Abstract
Background
As yet, no population-based prospective studies have been conducted to investigate the incidence and clinical outcome of glioblastoma (GBM) or the diffusion and impact of the current standard therapeutic approach in newly diagnosed patients younger than aged 70 years.
Methods
Data on all new cases of primary brain tumors observed from January 1, 2009, to December 31, 2010, in adults residing within the Emilia-Romagna region were recorded in a prospective registry in the Project of Emilia Romagna on Neuro-Oncology (PERNO). Based on the data from this registry, a prospective evaluation was made of the treatment efficacy and outcome in GBM patients.
Results
Two hundred sixty-seven GBM patients (median age, 64 y; range, 29–84 y) were enrolled. The median overall survival (OS) was 10.7 months (95% CI, 9.2–12.4). The 139 patients ≤aged 70 years who were given standard temozolomide treatment concomitant with and adjuvant to radiotherapy had a median OS of 16.4 months (95% CI, 14.0–18.5). With multivariate analysis, OS correlated significantly with KPS (HR = 0.458; 95% CI, 0.248–0.847; P = .0127), MGMT methylation status (HR = 0.612; 95% CI, 0.388–0.966; P = .0350), and treatment received in a high versus low-volume center (HR = 0.56; 95% CI, 0.328–0.986; P = .0446).
Conclusions
The median OS following standard temozolomide treatment concurrent with and adjuvant to radiotherapy given to (72.8% of) patients aged ≤70 years is consistent with findings reported from randomized phase III trials. The volume and expertise of the treatment center should be further investigated as a prognostic factor.
Keywords: center volume, glioblastoma, radiotherapy, surgery, temozolomide
Glioblastoma multiforme (GBM) is the most common and aggressive glioma in adults, with an incidence of 3 to 4 new cases per 100 000 inhabitants per year.1 Despite advances in treatment modalities, the prognosis for GBM remains poor, with a 10% probability of survival at 5 years.2
The standard-of-care treatment for newly diagnosed GBM was established in 2005, when findings made in a randomized phase III trial (EORTC 26981–22981 /NCIC CE3 [EORTC/NCIC]) demonstrated that temozolomide concurrent with and adjuvant to radiotherapy was more effective than radiotherapy alone.3 Results obtained from one phase III clinical trial neither warrant transferability to clinical practice nor reliably demonstrate a survival benefit for the entire GBM population. The impact of this combined approach has been evaluated in many retrospective population studies,4–6 but no findings from a prospective study have been reported in the literature so far.
Methods
A primary brain tumor (PBT) registry, formerly known as the Project of Emilia-Romagna Region on Neuro-Oncology (PERNO), funded by the Italian Ministry of Health, was created in the Emilia-Romagna region in 2008. The region had 4 223 591 inhabitants and a homogeneous distribution of specialized tertiary cancer care hospitals in 2008, with well-distributed neurosurgery, oncology, and radiotherapy departments and no socioeconomic or geographic variations in access to health care.
The cases were recorded on the basis of clinical findings and neuroimaging results leading to a suspicion of PBT. The following data were sent to the centralized registry database on an electronic clinical record form: diagnosis code by observing center, name of recording physician, date of first observation, initials of patient, date of birth, and sex. Capture-recapture analysis7 was performed using independent information sources to assess the completeness of each case record. In this multiple-record system, all individual specialists from the centers (n = 7) participating in the PERNO project communicated the contact details of their new patients to the registry staff to be incorporated into a single, comprehensive record for each case with input from multiple sources (neuroradiologist, neurosurgeon, neuropathologist, neurologist, radiotherapist, and oncologist) during the entire period of diagnosis and treatment. Virtually all PBT patients within the Emilia-Romagna region were thus registered in the above time interval.
The registry staff assigned each case an identification code and maintained communication with other research units to assure that all patients were included in the 5 parallel subprojects (ie, GBM clinical pathways, epilepsy outcome, translational research, neuroradiological CT perfusion). The completeness of case records in the registry was verified by checking International Classification of Disease discharge codes for PBTs from neurosurgical, neurological, radiotherapy, and oncological services. Furthermore, in order to check recruitment accuracy, information was exchanged with other tumor registries in the regional provinces.
As a PERNO subproject, a prospective study on the clinical pathways of patients with GBM (treatment evaluation and outcomes analysis included) was conducted between January 2009 and December 2010. For this prospective study, the main inclusion criteria were histologically proven GBM (according to the WHO classification), aged ≥18 years, KPS 30%–100%, no prior surgery for primary brain tumor, and residence within the Emilia-Romagna region.
When GBM patients entered the study, the following data were recorded: details on surgery, performance status, data and modalities of radiotherapy, and chemotherapy treatments. Completed data forms were sent to the central database. Electronic audit was used to detect outlier values, inconsistencies and omissions, and any queries were faxed to the study coordinators for resolution.
MGMT methylation status was assessed using methylation-specific PCR as described elsewhere.8 Approval for the project protocol was obtained from the ethical committee of each respective participating center. Before patient registration, fully informed consent in writing was obtained according to International Conference on Harmonisation/Good Clinical Practice, and national regulations.
Statistical Analysis
Survival data, processed using Kaplan-Meier methods to estimate survival functions, were analyzed by means of Cox models to assess the relevance of potential prognostic factors. Due to differences in the expected prognosis and treatments recommendations, a separate evaluation was made of patients aged ≤70 years who were treated with temozolomide concurrent with and adjuvant to radiotherapy. A comparison was made between the referring center and the other centers in terms of age (not dichotomized), KPS, and extent of surgery using Student t test for the first 2 variables and the chi-square test for the latter variables. The cutoff for significance level was P < .05. All analyses were made using SAS System software (version 9.2). At the time of analysis, 221 patients (82.8%) had died.
Results
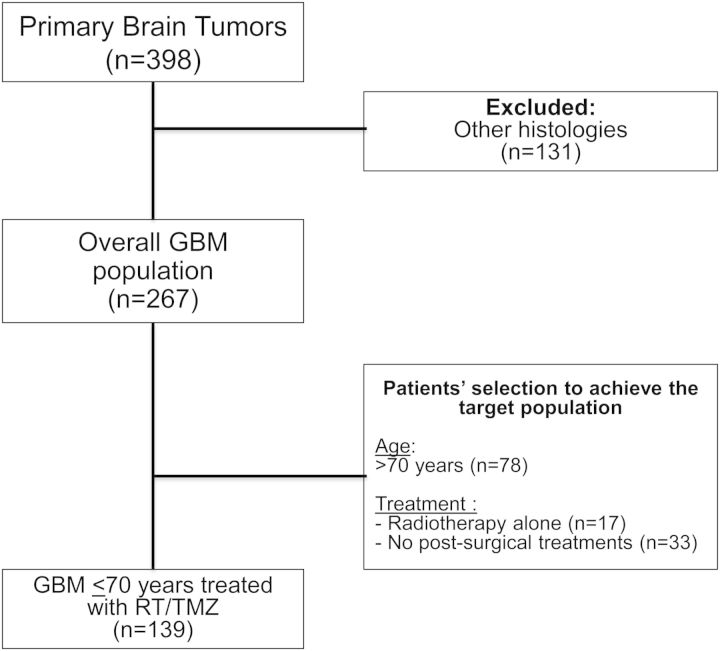
From January 1, 2009, to December 31, 2010, 398 PBT cases were included in the register, and 267 cases with histologically proven GBM were enrolled into the prospective subproject (Fig. 1) (Table 1). Median accrual by center was 28 (range, 6–63) cases. Ten patients migrated outside the Emilia-Romagna region for neurosurgery (3.7%), but all returned to the Emilia-Romagna region for postsurgical treatment.
Fig. 1.
Patient selection to achieve patients with glioblastoma aged ≤70 years and treated with radiotherapy/temozolomide.
Table 1.
Characteristics of patients in the entire glioblastoma population
| n = 267 | ||
|---|---|---|
| Age, y | Median age (range) | 64 (29–84) |
| Performance status | Median | 80% |
| Range | 30%–100% | |
| Extent of surgery, n (%) | Biopsy | 47 (17.6%) |
| Partial resection | 121 (45.3%) | |
| Complete resection | 98 (36.7%) | |
| Postsurgical treatment, n (%) | ≤70 years (n = 189) | |
| No postsurgical treatment | 33 (17.3%) | |
| Radiotherapy alone | 17 (8.9%) | |
| Temozolomide concomitant with and adjuvant to radiotherapy | 139 (72.8%) | |
| >70 years (n = 78) | ||
| No postsurgical treatment | 25 (32.9%) | |
| Radiotherapy alone | 25 (32.9%) | |
| Temozolomide concomitant with and adjuvant to radiotherapy | 28 (36.8%) |
Of the 257 (96.3%) patients who underwent resection within the Emilia-Romagna region, 241 (93.8%) had postsurgical scans with CT/MRI and 162 (67.2%) with contrast enhancement. The median time interval between surgery and histological diagnosis was 8 (range, 2–30) days.
Median OS in the entire population (n = 267) was 10.7 months (95% CI, 9.2–12.4; range, 0.1–40.6 mo).
Fifty-six of the 267 GBM patients (21.0%) did not receive postsurgical radiotherapy due to rapid clinical deterioration (n = 41; 15.4%), patient refusal (n = 8; 3.0%), postsurgical complications (n = 6; 2.2%), or tumor size (n = 1; 0.4%). The median OS in patients not receiving any postsurgical treatment was 3.0 months (95% CI, 2.3–4.5).
Two patients migrated to other Italian regions after surgery, and 209 patients (78.3%) received postsurgical treatment within the Emilia-Romagna region, with a median time interval between surgery and start of radiotherapy of 39 (range, 12.0–76.0) days and a median OS of 13.3 (95% CI, 11.9–15.6) months.
Forty-three of the 267 patients (20.6%, range per center, 0%–42.9%) were enrolled in clinical trials (31 patients [11.6%] were aged ≤70 years, and 12, [4.5%] were aged >70 years).
Assessment of MGMT status, undertaken in 181 of 209 (86.6%) patients, was achieved in 173 (82.8%) cases; 75 of 173 (43.3%) were methylated, and 98 of 173 (56.7%) were unmethylated.
Findings for univariate and multivariate analysis on the entire population are reported in Table 2.
Table 2.
Univariate and multivariate analyses of the entire GBM population
| Variable | Factor | n | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio |
P Value | Hazard Ratio |
P Value | |||||
| Estimate | 95% CI | Estimate | 95% CI | |||||
| Age, y | 29.4–83.9 | 267 | 1.040 | 1.025–1.056 | <.0001 | 1.024 | 1.009–1.040 | .0017 |
| KPS, % | 100 | 38 | 0.319 | 0.201–0.505 | <.0001 | 0.473 | 0.294–0.760 | .0020 |
| ≤90 | 229 | |||||||
| Extent of surgery | Complete | 98 | 0.300a | 0.205–0.439 | <.0001 | 0.383a | 0.259–0.566 | <.0001 |
| Partial | 121 | 0.565a | 0.397–0.804 | 0.604a | 0.424–0.860 | |||
| Biopsy | 47 | |||||||
| Postsurgical radiotherapy | Yes | 211 | 0.112 | 0.078–0.160 | <.0001 | 0.166 | 0.114–0.242 | <.0001 |
| No | 56 | |||||||
a“biopsy” is the reference class for the Hazard Ratio.
Abbreviation: CI, confidence interval.
Clinical Outcomes in Patients Aged ≤70 Years
One hundred eighty-nine patients were aged ≤70 years (Fig. 1) (Table 1). One hundred thirty-nine (Table 3) of 189 patients (72.8%) were treated according to the EORTC/NCIC study.8 Their median OS was 16.4 months (95% CI, 14.0–18.5), being 20 months (95% CI, 15.2–27.0) in patients (n = 56) with methylated MGMT and 15.4 months (95% CI, 11.9–17.6) in those with unmethylated MGMT status (n = 60); this difference in OS was statistically significant (P = .0052 and P = .0350 for univariate and multivariate analysis, respectively).
Table 3.
Characteristics of patients ≤70 years treated according to EORTC 26981-22981 /NCIC CE.3 trial
| n = 139 | ||
|---|---|---|
| Age | Median age | 59 years |
| Range | 29–70 years | |
| Gender | Male | 86 (61.9%) |
| Female | 53 (38.1%) | |
| Performance status, n (%) | Median (range) | 90 (50–100) |
| 90–100 | 78 (56.1%) | |
| 70–80 | 52 (37.4%) | |
| 50–60 | 9 (6.5%) | |
| Extent of surgery, n (%) | Biopsy | 22 (15.8%) |
| Partial resection | 49 (35.3%) | |
| Complete resection | 68 (48.9%) |
Survival rates at 1, 2, and 3 years were 66.2% (95% CI, 58.3%–74.0%), 32.5% (95% CI, 24.5%–40.5%), and 21.3% (95% CI, 12.8–29.8%), respectively. Survival rates at 1, 2, and 3 years in MGMT methylated patients were 71.4% (95% CI, 59.6%–83.3%), 45.8% (95% CI, 32.6%–59.0%), and 34.0% (95% CI, 19.8%–48.1%), respectively, while survival rates at 1, 2, and 3 years for MGMT unmethylated patients were 63.3% (95% CI, 51.1%–75.5%), 19.8% (95% CI, 8.8%–30.8%), and zero, respectively.
Findings for univariate and multivariate analysis are reported in Table 4.
Table 4.
Survival and uni- and multivariate analysis for patients ≤70 years treated according to EORTC26981–22981 /NCIC CE.3 protocol
| Variable | Factor | n | Survival |
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio |
P-value | Hazard ratio |
P-value | |||||||
| OS (mo) | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |||||
| Age | 29–70 y | 139 | 16.4 | 14.0–18.5 | 1.033 | 1.007–1.061 | 0.0134 | 1.015 | 0.983–1.047 | 0.3675 |
| KPS | 100 | 31 | 26.6 | 19.0–30.1 | 0.451 | 0.264–0.771 | 0.0036 | 0.458 | 0.248–0.847 | 0.0127 |
| ≤90 | 108 | 14.3 | 12.1–17.0 | |||||||
| MGMT status | Methylated | 56 | 20.0 | 15.2–27.0 | 0.530 | 0.339–0.827 | 0.0052 | 0.612 | 0.388–0.966 | 0.0350 |
| Unmethylated | 60 | 15.4 | 11.9–17.6 | |||||||
| Extent of surgery | Complete | 68 | 17.9 | 15.2–22.7 | 0.323a | 0.190–0.548 | 0.0001 | 0.463a | 0.240–0.892 | 0.0698 |
| Partial | 49 | 18.1 | 12.9–24.9 | 0.330a | 0.188–0.579 | 0.539a | 0.274–1.061 | |||
| Biopsy | 22 | 10.6 | 5.7–12.9 | |||||||
| Enrolled in clinical trial | Yes | 30 | 24.9 | 16.3–NA | 0.509 | 0.298–0.869 | 0.0134 | 0.756 | 0.412–1.386 | 0.3656 |
| No | 109 | 15.6 | 12.3–17.8 | |||||||
| Treated in high-volume center | Yes | 37 | 24.1 | 15.4–NA | 0.533 | 0.328–0.866 | 0.0110 | 0.569 | 0.328–0.986 | 0.0446 |
| No | 102 | 15.9 | 12.3–17.8 | |||||||
a“biopsy” is the reference class for the Hazard Ratio.
Abbreviations: CI, confidence interval; mo, months: NA, not achieved; y, year.
In the group of patients aged ≤70 years and treated according to the EORTC/NCIC study, survival rates were higher in the center that reported a higher number of patients with postsurgical treatment. With univariate analysis, it was found that having been treated in the high-volume referral center correlated with a median survival that was significantly longer than survivals obtained in other centers (24.1 months vs 15.9 months; HR, 0.533; 95% CI, 0.328–0.866; P = .0110). An exploratory analysis was therefore made to evaluate the accrual rate as a continuous variable; it was found to have no significant impact by univariate analysis on OS (P = .0673). Multivariate analysis was therefore performed to evaluate the accrual rate as a dichotomous variable (referral to high-volume center vs other centers): postsurgical treatment was correlated significantly with OS in the high-volume center (HR = 0.569; 95% CI, 0.328–0.986; P = .0446; see Table 4).
No significant differences were found between the referring center and other centers for relevant clinical factors such as age (P = .0681) and KPS (P = .8899), whereas differences were found between the centers for extent of surgery (P = .0164). Complete resections were performed in 37.8% of cases in the referral center and 52.9% of cases in other centers (P = .3462), partial resections were performed in 54.1% of cases in the referral center and 28.4% in other centers (P = .0156), and biopsies were performed in 8.1% of cases in the referral center and 18.6% of cases in other centers (P = .3996).
Discussion
The PERNO registry, the first in Italy to prospectively record the incidence of PBTs, was created in order to include all PBT cases within the Emilia-Romagna region. The present paper reports the results of a prospective subproject analyzing the newly diagnosed GBM population included in this registry and their clinical outcome.
In the Emilia-Romagna region, radiotherapy alone or combined with chemotherapy was given to 78.3% of the registered participants, the rate being 82.7% in patients aged ≤70 years and 67.1% in those >aged 70 years. The standard of care for newly diagnosed GBM patients aged ≤70 years has been established by the EORTC/NCIC3 trial, and 72.8% of cases considered in the present study underwent this treatment protocol (Table 1).
Our survival data are comparable with those obtained in the EORTC/NCIC trial and the more recent RTOG 0525 trial,9 as well data obtained in retrospective registries in North America (United States and Canada)10,11 and South America (Columbia),12 and seem better than data from Austria13 and Switzerland.14
In particular, these results are comparable with those reported in the retrospective Surveillance, Epidemiology and End Results (SEER) program,6 in which 65% of patients received the combined treatment according to EORTC/NCIC, followed by an OS of 15 months; 23% of patients who received no postsurgical treatment had an OS of 2 months.
Population-based data from Switzerland (Canton of Zurich)14 and the Austrian Brain Tumor Registry13 showed a dismal prognosis in the entire populations of GBM cases, with an OS in the general populations of 5–6 months The Swiss registry was compiled between 1980 and 1994, before the EORTC/NCIC trial results were published (2005), and the Austrian data cover the years 2005 to 2006. In the latter preliminary spatial epidemiological analysis, the authors suggested that potential regional disparities across the Austrian health care regions may have affected differences in survival outcomes.13
A low socioeconomic status and its impact on patient care was also be cited as a cause of worse outcome in United States veteran cases in the national SEER cohort in which the OS for the GBM veteran patients was 6.5 months compared with 9.0 months in the SEER cohort.15
All of the above registries, despite their larger sample sizes, may be biased due to their retrospective nature: data on survival and treatment were frequently collected in a post-hoc manner.
A major advantage of this prospective population study was the solid epidemiological method (capture-recapture method) that allowed inclusion of virtually all GBM cases diagnosed within the Emilia-Romagna region. Moreover, the prospective record of patients and their treatment data allowed more comprehensive collection of demographic, treatment, and follow-up information.
The findings reported in the present study also confirm the prognostic role of MGMT methylation status. Moreover, the 2-year survival rates obtained are comparable with those reported in the EORTC/NCIC and RTOG 0525 studies, both for patients with MGMT methylated (46% in the PERNO, 49% in the EORTC/NCIC,2,3 and 47% in the RTOG 0525 trials) and for those with MGMT unmethylated (20% in the PERNO and 15% in EORTC/NCIC trials and 20% in the RTOG 0525 trial) status. Although comparisons between clinical trials and population studies are always challenging, the similarities suggest that the results obtained in the EORTC/NCIC phase III trial are transferable to the general population of GBM patients aged ≤70 years.
Our data confirms the role of known prognostic factors (performance status and MGMT status), while the extent of surgery appears to have a borderline effect (by multivariate analysis; P = .698) in patients aged ≤70 years treated according to the EORTC/NCIC trial protocol. However, the extent of surgery was evaluated by taking into account postsurgical CT/MRI with or without contrast enhancement performed within 48 hours after surgery in 60% of patients and on the basis of the neurosurgical report in about 40% of patients.
For the first time in the field of neuro-oncology, we have investigated the impact of center volume and expertise in postsurgical treatment as a variable that may influence the outcome of GBM patients, as it does in other cancer types (eg, lung,16 rectal,17 and ovarian cancer18), and we found that center expertise has an impact (P = .0446) on the survival of patients.
The HR of 0.569, found by multivariate analysis, suggests a potential reduction of mortality in the range of 40%. From a clinical viewpoint, the median OS for cases treated in a center with high volume and expertise in postsurgical treatment showed a difference (∼ 8 months); this should be confirmed in a larger prospective study. However, the OS of ∼ 16 months found in cases treated in lower volume centers is in line with data reported in the EORTC/NCIC trial.2,3
Unlike settings for other cancer types, such as lung carcinoma, where hospitals with a high volume of surgical resections are followed by increased survival16 or, in the rectal and esophageal cancer setting where surgeon volume17,19 plays a significant role in prolonging survival, the results in our setting were not clearly attributable to the surgeon. By multivariate analysis, the extent of surgical resection had only a borderline effect on OS. In cases with ovarian carcinoma, findings at multivariate analysis demonstrated that OS correlated with adherence to NCCN guidelines, even if adherence was greater in high-volume rather than low-volume hospitals and in physicians who treated more patients. The same claim cannot be made for our GBM population, which was homogeneous (aged ≤70 years) and had standardized treatment with temozolomide concomitant and adjuvant to radiotherapy. Our findings may have depended on several factors such as expertise in neuroradiological interpretation, management of adverse events, and use of third-line treatments as well as improvements in administering the best supportive care. Moreover, the volume analysis was made following protocol, case accrual being achieved within the Emilia Romagna region, which is only partially representative of the size of the practice in the high-volume center.
Given the number of variables tested, there is concern regarding some potential prognostic associations that would be difficult to estimate within a relatively small patient population, and improvements in immediate postsurgical disease assessment are needed. Therefore, the exploratory data reported in the present prospective population-based registry study should be confirmed by a larger prospective study.
Funding
This study was funded by the Italian Ministry of Health with a national “Ricerca Finalizzata” grant.
Acknowledgments
The authors are indebted to Dr. Rossana De Palma (Regional Agency for Health and Social Care of Emilia-Romagna Region) and Dr. Giovanni Apolone (Scientific Director of (IRCCS-Arcispedale Santa Maria Nuova, Reggio Emilia, Italy) for their constructive criticism and for the continuous support given during and after the study.
Conflict of interest statement. None declared.
Contributor Information
Collaborators: A. Baruzzi, F. Albani, F. Calbucci, R. D'Alessandro, R. Michelucci, A. Brandes, V. Eusebi, S. Ceruti, E. Fainardi, R. Tamarozzi, E. Emiliani, M. Cavallo, E. Franceschi, A. Tosoni, M. Cavallo, F. Fiorica, A. Valentini, R. Depenni, C. Mucciarini, G. Crisi, E. Sasso, C. Biasini, L. Cavanna, D. Guidetti, N. Marcello, A. Pisanello, A.M. Cremonini, G. Guiducci, S. de Pasqua, S. Testoni, R. Agati, G. Ambrosetto, A. Bacci, E. Baldin, A. Baldrati, E. Barbieri, S. Bartolini, E. Bellavista, F. Bisulli, E. Bonora, F. Bunkheila, V. Carelli, M. Crisci, P. Dall'Occa, D. de Biase, S. Ferro, C. Franceschi, G. Frezza, V. Grasso, M. Leonardi, G. Marucci, V. Mazzocchi, L. Morandi, B. Mostacci, G. Palandri, E. Pasini, M. Pastore Trossello, A. Pession, M. Ragazzi, P. Riguzzi, R. Rinaldi, S. Rizzi, G. Romeo, F. Spagnolli, P. Tinuper, C. Trocino, S. Cerasoli, M. Dall'Agata, M. Faedi, M. Frattarelli, G. Gentili, A. Giovannini, P. Iorio, U. Pasquini, G. Galletti, C. Guidi, W. Neri, A. Patuelli, S. Strumia, M. Casmiro, A. Gamboni, F. Rasi, G. Cruciani, P. Cenni, C. Dazzi, AR. Guidi, F. Zumaglini, A. Amadori, G. Pasini, M. Pasquinelli, E. Pasquini, A. Polselli, A. Ravasio, B. Viti, M. Sintini, A. Ariatti, F. Bertolini, G. Bigliardi, P. Carpeggiani, F. Cavalleri, S. Meletti, P. Nichelli, E. Pettorelli, G. Pinna, E. Zunarelli, F. Artioli, I. Bernardini, M. Costa, G. Greco, R. Guerzoni, C. Stucchi, C. Iaccarino, R. Rizzi, G. Zuccoli, P. Api, F. Cartei, E. Fallica, E. Granieri, F. Latini, G. Lelli, C. Monetti, V. Ramponi, A. Saletti, R. Schivalocchi, S. Seraceni, M.R. Tola, B. Urbini, C. Giorgi, E. Montanari, D. Cerasti, P. Crafa, I. Dascola, I. Florindo, S. Mazza, F. Servadei, EM. Silini, P. Torelli, P. Immovilli, N. Morelli, and C. Vanzo
Appendix: The PERNO Study group
Steering committee
Baruzzi A. (Chair), Albani F., Calbucci F., D'Alessandro R., Michelucci R. (IRCCS Institute of Neurological Sciences, Bologna, Italy), Brandes A. (Department of Medical Oncology, Bellaria-Maggiore Hospitals, Bologna, Italy), Eusebi V. (Department of Hematology and Oncological Sciences “L. & A. Seragnoli,” Section of Anatomic Pathology at Bellaria Hospital, Bologna, Italy), Ceruti S., Fainardi E., Tamarozzi R. (Neuroradiology Unit, Department of Neurosciences and Rehabilitation, S. Anna Hospital, Ferrara, Italy), Emiliani E. (Istituto Oncologico Romagnolo, Department of Medical Oncology, Santa Maria delle Croci Hospital, Ravenna, Italy), Cavallo M. (Division of Neurosurgery, Department of Neurosciences and Rehabilitation, S. Anna Hospital, Ferrara, Italy).
Executive committee
Franceschi E., Tosoni A. (Department of Medical Oncology, Bellaria-Maggiore Hospitals, Bologna, Italy), Cavallo M. (Division of Neurosurgery, Department of Neurosciences and Rehabilitation, S. Anna Hospital, Ferrara, Italy), Fiorica F. (Department of Radiation Oncology, S. Anna Hospital, Ferrara, Italy), Valentini A. (Division of Neurosurgery, Nuovo Ospedale Civile S. Agostino-Estense, Baggiovara, Modena, Italy), Depenni R. (Department of Oncology, Policlinico di Modena, Italy), Mucciarini C. (Department of Oncology, Ramazzini Hospital, Carpi, Modena, Italy), Crisi G. (Department of Neuroradiology, Maggiore Hospital, Parma, Italy), Sasso E. (Department of Neurological Sciences, Maggiore Hospital, Parma, Italy), Biasini C., Cavanna L. (Department of Oncology and Hematology, Guglielmo da Saliceto Hospital, Piacenza, Italy), Guidetti D. (Department of Neurology, Guglielmo da Saliceto Hospital, Piacenza, Italy), Marcello N., Pisanello A. (Department of Neurology, Istituto in tecnologie avanzate e modelli assistenziali in oncologia, IRCCS, S. Maria Nuova Hospital, Reggio Emilia, Italy), Cremonini A.M., Guiducci G. (Division of Neurosurgery, M. Bufalini Hospital, Cesena, Italy). Registry Coordination Office: de Pasqua S., Testoni S. (IRCCS Institute of Neurological Sciences, Bologna, Italy).
Participants
Agati R., Ambrosetto G., Bacci A., Baldin E., Baldrati A., Barbieri E., Bartolini S., Bellavista E., Bisulli F., Bonora E., Bunkheila F., Carelli V., Crisci M., Dall'Occa P., de Biase D., Ferro S., Franceschi C., Frezza G., GrassoV., Leonardi M., Marucci G., Mazzocchi V., Morandi L., Mostacci B., Palandri G., Pasini E., Pastore Trossello M., Pession A., Ragazzi M., Riguzzi P., Rinaldi R., Rizzi S., Romeo G., Spagnolli F., Tinuper P., Trocino C. (Bologna), Cerasoli S., Dall'Agata M., Faedi M., Frattarelli M., Gentili G., Giovannini A., Iorio P., Pasquini U., Galletti G., Guidi C., Neri W., Patuelli A., Strumia S. (Forlì-Cesena), Casmiro M., Gamboni.A., Rasi F. (Faenza, RA), Cruciani G. (Lugo, RA), Cenni P., Dazzi C., Guidi AR., Zumaglini F. (Ravenna), Amadori A., Pasini G., Pasquinelli M., Pasquini E., Polselli A., Ravasio A., Viti B. (Rimini), Sintini M. (Cattolica, RN), Ariatti A., Bertolini F., Bigliardi G., Carpeggiani P., Cavalleri F., Meletti S., Nichelli P., Pettorelli E., Pinna G., Zunarelli E. (Modena), Artioli F., Bernardini I., Costa M., Greco G., Guerzoni R., Stucchi C. (Carpi, MO), Iaccarino C., Rizzi R., Zuccoli G. (Reggio Emilia), Api P., Cartei F., Fallica E., Granieri E., Latini F., Lelli G., Monetti C., Ramponi V., Saletti A., Schivalocchi R., Seraceni S., Tola M.R., Urbini B. (Ferrara), Giorgi C. Montanari E. (Fidenza, PR), Cerasti D., Crafa P., Dascola I., Florindo I., Mazza S., Servadei F., Silini EM., Torelli P. (Parma), Immovilli P., Morelli N., Vanzo C. (Piacenza).
The full affiliations and postal addresses of PERNO participants are available on the study website: www.perno.it.
References
- 1.Crocetti E, Trama A, Stiller C, et al. Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer. 2012;48(10):1532–1542. doi: 10.1016/j.ejca.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Graus F, Bruna J, Pardo J, et al. Patterns of care and outcome for patients with glioblastoma diagnosed during 2008–2010 in Spain. Neuro Oncol. 2013;15(6):797–805. doi: 10.1093/neuonc/not013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DR, O'Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2011;107(2):359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 6.Yabroff KR, Harlan L, Zeruto C, et al. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro Oncol. 2012;14(3):351–359. doi: 10.1093/neuonc/nor218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hook EB, Regal RR. The value of capture-recapture methods even for apparent exhaustive surveys. The need for adjustment for source of ascertainment intersection in attempted complete prevalence studies. Am J Epidemiol. 1992;135(9):1060–1067. doi: 10.1093/oxfordjournals.aje.a116400. [DOI] [PubMed] [Google Scholar]
- 8.Brandes AA, Tosoni A, Cavallo G, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: a prospective GICNO study. J Clin Oncol. 2006;24(29):4746–4753. doi: 10.1200/JCO.2006.06.3891. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lwin Z, MacFadden D, Al-Zahrani A, et al. Glioblastoma management in the temozolomide era: have we improved outcome? J Neurooncol. 2013;115(2):303–310. doi: 10.1007/s11060-013-1230-3. [DOI] [PubMed] [Google Scholar]
- 11.Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107(1):207–212. doi: 10.1007/s11060-011-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz LD, Cardona AF, Fadul CE, et al. Clinical outcome of concomitant chemoradiotherapy followed by adjuvant temozolomide (TMZ) therapy for high-grade gliomas (HGG) in Colombia (RedLANO registry) ASCO Meeting Abstracts. 2011;29(15_suppl):2092. [Google Scholar]
- 13.Woehrer A. Brain tumor epidemiology in Austria and the Austrian Brain Tumor Registry. Clin Neuropathol. 2013;32(4):269–285. doi: 10.5414/NP300600. [DOI] [PubMed] [Google Scholar]
- 14.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 15.Arrigo RT, Boakye M, Skirboll SL. Patterns of care and survival for glioblastoma patients in the Veterans population. J Neurooncol. 2011;106(3):627–635. doi: 10.1007/s11060-011-0702-6. [DOI] [PubMed] [Google Scholar]
- 16.Luchtenborg M, Riaz SP, Coupland VH, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. J Clin Oncol. 2013;31(25):3141–3146. doi: 10.1200/JCO.2013.49.0219. [DOI] [PubMed] [Google Scholar]
- 17.Martling A, Cedermark B, Johansson H, et al. The surgeon as a prognostic factor after the introduction of total mesorectal excision in the treatment of rectal cancer. Br J Surg. 2002;89(8):1008–1013. doi: 10.1046/j.1365-2168.2002.02151.x. [DOI] [PubMed] [Google Scholar]
- 18.Bristow RE, Chang J, Ziogas A, et al. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121(6):1226–1234. doi: 10.1097/AOG.0b013e3182922a17. [DOI] [PubMed] [Google Scholar]
- 19.Derogar M, Sadr-Azodi O, Johar A, et al. Hospital and Surgeon Volume in Relation to Survival After Esophageal Cancer Surgery in a Population-Based Study. J Clin Oncol. 2013;31(5):551–557. doi: 10.1200/JCO.2012.46.1517. [DOI] [PubMed] [Google Scholar]