BSM with Gamma Knife SRS (GK_SRS).
Methods
The records of 1962 patients with brain metastases treated with GK_SRS between 2009 and 2013 were retrospectively reviewed. Seventy-four patients with 77 BSMs and follow-up brain imaging were identified. Local control (LC), overall survival (OS), progression-free survival (PFS), and toxicity were assessed.
Results
Median follow-up was 5.5 months (range, 0.2–48.5 months). Median tumor volume was 0.13 cm3 (range, 0.003–5.58 cm3). Median treatment dose was 16 Gy (range, 10–20 Gy) prescribed to 50% isodose line (range, 40%–86%). Crude LC was 94% (72/77). Kaplan-Meier estimate of median OS was 8.5 months (95% CI, 5.6–9.4 months). Symptomatic lesions and larger lesions, especially size ≥2 cm3, were associated with worse LC (HR = 8.70, P = .05; HR = 14.55, P = .02; HR = 62.81, P < .001) and worse OS (HR = 2.00, P = .02; HR = 2.14, P = .03; HR = 2.81, P = .008). Thirty-six percent of BSMs were symptomatic, of which 36% (10/28) resolved after SRS and 50% (14/28) had stable or improved symptoms. Actuarial median PFS was 3.9 months (95% CI, 2.7–4.9 months). Midbrain location was significant for worse PFS (HR = 2.29, P = .03). Toxicity was low (8%, 6/74), with size and midbrain location associated with increased toxicity (HR 1.57, P = .05; HR = 5.25, P = .045).
Conclusions
GK_SRS is associated with high LC (94%) and low toxicity (8%) for BSMs. Presence of symptoms or lesion size ≥ 2 cm3 was predictive of worse LC and OS.
Keywords: brainstem metastases, Gamma Knife, radiosurgery
Brainstem metastases (BSMs) present a difficult problem given (i) their generally inoperable location and (ii) the need for high local control (LC) to minimize associated neurological deficits from tumor growth. Furthermore, the incidence of BSM is low, accounting for 3%–5% of all brain metastases.1 In 2009, our institution transitioned from the exclusive use of linear accelerator-based stereotactic radiosurgery (Linac_SRS) to Gamma Knife stereotactic radiosurgery (GK_SRS) for treatment of intracranial lesions deemed suitable for radiosurgery. We therefore undertook this study to evaluate our experience treating BSMs with radiosurgery in a more modern treatment period with GK_SRS. We hypothesized that treatment of BSMs with GK_SRS would provide similar, if not improved, levels of LC. With interim advances in brain imaging allowing earlier detection of smaller metastases, patients with BSMs may have improved outcomes.
We report our outcomes with the use of GK_SRS in treatment of patients with BSMs from 2009 to 2013, specifically evaluating risk factors for worse LC, overall survival (OS), progression-free survival (PFS), and treatment-related toxicity.
Methods
A retrospective review of 1962 consecutive patients with brain metastases treated with GK_SRS between June 2009 and October 2013 was performed using our institutional review board-approved Gamma Knife database. Seventy-four patients with BSMs and posttreatment follow-up imaging were identified for this analysis.
At our institution, patient selection for the use of GK_SRS was determined after consensus by neurosurgeons, neuroradiation oncologists, and neuroradiologists at a weekly multidisciplinary tumor board review of proposed radiosurgery cases. In general, patients were not deemed suitable for GK_SRS if the presence of multiple brain metastases was noted, especially in the setting of uncontrolled systemic disease, no prior whole-brain radiotherapy (WBRT), and/or poor performance status. GK_SRS maybe recommended if a patient had received previous WBRT with interval development of new and/or progressive brain metastases, had confirmed radio-resistant tumor histology, or had been treated for isolated brain metastases prior to enrollment on clinical protocols.
All patients were treated with the Elekta Gamma Knife Perfexion system using gadolinium-enhanced MRI-based planning. Dose was selected by the treating radiation oncologist and was limited by the maximum tolerated doses per maximum lesion size as determined by the Radiation Therapy Oncology Group study 90–05.2 In general, brainstem lesions were treated with 14–18 Gy, depending on size and location within the brainstem. Post-GK steroids were prescribed at the discretion of the treating radiation oncologist. If not currently on steroids, patients with symptomatic or large BSMs with concerns for treatment-induced edema received either (i) 10 milligrams of i.v. dexamethasone given at the time of GK_SRS or (ii) a 2–3 week steroid taper following GK_SRS. Patients were typically followed with MRI imaging 1 month after GK_SRS and every 3–6 months thereafter.
Local control was defined as the absence of local progression,3,4 excluding lesions with radionecrosis. Radionecrosis was defined by (i) report by the reading neuroradiologist or having radiographic changes concerning for necrosis or tumor progression with (ii) stable or improved clinical and radiographic findings after treatment with steroids, bevacizumab, or pentoxyfilline.5,6 BSMs were categorized as symptomatic if patients presented with neurological symptoms thought to be related to a brainstem lesion. Resolution of these symptoms was defined as resolution of pre-GK symptoms at follow-up. Stable or improved symptoms were defined as persistent symptoms without new neurological findings or decreased symptoms as compared with those prior to GK_SRS. New or progressive symptoms were defined as worsening of pre-GK symptoms at follow-up or new symptoms at follow-up. Treatment-related toxicity was defined as new or progressive clinical symptoms thought to be related to brainstem lesions and supported by imaging evidence of changes related to the treatment of the BSM. Local progression-free survival (LPFS) included time to local failure or censoring at time of last brain imaging. PFS included time to local and distant brain failure or censoring at time of last brain imaging.
Descriptive analyses were performed. Median follow-up times were determined using descriptive statistics. Kaplan-Meier curves were generated to provide actuarial estimates of LC with LPFS, OS, and PFS. Due to the low number of local failures, actuarial median LPFS could not be estimated with Kaplan-Meier methodology. Thus, descriptive median time to local failure and actuarial mean LPFS were presented. Actuarial estimates of median OS and PFS were presented. Variables evaluated included patient characteristics (age, sex, presence of symptoms that could be attributed to BSM, Karnofsky Performance Status (KPS), primary and extracranial disease tumor control status, number of brain metastases at GK_SRS), BSM characteristics (malignancy type, location, volume, and MRI evidence of edema, hemorrhage or necrosis prior to GK_SRS), and treatment characteristics (GK dose, receipt of WBRT at any time before or after GK_SRS). Univariate analysis using Cox proportional hazard models evaluated the associations between the above characteristics and LPFS, OS, PFS, and toxicity. A multivariate Cox proportional hazard was not performed due to the low number of local failures and radiation-related toxicity events. Nelson-Aalen curves were created to provide actuarial estimates of cumulative toxicity. All analyses were performed with STATA, version 12.1. P values < .05 were considered statistically significant.
Results
Baseline patient, tumor, and treatment characteristics are detailed in Table 1. Seventy-four patients with 77 lesions involving the brainstem were included in the analysis. Median age was 59 years (range, 27–85 years). Baseline median KPS was 90% (range 50%–100%). Tumor histologies included lung (34%, n = 25), melanoma (31%, n = 23), breast (18%, n = 13), and other (18%, n = 13). The majority of the lesions were located in the pons (78%, n = 60), followed by the midbrain (14%, n = 11) and medulla (8%, n = 6). Median tumor volume was 0.13 cm3 (range, 0.003–5.58 cm3). The median treatment dose was 16 Gy (range, 10–20 Gy) prescribed to 50% isodose line (range, 40%–86%). One (1%) of 74 patients had synchronous brainstem lesions, and 10 (13%) had metachronous brainstem lesions. Thirty-six percent of the brainstem lesions were symptomatic, with predominant symptoms being headache (18%), balance issues (18%), and cranial nerve dysfunction (13%). Four patients (5%, 3 before and 1 after GK_SRS) received WBRT as a planned component of the BSM treatment immediately sequenced with GK_SRS. For the remainder of patients who received any WBRT, 34% (n = 25 of 74) received WBRT prior to GK, and 23% (n = 17 of 74) received WBRT after GK. Three patients (4%) had WBRT before and after GK_SRS.
Table 1.
Patient, tumor, and treatment characteristics
| Characteristic | No. |
|---|---|
| Patients (male/female) | 74 (37/37) |
| Age, median (range) | 59 (27–85) |
| Primary malignancy | |
| Lung | 25 (34%) |
| Melanoma | 23 (31%) |
| Breast | 13 (18%) |
| Other | 13 (18%) |
| Karnofsky Performance Status, median (range)* | 90 (50–100) |
| Brainstem lesions | 77 |
| Midbrain | 11 (14%) |
| Pons | 60 (78%) |
| Medulla | 6 (8%) |
| Synchronous brainstem lesion | 1 (1%) |
| Metachronous brainstem lesion | 10 (13%) |
| Symptoms at GK radiosurgery | 28 (36%) |
| No symptoms | 49 (64%) |
| Headache | 14 (18%) |
| Balance issues | 14 (18%) |
| Cranial nerve dysfunction | 10 (13%) |
| Hydrocephalus | 1 (1%) |
| Hemiparesis | 1 (1%) |
| Seizures | 3 (4%) |
| Nausea/vomiting | 2 (3%) |
| Other symptoms | 4 (5%) |
| Pre-GK MRI findings | |
| Gadolinum enhancement | 77 (100%) |
| Edema | 22 (28%) |
| Tumor necrosis | 1 (1%) |
| Hemorrhage | 1 (1%) |
| Other intracranial metastases at the time of GK | 63 (82%) |
| Uncontrolled primary disease | 32 (43%) |
| Uncontrolled extracranial disease | 55 (74%) |
| Median tumor volume, cm3 (range) | 0.13 (0.003–5.58) |
| Median target volume, cm3 (range) | 0.22 (0.02–7.56) |
| Median dose, Gy (range) | 16 (10–20) |
| Median percent isodose line, (range) | 50 (40–86) |
| Prophylactic steroid given immediately after Gk | 50 (65%) |
| WBRT for BSM immediately sequenced with GK | 4 (5%) |
| WBRT prior to GK, any | 28 (38%) |
| WBRT, any | 43 (58%) |
| Follow-up, median months (range) | 5.5 (0.2–48.5) |
*73 of 74 with documented KPS;
Abbreviations; BSM, brainstem metastasis; GK, Gamma Knife; cm3, cubic centimeters; WBRT, whole-brain radiation therapy.
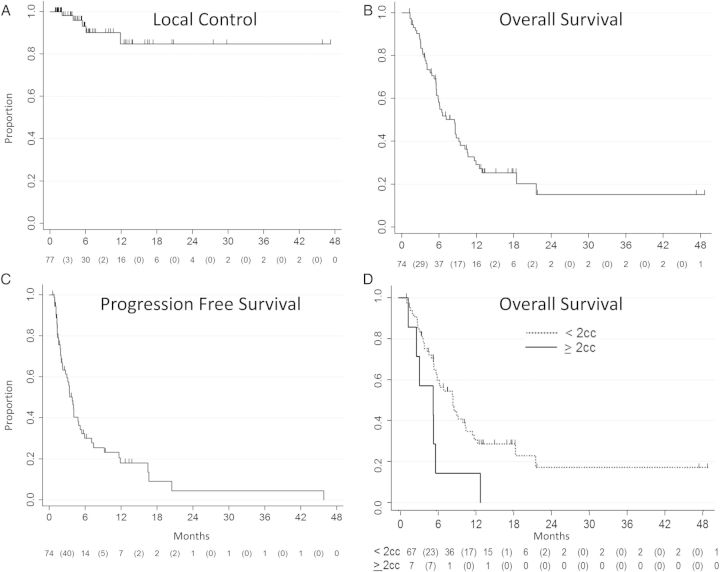
Patient and treatment outcomes are detailed in Table 2. Median follow-up of all patients was 5.5 months (range, 0.2–48.5 months). The median follow-up of patients who were alive at last follow-up was 3.8 months (range, 0.2–16.8 months). There were 5 local failures with a crude LC rate of 94% (n = 72 out of 77). Median actuarial local PFS using Kaplan-Meier estimation could not be calculated due to the low numbers of local failures. The 6-month and 1-year actuarial local PFS were 93% (95% CI, 80%–98%) and 85% (95% CI, 63%–93%), respectively; Figure 1A. Descriptive median time to failure for these 5 local failures was 5.5 months. The 5 failures occurred at 2.1, 3.1, 5.5, 6.1, and 11.9 months.
Table 2.
Patient and tumor outcomes
| Characteristic | No. |
|---|---|
| Median imaging follow-up after GK, month (range) | 4.9 (0.8–45.7) |
| Symptom change after GK of n = 28 with symptomatic lesions | |
| Resolution of symptoms | 10 (36%) |
| Stable/improved symptoms | 14 (50%) |
| New/progression of symptoms | 4 (14%) |
| Complication during GK | 0 (%) |
| Post GK symptoms* | |
| Headache | 17 (22%) |
| Balance issues | 12 (16%) |
| Cranial nerve dysfunction | 10 (13%) |
| Hydrocephalus | 0 (0%) |
| Hemiparesis | 1 (1%) |
| Seizure | 0 (0%) |
| Nausea/vomiting | 4 (5%) |
| Other symptoms | 3 (4%) |
| Local control | 72 (94%) |
| Distant failure | 40 (54%) |
| Death | 50 (68%) |
| GK toxicity | 6 (8%) |
Abbreviation: GK, Gamma Knife.
*73 of 74 patients with symptom follow-up, 76 of 77 lesions with symptom follow-up.
Figure 1.
Kaplan-Meier curves demonstrating actuarial (A) local control or local progression-free survival, (B) overall survival, (C) progression-free survival of patients after treatment with Gamma Knife stereotactic radiosurgery. The numbers at risk at each time point are displayed below the curve, with events displayed in between parentheses, and (D) overall survival stratified by brainstem lesions ≥2 cm3 as compared with, <2 cm3.
Median tumor volume of these lesions was 3.50 cm3 (range, 0.51–5.12 cm3). The median treatment dose for these lesions was 14 Gy (range, 10–18 Gy) prescribed to the 50% isodose line (range, 49%–50%). Primary tumor histologies of local progression included melanoma (n = 2), renal cell, non–small cell lung cancer, and ex pleomorphic adenoma of the head and neck. All 5 failed lesions were located in the pons. On univariate analysis, larger tumor volume was associated with worse LC (HR, 2.09; 95% CI, 1.39–3.14; P < .001) with increased risk of failure for lesions ≥1 cm3, ≥2 cm3, and ≥3 cm3 (HR = 14.6, P = .02; HR = 62.8, P < .001, and HR = 29.5, P < .001, respectively). Presenting with symptoms was also associated with worse LC (HR, 8.70; 95% CI, 0.97–77.94; P = .053). Univariate analysis results are shown in Table 3.
Table 3.
Univariate analysis of local failure, death, and any brain progression
| Characteristic | Local Failure |
Death |
Any Brain Progression |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Karnofsky performance status | 0.96 (0.89–1.05) | .43 | 0.96 (0.95–0.99) | .01 | 0.99 (0.96–1.01) | .32 |
| ≥80% | 0.20 (0.03–1.23) | .08 | 0.50 (0.26–0.98) | .04 | 0.79 (0.37–1.71) | .55 |
| Tumor location | ||||||
| Pons | # | # | 0.92 (0.47–1.80) | .81 | 0.58 (0.29–1.16) | .12 |
| Midbrain | # | # | 0.99 (0.45–2.21) | .99 | 2.29 (1.08–4.87) | .03 |
| Medulla | # | # | 1.24 (0.44–3.46) | .68 | 0.73 (0.18–3.04) | .67 |
| Symptoms at GK | 8.70 (0.97–77.94) | .053 | 2.00 (1.15–3.48) | .02 | 1.41 (0.78–2.54) | .25 |
| Tumor volume | 2.09 (1.39–3.14) | <.001 | 2.14 (1.02–1.49) | .03 | 0.95 (0.74–1.22) | .67 |
| ≥1 cm3 | 14.55 (1.62–130.30) | .02 | 1.56 (0.85–2.87) | .15 | 0.80 (0.40–1.62) | .54 |
| ≥2 cm3 | 62.81 (6.82–578.6) | <.001 | 2.81 (1.31–6.06) | .008 | 1.09 (0.43–2.77) | .85 |
| ≥3 cm3 | 29.50 (4.77–182.56) | <.001 | 2.51 (1.06–5.94) | .04 | 1.16 (0.41–3.24) | .78 |
Abbreviations: CI, confidence interval; cm3, cubic centimeters; GK, Gamma Knife; HR, hazards ratio; #, analysis could not be performed due to limited number of failure events.
Reference group for Cox regression analysis with categorical variables was complement of the analyzed group.
Of the 28 patients presenting with symptoms, 86% had either stable or improved symptoms (50%, n = 14) or complete resolution of symptoms (36%, n = 10) after GK_SRS. Four of the 28 (14%) symptomatic patients had progression or new symptoms after GK. Of those 4 patients, one later had a recurrence, one had increasing headaches, emesis, and lightheadedness with stable imaging, and 2 had worsened balance issues with known concurrent cerebellar metastases or leptomeningeal disease. The majority of patients (n = 50, 65%) were given prophylactic steroids immediately after GK_SRS as per prescribing physician's discretion. On univariate analysis, receipt of prophylactic steroids did not influence the change, improvement, or resolution of initial presenting symptoms.
Median OS, as determined by Kaplan-Meier estimation, was 8.5 months (95% CI, 5.6–9.4 months). The 6-month and 1-year actuarial OS were 58% (95% CI, 46%–69%) and 29% (95% CI, 18%–41%), respectively; see Figure 1B. As found in univariate analysis of local failure, larger tumor size as a continuous variable, especially volumes ≥2 cm3 (see Figure 1D) or ≥3 cm3, and presence of symptoms were also associated with worse OS on univariate analysis, (HR = 2.14, P = .03; HR = 2.81, P = .008; HR = 2.51, P = .04; and HR = 2.00, P = .02, respectively). Higher performance status was associated with improved survival (HR = 0.96, P = .01), which was greater for patients with KPS of ≥80% (HR = 0.50; 95% CI, 0.26–0.98; P = .04). Seventy-nine percent (n = 58) of patients had KPS ≥80%. Nineteen patients (25%) with no or controlled extracranial disease had improved actuarial median OS of 12.9 months, with actuarial 6-month OS of 72% (95% CI, 45%–87%) and actuarial 1-year OS of 53% (95% CI, 27%–73%).
Eighteen percent (n = 14) of BSMs were single lesions treated at the time of GK_SRS. The remainder (82% of BSMs) had 1 or more synchronous brain metastases treated at the time of GK_SRS. Actuarial median PFS was 3.9 months (95% CI, 2.7–4.9 months); see Figure 1C. Midbrain location was significant for worse PFS (HR = 2.29, P = .03). On univariate analysis, receipt of WBRT was not significantly associated with LPFS, OS, or PFS.
Toxicity
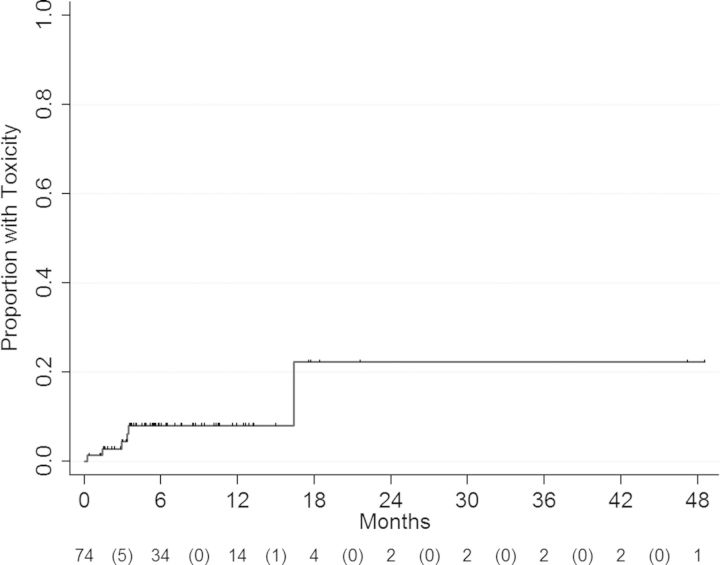
There were 6 treatment-related toxicities in 74 patients (8%), with most events occurring within the first 4 months after GK. The 3- and 6-month/1-year cumulative hazard rate of toxicity was 4.4% (95% CI, 1.4%–13.7%) and 8.0% (95% CI, 3.3%–19.4%), respectively (Figure 2). Two patients developed radionecrosis at 9.2 months and 16.4 months after GK-SRS (see Table 4, case numbers 1 and 3, respectively). On univariate analysis, larger tumor size was associated with a risk of increased toxicity (HR = 1.57, P = . 05), which was more pronounced for size ≥0.05 cm3 versus < 0.05 cm3 (HR = 6.29, P = .04). As for PFS, midbrain location was also significant for increased toxicity risk (HR = 5.25, P = .045). Description and univariate analyses of treatment toxicity are summarized in Tables 4 and 5.
Figure 2.
Cumulative hazard curve demonstrates actuarial cumulative toxicity. The numbers at risk at each time point are displayed below the curve, with numbers of events being displayed inside parentheses.
Table 4.
Toxicity description
| No. | Sex | Primary Malignancy | Location | Tumor Vol (cm3) | Dose (Gy) | Prescribed Isodose Line (%) | Target Vol (cm3) | Pre-GK Symptoms | Toxicity (Imaging Features) | Time to Toxicity (month) | Local Failure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Lung | Midbrain | 0.10 | 16 | 50 | 0.13 | No | Headache, emesis, diagnosed radionecrosis at an outside institution, placed on pentoxifylline (subacute hemorrhage) | 16.4 | No |
| 2 | M | Thyroid | Midbrain | 0.14 | 20 | 50 | 0.21 | No | Subjective imbalance, left leg weakness (increased perilesional edema) | 3.4 | No |
| 3 | F | Breast | Pons | 0.63 | 16 | 45 | 0.91 | Yes | (1) Right-sided Horner's syndrome at 0.3 month (treatment effect; edema); (2) Visual/depth perception changes at 9.2 months (radiographically diagnosed radionecrosis) improved with dexamethasone and avastin | 0.3 | No |
| 4 | F | Sarcoma | Pons | 0.85 | 18 | 50 | 1.10 | Yes | Increased gait imbalance, placed on dexamethasone (increased perilesional edema) | 3.0 | No |
| 5 | M | Melanoma | Midbrain | 1.60 | 15 | 50 | 2.50 | No | Imbalance and falls (increasing perilesional edema) | 3.4 | No |
| 6 | M | Melanoma | Pons | 5.58 | 14 | 50 | 7.56 | No | Headaches (perilesional edema, microhemorrhage) | 1.4 | No |
Abbreviations: GK, Gamma Knife; No., Number; Vol, volume.
Table 5.
Univariate analysis of toxicity
| HR (95% CI) | P value | |
|---|---|---|
| Midbrain tumor location | 5.25 (1.04–26.60) | .045 |
| Edema on Pre-GK Imaging | 5.31 (0.97–29.04) | .054 |
| Symptoms at GK | 0.91 (0.17–4.97) | .91 |
| Uncontrolled extracranial disease | 0.19 (0.03–1.04) | .06 |
| Tumor volume, continuous variable | 1.57 (1.00–2.46) | .05 |
| ≥0.5 cm3 | 6.29 (1.13–35.00) | .04 |
| Prescribed isodose line | 0.64 (0.48–0.84) | .06 |
Abbreviations: CI, confidence interval; cm3, cubic centimeters; GK, Gamma Knife; HR, hazard ratio.
Reference group for Cox regression analysis with categorical variables was complement of the analyzed group.
Discussion
In this retrospective analysis, BSMs treated with GK_SRS demonstrated excellent LC (crude rate of 94%). Both larger tumor volume (especially lesions ≥2 cm3) and presence of symptoms were associated with risk of increased local failure as well as shorter survival. The majority (86%) of patients who initially presented with symptoms benefitted from GK_SRS with either stable, improved, or resolved symptoms after treatment. Treatment-related toxicity rates were low (crude rate 8%, 1-year actuarial rate 8%).
Larger brainstem lesion volume and KPS <80% were associated with worse LC and OS, which were similar to findings from our historical single-institution retrospective study that evaluated outcomes of BSMs treated with Linac_SRS using CT-based planning. However, in our updated study, lesion cutoff size ≥2 cm3 rather than ≥4 cm3 was prognostic of worse LC and OS. On univariate analysis, lesions ≥1 cm3 were also associated with worse LC but not worse OS.
Local control is improved in the current study when compared with our historical Linac_SRS cohort (crude LC 94% vs historical control of 76%; 1-year actuarial LPFS of 85%, 95% CI 65%–93% vs historic 1-year LPFS of 35%, 95% CI 16%–53%).7 Several factors could have contributed to this difference, including lesion size. The size of BSMs treated in this current study was smaller (median tumor 0.13 cm3, range 0.003–5.58 cm3; calculated median diameter 0.49 cm) than our Linac_SRS cohort (median tumor 1 cm3; range, 0.1-8.7 cm3; calculated median diameter 1 cm). Differences in baseline patient characteristics of our current and Linac_SRS cohorts are likely attributed to differences in target lesion size. Our Linac_SRS cohort had higher rates of presenting symptomatic lesions (72% vs 36%) such as cranial neuropathies (38% vs 13%), increased pretreatment abnormal imaging findings such as edema (42% vs 28%), and posttreatment toxicity (20% vs 8%). The detection of smaller BSMs may be attributed to improved brain imaging and MRI-based SRS planning capabilities, as 82% of patients in this current study had other intracranial metastases at the time of SRS as compared with 42% of patients in the Linac_SRS cohort. Other baseline characteristics (including median age, KPS, lesion location, and tumor histology profile) were similar in these 2 studies. Unlike our historic control, GK_SRS dose was not associated with LC on univariate analysis. Although the typical prescribing isodose lines for GK_SRS and Linac_SRS are 50% and 80%, leading to higher dose maximum and anticipated sharper dose falloff for GK_SRS, there is no convincing evidence that this dosimetric difference is transformed into a clinical benefit.1,8–22
The crude LC rate of BSMs in this current study (94%) is slightly higher than previous studies utilizing GK_SRS, (median LC 88%, mean LC 89%, based on Table 6). Our study cohort had smaller median target volume size and is more contemporary with the treatment years being 2009–2013.11,13,15,18–21,23 The improvement in quality of MRI scans and MRI-based radiosurgery is likely a primary factor for identification and hence treatment of BSMs at a smaller size. Given the limitation in resources, most institutions that treat brain metastases with radiosurgery nearly exclusively utilize either GK_SRS or Linac_SRS. This study demonstrates the evolution in diagnosis of BSMs at smaller sizes and resultant outcomes when descriptively compared against historic single institution Linac_SRS outcomes.
Table 6.
Review of Gamma Knife and Linear Accelerator-based stereotactic radiosurgery studies
| Study | SRS Type | Treatment Years | Patients/ Lesions | Median Tumor Size (cm3) | Median Dose (Gy) | Median Prescribed Isodose | Median Follow-up (mo) | Crude Local Control | Actuarial 1-year LC | Median OS (mo) | WBRT prior to SRS (%, n) | Crude Toxicity Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hatiboglu 2011 | Linac | 1994–2007 | 60/60 | 1.0 (0.1–8.7) | 15 (8–18) | 85 (77–95) | 12.8 | 76% (37/49) | 35% | 4.2 | 15% (9) | 20% (12) |
| Lin 2012 | Linac | 1997–2008 | 45/48 | 0.40 (0.02–5.70) | 14 (10–17) | 90 (90–90) | NR | 91% (40/44) | 88% | 11.6 | 38% (17) | 4% (2/45) |
| Kelly 2011 | Linac | 2001–2009 | 24/24 | 0.2 (0.02–2.39) | 13 (8–16) | 73 (52–90) | NR | 82% (18/22) | 79% | 5.3 | 96% (23) | 9% (2/24) |
| Valery 2011 | Linac | 2005–2007 | 30/43 | 2.8 (0.06–18) | 13.4 (8.2–15) | NR | NR | 90% (NR) | 79% | 10 | 27% (8) | 0% |
| Voong 2015 (current study) | GK | 2009–2013 | 74/77 | 0.13 (0.003–5.58) | 16 (10–20) | 50 (40–86) | 5.5 | 94% (72/77) | 85% | 8.5 | 30% (22) | 10% (7/74) |
| Kilburn 2014 | GK | 2000–2010 | 44/52 | 0.13 (0.01–6.60) | 18 (10–22) | 50 (NR) | 6.0 | 82% (36/44) | 74% | 6 | 57% (25) | 9% (4/44) |
| Sengoz 2013 | GK | 2005–2011 | 44/46 | 0.6 (0.34–7.3) | 16 (10–20) | 50 | NR | 96 (44/46) | NR | 8 | 52% (23/44) | 0% |
| Li 2012 | GK | 1995–2008 | 28/32 | 0.78 (0.03–5.6) | 16 (12–20) | 50 (45–90) | NR | 90.6% (29/32) | NR | 9 | 0% (0) | 4% (1/28) |
| Jung 2012 | GK | 2003–2010 | 32/32 | 0.71 (0.01–20.01) | 13 (8–20) | 50 (NR) | 12.5 | 88% (28/32) | NR | 5.2 | 53% (17) | 0% |
| Kawabe 2012 | GK | 1998–2011 | 200/222 | 0.2 (0.005–10.7) | 18 (12–25) | NR | NR | 82% (107/129) | 83% | 6 | 7% (13) | 1% (1/200) |
| Yoo 2011 | GK | 1992–2010 | 32/32 | 1.52* (0.01–6.0) | 15.9* (6–23) | 50 (NR) | 87.5% (21/24) | NR | 7.7 | NA | 3% (1/32) | |
| Koyfman 2010 | GK | 1997–2007 | 43/43 | 0.37 (0.01–8.8) | 15 (9.6–24) | NR | 5.3 | 100% (33/33) | 85% | 5.8 | 51% (22) | 0% |
| Lorenzoni 2009 | GK | 1999–2006 | 25/27 | 0.6 (0.01–3.6) | 20 (15–24) | 50 (50–70) | 10.5 | 95% (20/21) | NR | 11.1 | 44% (11) | NR |
| Kased 2008 | GK | 1991–2005 | 42/44 | 0.26 (0.02–2.8) | 16 (10–19.8) | 50 (39–90) | 6.9 | 85% (22/26) | 77% | 9 | 45% (19/42) | 10% (4/42) |
| Hussain 2007 | GK | 1991–2004 | 22/25 | 0.9 (0.1–3.3) | 16 (14–23) | 50 (40–80) | 8.5 | 100% (22/22) | NR | 8.5 | NR | 5% (1/22) |
| Fuentes 2006 | GK | 1992–2001 | 28/28 | 2.1* (0.4–11) | 19.6* (11–30) | 50 (NR) | 11 | 92% (22/24) | NR | 12 | 21% (6) | 11% (3/28) |
| Yen 2006 | GK | 1989–2005 | 53/53 | 17.6* (9–25) | 2.8* (0.5–21) | 40 (26–85) | 9.8 | 86% (32/37) | NR | 11 | 40% (21) | NR |
| Shuto 2003 | GK | 1992–2001 | 25/31 | 2.1 (0.02–12) | 13 (8–18) | NR | 5.2 | 77.4% (24/31) | NR | 4.9 | 36% (9) | 3% (2/25) |
| Huang 1999 | GK | 1988–1998 | 26/27 | 1.1 (0.3–9.7) | 16 (12–20) | 50 (NR) | 9.5 | 95% (21/22) | NR | 9 | 92% (24) | 12% (3/26) |
Abbreviations: LC, local control; mo, months; NR, data not reported; OS, overall survival.
*Mean values reported.
Interestingly, normalization of the target lesion to a higher isodose line was trending toward association with decreased toxicity (HR, 0.64; P = .06). One of the 6 patients with toxicities following treatment (Horner's syndrome at 0.3 months, with subsequent development of radionecrosis on imaging at 9.2 months) had the dose normalized to 45% isodose lines. Normalization to less than the 50% isodose line, the standard normalization line for GK_SRS, increases the maximum dose delivered to the target and thus may be a risk factor for increased toxicity after stereotactic radiosurgery in this exquisitely eloquent territory of the brain. A recent retrospective study analyzing GK_SRS for treatment of metastases located anywhere in the brain found that less conformal GK_SRS plans (ie, normalization to isodose lines >50%) was associated with decreased rates of radionecrosis on univariate analysis without compromise on LC.3 Shiue et al also noted that metastases in the brainstem, compared with other brain locations, was associated with worse LC.3 Validation of the risk of increased GK_SRS-related toxicity in the brainstem, due to normalization to lower isodose lines, would benefit from larger pooled studies, given the low rate (1%–12%) of radionecrosis as well as toxicity after GK_SRS for BSMs.11,13,15,18–21,23
Limitations of this study include the retrospective nature of the analysis and low local failure events, which may have limited detection of prognostic factors, such as SRS dose, during analysis. The median follow-up time of the cohort, which is lower than the median OS, may have limited the detection of failure events. This shorter follow-up time may be attributed to (i) the poor overall prognosis of the patients with BSMs (median follow-up of patients alive at time of last follow-up was 3.8 months as compared with 5.5 months for all patients) and (ii) our institution's large catchment area as a tertiary cancer center with a high (36%) Gamma Knife patient population referred from out of state. The control and toxicity results of this analysis must therefore be evaluated in the context of the short median patient follow-up time of 5.5 months. Multivariate analysis of LC and toxicity was not feasible due to the small number of observed events. Given the rarity of BSMs, larger pooled analysis would provide better predictors for outcomes including LC and toxicity, which are important in treatment of non-operable lesions.
Conclusion
In conclusion, GK_SRS provides excellent LC of BSMs, with stabilization or improvement of initial presenting symptoms and low toxicity rates. Larger tumor volume (especially ≥2 cm3) and symptomatic lesions at presentation were associated with worse LC and OS.
Funding
KRV and BF were recipients of Elekta resident travel grants. AL was the recipient of an Elekta honorarium.
Acknowledgments
This work was presented at the 96th Annual Meeting of American Radium Society, April 26–29, 2014, St. Thomas, US Virgin Islands, and the 17th International Leksell Gamma Knife Society Meeting, May 11–15, 2014, New York, New York.
Conflicts of interest statement. No additional potential conflicts of interests have been identified by the authors of this original work.
References
- 1.Fuentes S, Delsanti C, Metellus P, et al. Brainstem metastases: management using gamma knife radiosurgery. Neurosurgery. 2006;58(1):37–42. doi: 10.1227/01.neu.0000190655.95669.5c. discussion 37–42. [DOI] [PubMed] [Google Scholar]
- 2.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Physics. 2000;47(2):291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 3.Shiue K, Barnett GH, Suh JH, et al. Using higher isodose lines for gamma knife treatment of 1 to 3 brain metastases is safe and effective. Neurosurgery. 2014;74(4):360–366. doi: 10.1227/NEU.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Delanian S, Lefaix JL. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Sem Radiat Oncol. 2007;17(2):99–107. doi: 10.1016/j.semradonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15(9):1257–1263. doi: 10.1093/neuonc/not085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatiboglu MA, Chang EL, Suki D, Sawaya R, Wildrick DM, Weinberg JS. Outcomes and prognostic factors for patients with brainstem metastases undergoing stereotactic radiosurgery. Neurosurgery. 2011;69(4):796–806. doi: 10.1227/NEU.0b013e31821d31de. discussion 806. [DOI] [PubMed] [Google Scholar]
- 8.Lin CS, Selch MT, Lee SP, et al. Accelerator-based stereotactic radiosurgery for brainstem metastases. Neurosurgery. 2012;70(4):953–958. doi: 10.1227/NEU.0b013e31823c40fe. discussion 958. [DOI] [PubMed] [Google Scholar]
- 9.Kelly PJ, Lin YB, Yu AY, et al. Linear acm3elerator-based stereotactic radiosurgery for brainstem metastases: the Dana-Farber/Brigham and Women's Cancer Center experience. J Neurooncol. 2011;104(2):553–557. doi: 10.1007/s11060-010-0514-0. [DOI] [PubMed] [Google Scholar]
- 10.Valery CA, Boskos C, Boisserie G, et al. Minimized doses for linear accelerator radiosurgery of brainstem metastasis. Int J Radiat Oncol Biol Phy. 2011;80(2):362–368. doi: 10.1016/j.ijrobp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Kilburn JM, Ellis TL, Lovato JF, et al. Local control and toxicity outcomes in brainstem metastases treated with single fraction radiosurgery: is there a volume threshold for toxicity? J Neurooncol. 2014;117(1):167–174. doi: 10.1007/s11060-014-1373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Xu D, Zhang Z, et al. Gamma Knife surgery for brainstem metastases. J Neurosurg. 2012;117(Suppl):13–16. doi: 10.3171/2012.7.GKS121020. [DOI] [PubMed] [Google Scholar]
- 13.Jung EW, Rakowski JT, Delly F, et al. Gamma Knife radiosurgery in the management of brainstem metastases. Clin Neurol Neurosurg. 2013;115(10):2023–2028. doi: 10.1016/j.clineuro.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Kawabe T, Yamamoto M, Sato Y, et al. Gamma Knife surgery for patients with brainstem metastases. J Neurosurg. 2012;117(Suppl):23–30. doi: 10.3171/2012.7.GKS12977. [DOI] [PubMed] [Google Scholar]
- 15.Yoo TW, Park ES, Kwon do H, Kim CJ. Gamma knife radiosurgery for brainstem metastasis. J Korean Neurosurg Soc. 2011;50(4):299–303. doi: 10.3340/jkns.2011.50.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyfman SA, Tendulkar RD, Chao ST, et al. Stereotactic radiosurgery for single brainstem metastases: the Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2010;78(2):409–414. doi: 10.1016/j.ijrobp.2009.07.1750. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzoni JG, Devriendt D, Massager N, et al. Brain stem metastases treated with radiosurgery: prognostic factors of survival and life expectancy estimation. Surg Neurol. 2009;71(2):188–195. doi: 10.1016/j.surneu.2008.01.029. discussion 195, 195–186. [DOI] [PubMed] [Google Scholar]
- 18.Kased N, Huang K, Nakamura JL, et al. Gamma knife radiosurgery for brainstem metastases: the UCSF experience. J Neurooncol. 2008;86(2):195–205. doi: 10.1007/s11060-007-9458-4. [DOI] [PubMed] [Google Scholar]
- 19.Hussain A, Brown PD, Stafford SL, Pollock BE. Stereotactic radiosurgery for brainstem metastases: Survival, tumor control, and patient outcomes. Int J Radiat Oncol Biol Phys. 2007;67(2):521–524. doi: 10.1016/j.ijrobp.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 20.Yen CP, Sheehan J, Patterson G, Steiner L. Gamma knife surgery for metastatic brainstem tumors. J Neurosurg. 2006;105(2):213–219. doi: 10.3171/jns.2006.105.2.213. [DOI] [PubMed] [Google Scholar]
- 21.Shuto T, Fujino H, Asada H, Inomori S, Nagano H. Gamma knife radiosurgery for metastatic tumours in the brain stem. Acta Neurochir (Wien) 2003;145(9):755–760. doi: 10.1007/s00701-003-0034-1. [DOI] [PubMed] [Google Scholar]
- 22.Huang CF, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brainstem metastases. J Neurosurg. 1999;91(4):563–568. doi: 10.3171/jns.1999.91.4.0563. [DOI] [PubMed] [Google Scholar]
- 23.Sengoz M, Kabalay IA, Tezcanli E, Peker S, Pamir N. Treatment of brainstem metastases with gamma-knife radiosurgery. J Neurooncol. 2013;113(1):33–38. doi: 10.1007/s11060-013-1086-6. [DOI] [PubMed] [Google Scholar]