Abstract
Background
Clinical studies of re-irradiation (ReRT) for relapsed high-grade glioma (HGG) have generally reported the use of small volume ReRT techniques such as stereotactic radiosurgery in selected patients with isolated focal relapse. This study reports the outcome with large-volume ReRT to manage the more common mescenario of extensive diffuse relapse of HGG.
Methods
All HGG patients managed with an overlapping second course of radiation therapy (RT) for refractory progression of HGG between October 2009 and April 2013 were included. ReRT was initially used with bevacizumab (BEV), then used when disease was refractory to BEV, and finally used upfront with BEV-naïve patients. Tumor volume (GTV) and specific RT dosimetry factors, including the target volume treated (PTV), and cumulative RT dose maximum (Dmax), were analyzed. Median survival post ReRT was calculated using the Kaplan-Meier method and SPPS v19 software.
Results
Eighteen HGG participants with refractory, bulky contrast-enhancing disease received ReRT. Thirteen participants had a maximum tumor diameter >5 cm, and median GTV was 54 cm3. Seven participants had BEV-refractory disease, and 8 participants were BEV naïve. ReRT dose was 35–40 Gy in 15 fractions; median PTV was 133 cm3, and median Dmax was 98.2 Gy. Median survival post ReRT for all participants was 8 months (95%CI, 5.8–10.2 months); with 10 months and 3 months for the BEV-naïve and BEV-refractory participants, respectively (P = .024). Two early participants, who were managed without BEV, were later salvaged with BEV, including one who required craniotomy for radiation necrosis at 6 weeks post RT. No other significant morbidity was reported.
Conclusion
ReRT combined with BEV is a feasible salvage treatment option for diffuse refractory HGG.
Keywords: bevacizumab, glioma, re-irradiation, salvage
Improvements in the initial management of patients with newly diagnosed glioblastoma multiforme (GBM) in recent years have increased the expectation of aggressive salvage therapies at the time of relapse.1–6 Routine MRI surveillance after initial therapy has also added to this expectation with detection of asymptomatic relapse in the presence of excellent performance status. Options for second- or third-line relapse therapies are evolving, but no specific single-agent therapy has provided any durable disease control.7–11 Bevacizumab (BEV) has been investigated in both the initial management of glioblastoma and at the time of relapse. With regard to initial management, the results of 2 large randomized controlled trials were presented at the 2013 annual meeting of the American Society of Clinical Oncology ASCO: the RTOG 0825 and the AVAGlio studies.12 Both trials demonstrated prolonged, progression-free survival, but not overall survival (OS), when BEV was combined with temozolomide. In the setting of relapsed disease, BEV has not provided durable benefit despite promising short-term symptomatic and radiological responses.13,14 Combination therapy with a systemic cytotoxic such as irinotecan, lomustine, or carboplatin has provided only limited extension of median survival.13–19 Significant acute morbidity may be evident with the systemic agents as well as the inconvenience of cyclical treatment administration and toxicity monitoring.
Historically, selected patients with focal, inoperable relapse have been managed with small-volume re-irradiation (ReRT) in specialized centers utilizing stereotactic radiosurgery/radiation therapy, but this has generally been limited to target lesions of <3 cm in diameter or <40 cm3in volume.20–26 Re-RT to larger overlapping volumes would potentially increase the risk of morbidity with acute edema or necrosis and thus reduce the therapeutic benefit. However, the recent awareness of BEV as a management option for radiation necrosis has expanded the potential for large-volume relapses to be managed with ReRT in combination with BEV to minimize the acute morbidity.27–29
This study details the experience with large volume ReRT and explores the impact on outcome in regard to timing of ReRT in relation to BEV implementation.
Methods
Newly diagnosed adult patients with a primary brain tumor, who had been referred to the Department of Radiation Oncology at the Northern Sydney Cancer Centre after May 2007, were entered into a prospective database that had been previously approved by the Institutional Ethics Review Board. All patients had given informed consent for their information to be recorded. Selected patients with high-grade glioma (HGG), as confirmed by sequential MR imaging with progressive gadolinium contrast-enhancing mass lesions refractory to systemic chemotherapy and offered re-irradiation, were formally included in this study. All participants were managed by one subspecialist radiation oncologist and one medical oncologist as part of a multidisciplinary neuro-oncology tumor team. Patient, tumor, and treatment factors were recorded in the database.
Patient Selection
Patients with recurrent high-grade glioma post standard dose (54–60 Gy) adjuvant RT and temozolomide had individualized interventions based on tumor and prior treatment factors. Generally, the Neuro-oncology Unit's policy was surgical resection at first relapse if isolated and operable, followed by second- and third-line chemotherapy with options including procarbazine, carboplatin, and lomustine. BEV was preferentially offered late with refractory disease rather than at first relapse, but utilization was dependent upon logistical factors such as the participant co-funding.
Diagnosis of Recurrent Disease
Sequential T3 MRI was the standard modality utilized to confirm progressive disease with disease measurements based on T1 gadolinium-enhanced sequences as well as T2/FLAIR images. MRI was performed after initial therapy at 1 month post adjuvant RT and then every second month during adjuvant temozolomide, followed by every 3 months until year 3 post treatment. Any progressive contrast enhancement in the 6 months post RT was presumed to be pseudoprogression unless residual tumor with a proliferative Ki67 level was confirmed by repeat craniotomy. No patients were considered for ReRT in the initial 9 months post diagnosis.
Planning Target Volume Determination
The gross tumor volume (GTV) was based on sequential MRI imaging results encompassing all gadolinium-enhanced tumor as well as any significant nonenhancing T2/FLAIR suggestive of infiltrative tumor. To clarify the nonenhancing tumor, amino acid (FET) PET was performed with tumor confirmed as FET-uptake in the presence of T2/FLAIR. The GTV (cm3) and maximum tumor diameter (cm) were recorded. The GTV was expanded by 5 mm to anatomical boundaries and a further 3 mm to planning target volume (PTV).
Radiation Therapy Dosimetry
Patients were managed with intensity-modulated radiation therapy (IMRT), optimally using a fractionated schedule involving a dose of 35 Gy in 15 treatments over 3 weeks. Other fractionation schedules (25 Gy/5, 40 Gy/20, and 45 Gy/25) were utilized in selected participants. Prior RT dosimetry was utilized in treatment design to produce a summated plan. No dose limitation was applied to the PTV or cortical brain, but the sum dose to optic chiasm/nerves was limited to a maximum dose of 75 Gy and to 1 cm3 volume of 85 Gy to the brainstem. Dosimetry factors were recorded including ReRT PTV (cm3), sum plan dose maximum (Gy), sum mean brain dose, volume of brain receiving 80 Gy (V80), and 90 Gy (V90).
Systemic Therapy
Participants were managed with ReRT at the time of refractory disease, generally following a minimum of 2 salvage regimens. The integration of ReRT with BEV was adjusted over the timeframe of the study with initial participants in 2009 being managed with ReRT alone; ReRT was delivered with BEV in BEV-refractory disease in 2010 and delivered prior to BEV in BEV-naïve participants in 2012. The BEV dose used was 10 mg/m2 every 2 weeks, and BEV was commenced 2 weeks prior to ReRT in BEV-naïve participants. It was continued indefinitely on a every 3 weeks regimen after ReRT, even in the presence of radiological or clinical progression. Cessation occurred when participants reached a poor performance status of Eastern Cooperative Oncology Group (ECOG) 3. Concurrent systemic cytotoxics (except CCNU) were avoided during ReRT.
Follow-up
All participants were followed clinically until death or the censure date of the study (October 15, 2013). Participants were assessed for toxicity every 3 weeks after ReRT at the time of BEV delivery except those managed with RT alone, who were reviewed monthly. Radiological assessment with MRI was performed at 1 month after ReRT and then second monthly.
RT toxicity endpoints collected were specifically unplanned admission to hospital within 60 days of ReRT, ECOG performance status at month+1, and radiation necrosis confirmed by pathology at the time of craniotomy or FDG-PET. Initial relapse was recorded as infield (within 95% isodose of high-dose RT), marginal (within 20 mm from 95% isodose), or distant (>20 mm from 95% isodose).
Statistical Considerations
All participants had their clinical and dosimetric data entered on an Excel database at Northern Sydney Cancer Centre and updated for outcome events. The primary endpoint for the study was OS, which was defined as the start of ReRT until death or censoring date. Survival curves were generated using the Kaplan-Meier method. Univariate predictors of survival duration were evaluated using log-rank comparisons. All reported P values were 2-tailed. Statistical significance was defined as P ≤ .05 in all cases. SPSS version 21 was used for statistical analysis.
Results
Eighteen participants with refractory, contrast-enhancing HGG were managed with ReRT between October 2009 and April 2013 and were included in the analysis. Patient and tumor characteristics are summarized in Table 1. There were 14 males and 4 females, and the age at ReRT ranged from 32 to 73 years with a median age of 50 years. The initial pathology was GBM and anaplastic astrocytoma in 72% and 28% of participants, respectively. The median time from initial diagnosis to ReRT was 22 months (range, 8–154 months); from diagnosis of glioblastoma or high-grade enhancing relapse to ReRT, the median time was 15 months (range, 0–108 months). Only one participant had a surgical procedure within 6 weeks of ReRT, and this was a subtotal resection with residual disease evident on MRI. Pre-ReRT performance status was excellent (ECOG 0–1) in the majority of participants (61%), and only one participant was managed initially as an inpatient. Four participants were in paid employment prior to ReRT.
Table 1.
Patient characteristics
| Characteristic | n = 18 | % |
|---|---|---|
| Male | 14 | 78 |
| Female | 4 | 22 |
| Age range (years) | 32–73 | |
| Median age (years) | 50 | |
| Time from diagnosis to ReRT (months): | ||
| Range | 8–154 | |
| Median | 22 | |
| Time from GBM to ReRT (months): | ||
| Range | 0–108 | |
| Median | 15 | |
| ECOG pre-ReRT: | ||
| 0 | 3 | 17 |
| 1 | 8 | 44 |
| 2 | 6 | 33 |
| 3 | 1 | 6 |
| Initial histopathology: | ||
| GBM | 13 | 72 |
| Anaplastic glioma | 5 | 28 |
| Refractory disease location: | ||
| Frontal | 7 | 39 |
| Temporal | 3 | 17 |
| Occipital | 3 | 17 |
| Parietal | 4 | 22 |
| Thalamus/brainstem | 1 | 6 |
| ReRT dose: | ||
| 25 Gy | 1 | 6 |
| 35 Gy | 14 | 78 |
| 40 Gy | 2 | 11 |
| 45 Gy | 1 | 6 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; GBM, glioblastoma multiforme; ReRT, re-irradiation.
Site of refractory HGG was evenly distributed in the brain, with the majority being frontal lobe tumors (39%). The median diameter was 5.3 cm, with only 5 participants having maximum diameters < 5 cm. The median GTV was 54 cm3, with a range of 2–192 cm3.
All participants had progressed on at least 2 salvage chemotherapy regimens except for one who had debulking surgery prior to ReRT. This man had early progression at 5 months after adjuvant RT with dural-based relapse and had surgery followed by ReRT without BEV to the residual disease. Seven of the 18 participants had refractory disease on BEV prior to ReRT.
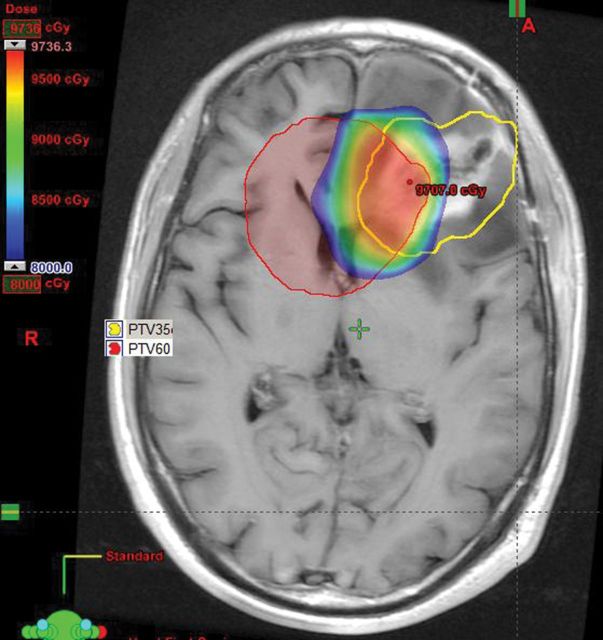
All ReRT was delivered with IMRT, and 13 participants had IMRT as part of initial definitive RT. The dose/fractionation was 35 Gy in 15 fractions in 14 participants. Two participants received 40 Gy in 20 fractions, one participant 45 Gy in 25 fractions, and one participant in 25 Gy 5 fractions. The RT dosimetry parameters are detailed in Table 2. The median PTV was 136 cm3, the extent of overlap of the PTVs between the primary treatment and the reirradiation was demonstrated by the median V80 and V90 of 162 cm3 and 101 cm3, respectively (Figure 1).
Table 2.
Treatment dosimetry characteristics
| Characteristic | Range | Median |
|---|---|---|
| Maximum diameter (cm) | 1.3–9.2 | 5.3 |
| GTV (cm3) | 2–192 | 54 |
| PTV (cm3) | 12–315 | 136 |
| Maximum brain dose (Gy) | 70.1–111.6 | 98.3 |
| Mean brain dose (Gy) | 22.4–43.9 | 32.5 |
| V90 (cm3) | 0–329 | 101 |
| V80 (cm3) | 0–456 | 162 |
| Maximum chiasm dose (Gy) | 5.1–65.9 | 46.6 |
| Maximum brainstem dose (Gy) | 11.9–84.1 | 51.7 |
Figure 1.
Example of re-irradation (ReRT) planning target volume (PTV) in left frontal lobe (yellow); initial RT PTV (red) and overlap dose >80Gy (dose wash).
The policy for integrating ReRT with BEV was modified over the 4-year period. ReRT without BEV was used in 3 participants, but 2 of these participants commenced BEV within 2 months because of necrosis or steroid dependency. The third participant did not start BEV due to further surgical procedures. The remaining participants had ReRT at the time of disease progression on BEV in 7 participants, and upfront with initial BEV utilization in 8 participants.
Overall Survival
Fourteen of the 18 participants had died from progressive HGG at the time of censure for analysis, with the survivors having a median follow-up of 5.2 months. Three of the surviving participants were free of radiological progression.
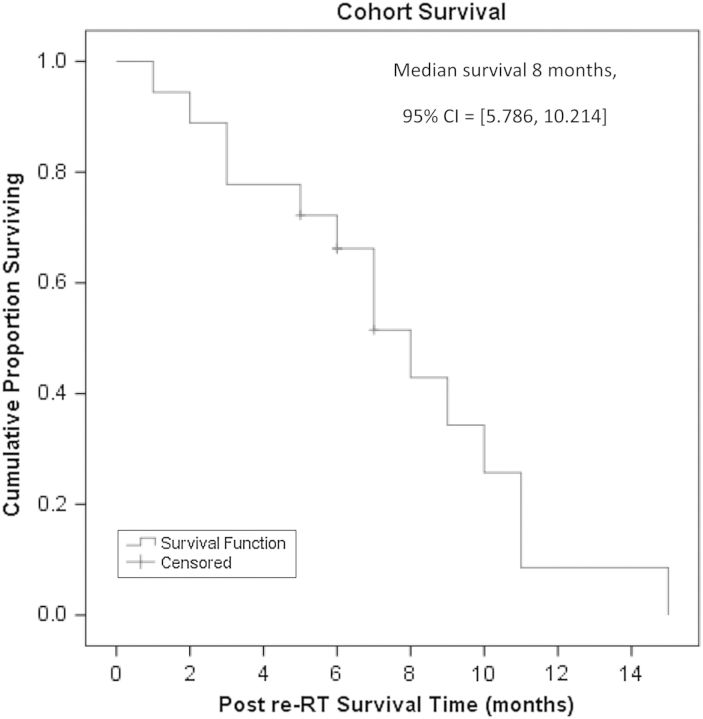
The median OS of the group from ReRT was 8 months (95% CI, 5.8–10.2 months) with 72% of participants surviving longer than 6 months (Figure 2). Univariate analysis of the entire cohort found that being BEV naïve and having a longer time from diagnosis were predictive for improved survival. Median survival in the BEV-naïve participants was 10 months (95% CI, 3.9–16.1 months), compared with 3 months (95% CI,1.7–4.3) for the BEV-refractory participants (P = .024). Participants with survival longer than the median of 22 months from diagnosis had longer survival post ReRT compared with those who had shorter intervals between diagnosis and ReRT. The median survival in the former was 9 months (95% CI, 6.6–11.4 months) versus 5 months in the latter (95% CI, 0.9–9.1) (P = .044).
Figure 2.
Overall survival curve following re-irradiation.
Tumor bulk, as measured by GTV, was not associated with survival post RT (P = .462), and neither was pre-ReRT ECOG 0–1 versus 2–3 (P = .148), smaller versus larger PTV (P = .913), mean brain dose (P = .619), or site of refractory disease (P = .342).
Toxicity
ReRT was well tolerated. Nine participants required dexamethasone (DEX) at commencement of ReRT; at the end of 3 weeks, 3 participants had reduced their dose, and 5 were stable, but one had increased the dose of DEX. Additionally, 4 of the 9 participants who were not taking DEX at the start of ReRT were requiring DEX at completion. Of the 5 participants increasing DEX during ReRT, 4 were BEV refractory or were not taking BEV. One participant, who was managed without BEV, required craniotomy for an enlarging, contrast-enhancing mass at 6 weeks after RT. This confirmed radiation necrosis; BEV was commenced, and the participant survived another 9 months.
A second participant, who was managed without BEV, was steroid dependent and commenced BEV at 4 months after ReRT. The participant survived an additional 8 months. Two participants had an unplanned admission in the 60 days after ReRT: one required craniotomy for radiation necrosis, and the other had a generalized seizure.
With regard to toxicity from BEV, there were 2 (13%) reported grade 3 toxicities: 1 grade 3 epistaxis and 1 deep venous thrombosis.
Of the 4 participants who were in paid employment prior to ReRT, 2 continued to work for a minimum of 3 months, but the other 2 participants stopped working because of progressive disease.
Discussion
This study demonstrates that ReRT in combination with BEV is a feasible treatment option for refractory, bulky, contrast-enhancing HGG with a median survival of 10 months in the participants re-irradiated with BEV-naïve disease. This is consistent with other ReRT series utilizing BEV, which described relatively long median survivals in the presence of relapsed disease and minimal toxicity.28,29
Even though the patient cohort was small, this study is significant because the tumors were bulky, with a median PTV of 133 cm2 being larger than prior ReRT series utilizing precision RT to small volumes. These studies generally showed median PTVs in the range 15–40 cm3,20–26,28,29 with only a series from Leipzig demonstrating a median PTV of >110 cm3.30 The impact of surgery was potentially a factor in that study because the majority of participants were managed at first relapse, and 67% had a surgical procedure prior to ReRT. The GTV included the surgical cavity, so the residual disease bulk would have been low. Only one participant from the current series had debulking surgery prior to ReRT and still had a significant residual disease component. Despite the large volumes treated, participants in the Leipzig study did not receive BEV, and the median survival in that study was 7.7 months. The role of surgery in reducing the residual bulk of disease may have also contributed to fewer requirements for BEV. In the current series, the 3 participants who did not receive BEV with ReRT were either salvaged with BEV or had early progression post ReRT (the inference being that the risk of radiation necrosis may be more evident when bulky tumors are treated without the co-delivery of BEV).
The other significant feature of this study is that participants were managed at the time of disease progression following a minimum of 2 salvage systemic therapy regimens, which is different from the prior salvage BEV-controlled studies that utilized BEV at the time of initial GBM relapse after adjuvant chemoradiation therapy.13–19 These studies utilized BEV alone, combined BEV and cytotoxic chemotherapy (irinotecan, lomustine, or carboplatin), or cytotoxic chemotherapy alone. Despite being implemented as the initial salvage therapy, the median survival in these studies was generally between 6 and 11 months, with the combined arm on the Dutch BELOB study (lomustine and BEV) performing most favorably with a median survival of 11 months.14 The CABARET study utilizing salvage carboplatin and BEV at initial relapse of GBM had a median survival of 6.9 months, suggesting a ceiling to the benefit that could be obtained with the addition of currently available cytotoxics at first relapse.19 Both the irinotecan BRAIN protocol15 and the BELOB study14 infer that combined therapy may be more favorable and that BEV requires a cytotoxic to achieve improved outcome. Combining RT as the cytotoxic with BEV has potential advantages in that the duration of therapy is brief and requires less monitoring. Using reRT with BEV allows prior or subsequent trial of other systemic agents as even in bulky unresectable disease, delayed BEV and ReRT reults in an acceptable duration of median survival.
The combination of BEV and ReRT is currently being examined in a randomized phase II study (RTOG 1205) with assignment of participants to BEV alone or BEV combined with ReRT (35 Gy in 10 fractions) at the time of first relapse of GBM.31 The study endpoint is median survival, with a hypothesis that the combined therapy will produce a 31% reduction in the hazard ratio to 0.69 and a median survival of 13 months. The participant selection is broad; even though it allows patients with larger target volumes, the maximum diameter of the tumor is restricted to 5 cm. Even with this criterion, only 5 of the 18 participants in the current series would have been eligible.
The role of ReRT in BEV-refractory disease remains uncertain since the current series of 7 participants was associated with poor outcome with a median survival of 3 months. The outcome of patients with progression on BEV is extremely poor, but patients who are being actively monitored with MRI may be diagnosed with asymptomatic radiological progression. In this study cohort, 3 of the 7 participants with BEV-refractory disease were ECOG status 0 or 1, and all survived longer than 6 months. In the absence of data, patient selection based on performance status may be crucial for delineating a more favorable group of BEV-refractory patients.
Conclusion
ReRT with BEV is a feasible salvage therapy for patients with refractory bulky HGG. While ReRT reports have described favorable median survival as a single modality, these have primarily been with small volume relapsed disease. Therefore, the addition of BEV should be considered when managing patients with large volume treatments.
References
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Dirks P, Bernstein M, Muller PJ, Tucker WS. The value of reoperation for recurrent glioblastoma. Can J Surg. 1993;36(3):271–275. [PubMed] [Google Scholar]
- 3.Brandes AA, Bartolotti M, Franceschi E. Second surgery for recurrent glioblastoma: advantages and pitfalls. Expert Rev Anticancer Ther. 2013;13(5):583–587. doi: 10.1586/era.13.32. [DOI] [PubMed] [Google Scholar]
- 4.Chaichana KL, Zadnik P, Weingart JD, et al. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2013;118(4):812–820. doi: 10.3171/2012.9.JNS1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch O, Han SJ, Cha S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117(6):1032–1038. doi: 10.3171/2012.9.JNS12504. [DOI] [PubMed] [Google Scholar]
- 6.Harsh GR, 4th, Levin VA, Gutin PH, et al. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21(5):615–621. doi: 10.1227/00006123-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt F, Fischer J, Herrlinger U, et al. PCV chemotherapy for recurrent glioblastoma. Neurology. 2006;66(4):587–589. doi: 10.1212/01.wnl.0000197792.73656.c2. [DOI] [PubMed] [Google Scholar]
- 8.Trent S, Kong A, Short SC, et al. Temozolomide as second-line chemotherapy for relapsed gliomas. J Neurooncol. 2002;57(3):247–251. doi: 10.1023/a:1015788814667. [DOI] [PubMed] [Google Scholar]
- 9.Kappelle AC, Postma TJ, Taphoorn MJ, et al. PCV chemotherapy for recurrent glioblastoma multiforme. Neurology. 2001;56(1):118–120. doi: 10.1212/wnl.56.1.118. [DOI] [PubMed] [Google Scholar]
- 10.Schäfer N, Tichy J, Thanendrarajan S, et al. Ifosfamide, carboplatin and etoposide in recurrent malignant glioma. Oncology. 2011;80(5–6):330–332. doi: 10.1159/000330358. [DOI] [PubMed] [Google Scholar]
- 11.Oh J, Sahgal A, Sanghera P, et al. Glioblastoma: patterns of recurrence and efficacy of salvage treatments. Can J Neurol Sci. 2011;38(4):621–625. doi: 10.1017/s0317167100012166. [DOI] [PubMed] [Google Scholar]
- 12.Weller M, Yung WKA. Angiogenesis inhibition for glioblastoma at the edge: beyond AVAGlio and RTOG 0825. Neuro Oncol. 2013;15(8):971. doi: 10.1093/neuonc/not106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang G, Huang S, Wang Z. A meta-analysis of bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent glioblastoma multiforme. J Clin Neurosci. 2012;19(12):1636–1640. doi: 10.1016/j.jocn.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Taal W, Oosterkamp HM, Walenkamp AME, et al. A randomized phase II study of bevacizumab versus bevacizumab plus lomustine versus lomustine single agent in recurrent glioblastoma: The Dutch BELOB study. J Clin Oncol [Internet] 2013 [cited 2013 Dec 4];31(suppl; abstr 2001).: Available from: http://meetinglibrary.asco.org/content/109069-132 . [Google Scholar]
- 15.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain MC. Bevacizumab plus irinotecan in recurrent glioblastoma. J Clin Oncol. 2008;26(6):1012–1013. doi: 10.1200/JCO.2007.15.1605. author reply 1013. [DOI] [PubMed] [Google Scholar]
- 17.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 18.Gil MJ, de Las Peñas R, Reynés G, et al. Bevacizumab plus irinotecan in recurrent malignant glioma shows high overall survival in a multicenter retrospective pooled series of the Spanish Neuro-Oncology Research Group (GEINO) Anticancer Drugs. 2012;23(6):659–665. doi: 10.1097/CAD.0b013e3283534d3e. [DOI] [PubMed] [Google Scholar]
- 19.Field KM, Simes J, Wheeler H, et al. A randomized phase II study of carboplatin and bevacizumab in recurrent glioblastoma multiforme (CABARET) J Clin Oncol [Internet] 2013 [cited 2013 Dec 4];31(suppl; abstr 2017). Available from: http://meetinglibrary.asco.org/content/115649-132 . [Google Scholar]
- 20.Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28(18):3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amelio D, Amichetti M. Radiation therapy for the treatment of recurrent glioblastoma: an overview. Cancers (Basel) 2012;4(1):257–280. doi: 10.3390/cancers4010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamberlain MC, Barba D, Kormanik P, et al. Stereotactic radiosurgery for recurrent gliomas. Cancer. 1994;74(4):1342–1347. doi: 10.1002/1097-0142(19940815)74:4<1342::aid-cncr2820740426>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23.Hall WA, Djalilian HR, Sperduto PW, et al. Stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol. 1995;13(7):1642–1648. doi: 10.1200/JCO.1995.13.7.1642. [DOI] [PubMed] [Google Scholar]
- 24.Combs SE, Thilmann C, Edler L, et al. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23(34):8863–8869. doi: 10.1200/JCO.2005.03.4157. [DOI] [PubMed] [Google Scholar]
- 25.Hudes RS, Corn BW, Werner-Wasik M, et al. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 1999;43(2):293–298. doi: 10.1016/s0360-3016(98)00416-7. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd SF, Laing RW, Cosgrove VP, et al. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Phys. 1997;37(2):393–398. doi: 10.1016/s0360-3016(96)00455-5. [DOI] [PubMed] [Google Scholar]
- 27.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niyazi M, Ganswindt U, Schwarz SB, et al. Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys. 2012;82(1):67–76. doi: 10.1016/j.ijrobp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82(5):2018–2024. doi: 10.1016/j.ijrobp.2010.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholtyssek F, Zwiener I, Schlamann A, et al. Reirradiation in progressive high-grade gliomas: outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol. 2013;8:161. doi: 10.1186/1748-717X-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.StudyDetails.pdf [Internet] [cited 2013 Dec 20]. Available from http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?action=openFile&FileID=10674 .