Abstract
Flowering time and seed size are traits related to domestication. However, identification of domestication-related loci/genes of controlling the traits in soybean is rarely reported. In this study, we identified a total of 48 domestication-related loci based on RAD-seq genotyping of a natural population comprising 286 accessions. Among these, four on chromosome 12 and additional two on chromosomes 11 and 15 were associated with flowering time, and four on chromosomes 11 and 16 were associated with seed size. Of the five genes associated with flowering time and the three genes associated with seed size, three genes Glyma11g18720, Glyma11g15480 and Glyma15g35080 were homologous to Arabidopsis genes, additional five genes were found for the first time to be associated with these two traits. Glyma11g18720 and Glyma05g28130 were co-expressed with five genes homologous to flowering time genes in Arabidopsis, and Glyma11g15480 was co-expressed with 24 genes homologous to seed development genes in Arabidopsis. This study indicates that integration of population divergence analysis, genome-wide association study and expression analysis is an efficient approach to identify candidate domestication-related genes.
The transitions from vegetative to reproductive growth (days to flowering) and from generation to generation through seeds are important stages of the plant life cycle. Flowering time reflects the adaptation of a plant to its environment, and the time required to mature varies widely among cultivars1. Seeds are important in the reproduction and spread of flowering plants, and seed size partly reflects the efficiency of plant production2. Both flowering time and seed size are important traits involved in domestication, a process accompanied by reduction in genetic diversity and loss of important traits preserved in wild relatives.
Morphological, physiological and molecular markers have been widely used to measure the genetic diversity of wild and cultivated plants in rice3, soybean4, and wheat5, to deduce the geographic regions of domestication, and to screen for breeding material. Linkage and association studies have identified quantitative trait loci (QTL) associated with domestication-related (DR) traits in various plants6,7and animals8, such as the lateral branching locus teosinte branched1 (tb1) in maize9, the seed shattering locus qSH1 in rice10, and the milk-production locus DGAT1 in dairy cattle11. Numerous DR genes have also been identified, including GW8 in rice12, Q in wheat13, vrs1 in barley14, and RYR1 in pig15. As whole genome sequences from almost all major plants have become available, substantial progress has been achieved, including 1) Hundreds of DR genes have been identified by comparative analysis of genomes in plants16 and animals17; 2) Candidate genes resulting from selection were detected by comparative analysis and functional tests7, for example, the genes LOC_Os05g20290 and LOC_Os08g40710 in rice18 and the genes GRMZM2G448355 and abph1 (GRMZM2G035688) in maize19; and 3) The molecular mechanisms underlying DR traits have been reported20,21. In addition, the ancient DNA and genomes of fossils have contributed to the histories of domestication and evolution in horses22, cattle23, and Arabidopsis thaliana24. However, little is known about the loci/genes underlying DR traits in soybean.
The soybean plant originated in China and was first domesticated by Chinese farmers between 6,000 and 9,000 years ago25. The modern domesticated soybean (Glycine max) is an economically important crop because it has high protein and oil contents, can fix nitrogen through microorganisms in the soil26,27, and is a model plant for legume research. The cultivated soybean (G. max) differs from the wild soybean (G. soja) in many traits, for example, the cultivated soybean forms flowers earlier and has larger seeds than the wild soybean28. Previous research has led to the discovery of many QTL correlated to DR traits such as seed size29,30,31,32,33 and other traits (http://www.SoyBase.org). Some genes controlling DR traits in soybean have also been discovered, e.g., E1–E434,35,36,37, FLC, VRNA, ELF8, PHYE, PHYA16, CDF3, VRN1, SVP, AP3 and PIF3 for flowering time38, and Dt1 for determinate growth habit39. Although the genes and their molecular mechanisms for some DR traits in soybean have been investigated38,39, the genes/loci underlying many other DR traits remain to be addressed.
In this study, restriction-site-associated DNA (RAD) tags from 14 wild, 153 landrace, and 119 bred accessions were sequenced, and the sequence variants were analyzed to detect DR loci by testing the independence between the SNPs and soybean evolutionary classes (wild, landrace, and bred) and comparing the genetic diversity between the wild and cultivated soybeans. Genome-wide association of the detected DR loci with DR traits (flowering time and seed size) were also studied. Candidate genes predicted to be involved in these two traits were pinpointed using comparative genomics technology. Co-expression analysis for individual candidate genes was also conducted.
Results and Discussion
Phenotypic characteristics of flowering time and seed size
Flowering time was measured by the days from the date of emergency to the date of first and full flowering in this study. The average plus standard error were 51.83 ± 3.73, 46.22 ± 0.83 and 35.60 ± 0.64 (days) for first flower and 55.95 ± 4.00, 49.96 ± 0.86 and 38.57 ± 0.65 (days) for full flower in wild, landrace and bred soybeans in 2010–2012, respectively. G. soja flowered later than G. max.
Seed size was characterized by seed length (SL), seed width (SW) and 100-seed weight (100 SW) in this study. The averages plus standard errors in wild, landrace and bred soybeans in 2008–2012 were 4.76 ± 0.18, 7.61 ± 0.08 and 7.85 ± 0.06 (mm) for SL, 3.56 ± 0.17, 6.54 ± 0.07 and 6.63 ± 0.05 (mm) for SW, and 2.99 ± 0.45, 15.43 ± 0.48 and 16.85 ± 0.39 (g) for 100 SW, respectively, indicating much smaller seeds in G. soja than in G. max.
Distinct difference of these traits between G. soja and G. max suggested that these traits are domestication-related. Flowering time and seed size were also considered as DR traits in other reports25,28,40,41,42, although domestication traits in soybean include more than these two traits, such as indeterminate habit and pod dehiscence.
Detection and distribution of domestication-associated loci
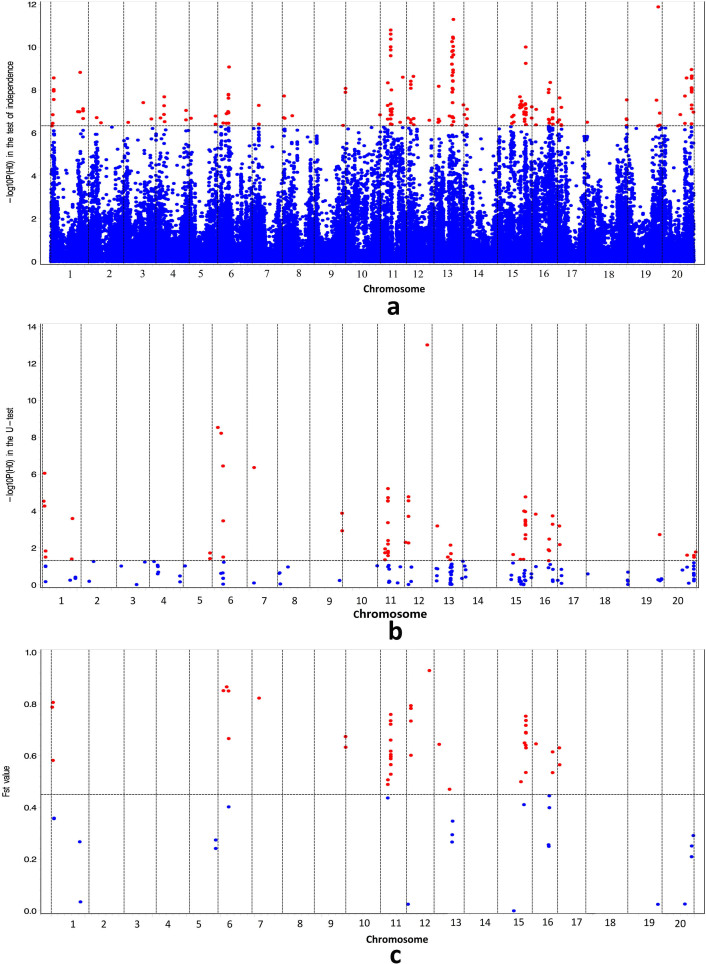
Based on the sequence obtained from 286 accessions through the RAD-seq genotyping approach, a total of 106,013 SNPs were identified. χ2 tests of independence between the SNPs and evolutionary classes (wild, landrace, and bred) of soybean showed that 198 SNPs were significant at P-value ≤ 4.72 × 10−7 (Fig. 1a). A U-test determined that the allelic frequency for 72 of the 198 SNPs were significantly different between the wild and cultivated classes (Fig. 1b). The fixation index (FST) analysis demonstrated that 48 of the 72 SNPs had an FST value greater than 0.45 (Fig. 1c; Table S1).
Figure 1.
Detection of domestication-related loci in the soybean genome using χ2 test of independence (a), U-test (b) and genetic diversity analysis (c). Significances of all the SNPs in the above three analyses were marked by red (significant) and blue (not significant) dots. The genomic positions of SNPs on chromosomes 1 to 20 were 7166 ~ 55874947, 2395 ~ 51643854, 45230 ~ 47773436, 15992 ~ 49228296, 9016 ~ 41933701, 1142 ~ 50641309, 37534 ~ 44659030, 3583 ~ 46944564, 25976 ~ 46841908, 24539 ~ 50962464, 30293 ~ 39163227, 13701 ~ 40093893, 10047 ~ 44402574, 13165 ~ 49710404, 8319 ~ 50879005, 14132 ~ 37370388, 47058 ~ 41905331, 426 ~ 62303776, 9282 ~ 50584403 and 18252 ~ 46703751 (bp), respectively.
Of the 48 DR loci, 3, 4, 1, 2, 14, 5, 2, 11, 3 and 3 loci were located on chromosomes 1, 6, 7, 9, 11, 12, 13, 15, 16 and 17, respectively (Fig. 1c). However, 52% of the 48 loci were located on chromosomes 11 or 15, and these two chromosomes harbour more DR loci than other chromosomes. Similar results were also observed by Kim et al.43, Chung et al.44 and Li et al.45. On the two chromosomes, Kim et al.43 identified four DR genes (Glyma15g14980, Glyma15g23400, Glyma11g05720 and Glyma15g04930) that were associated with flowering time; Chung et al.44 detected 204 soybean candidate DR genes; and Li et al.45 found three DR genes (Glyma11g03340, Glyma11g03330 and Glyma11g03320) near the reported flower colour QTL.
A total of 12 DR loci were identified in 11 genes with 4 loci in the coding regions of the genes. Of the 11 genes whose functions were previously annotated in the Pfam and NCBI non-redundant databases, three and two genes were located on chromosomes 11 and 15, respectively. The common allele for each of the 12 loci was different in the bred and wild soybean, and common alleles for each of the 3 DR loci on chromosome 11 were the same in both the landrace and wild soybeans.
Genome-wide association studies (GWAS) for flowering time and seed size
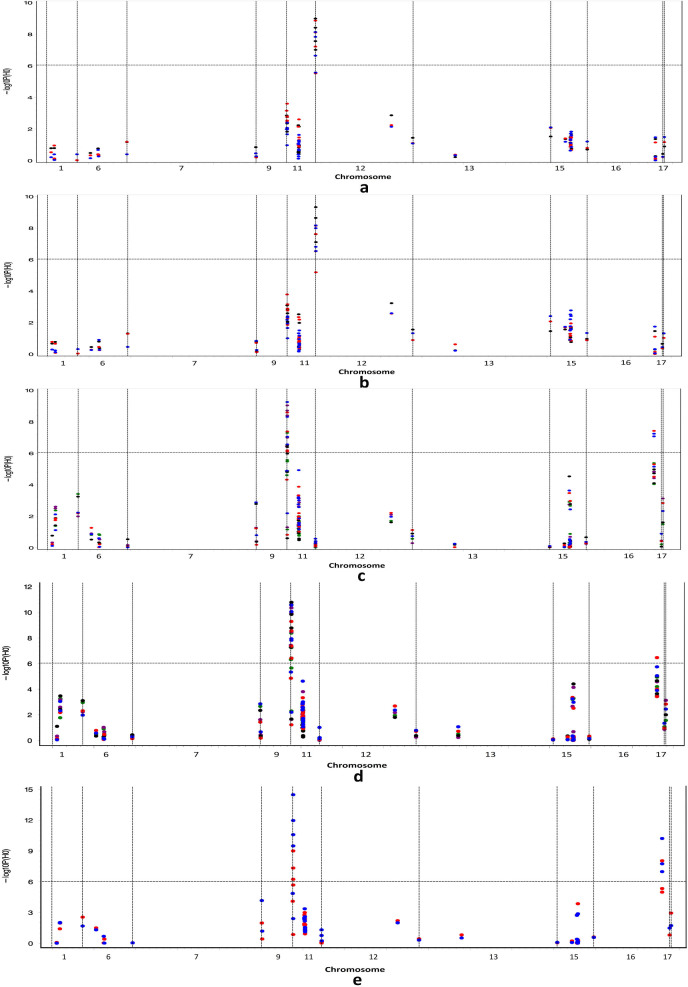
GWAS for flowering time (first and full) and seed size (SL, SW and 100 SW) was performed based on a dataset containing 286 accessions genotyped with 55,052 SNPs (Fig. S1). Because our purpose was to investigate the association of the DR loci with the two DR traits, we only analyzed the association of the DR loci listed in Fig. 2 and Table 1.
Figure 2.
Genome-wide association of first (a) and full (b) flowering times, seed length (c), seed width (d) and 100-seed weight (e) in soybean during 2008–2012 with domestication-related SNPs on chromosomes 1, 6, 7, 9, 11, 12, 13, 15, 16 and 17. Dots with green, purple, black, red, and blue colors denote 2008, 2009, 2010, 2011, and 2012, respectively.
Table 1. Genome-wide association of flowering time (FT) and seed size (SS) traits with domestication-related SNPs (bold) in soybean.
| SNP | Domestication analysis | Genome-wide association study | Homologous gene in Arabidopsis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr. | Position (bp) | χ2 (−log10P) | U (−log10P) | FST | Trait | Allele | Frequency | Effect | −log10P | r2 (%) | Candidate gene | Gene | Reference |
| 11 | 10957940 | 30.60(6.64) | −2.36(1.74) | 0.4890 | SS | T | 0.45 | −9.12 ~ −5.84 | 6.32 ~ 7.43 | 6.24 ~ 11.80 | Glyma11g15300 Glyma11g15310 | ||
| 11 | 11100801 | SS | G | 0.58 | −2.10 ~ −0.58 | 6.52 ~ 14.49 | 8.14 ~ 16.26 | Glyma11g15480 | NOT2A (AT1G07705) | 49 | |||
| 11 | 11111516 | 38.40(8.34) | −2.53(1.95) | 0.5069 | SS | C | 0.38 | 0.90 ~ 3.90 | 0.60 ~ 2.40 | 0.91 ~ 3.20 | Glyma11g15480 | NOT2A (AT1G07705) | 49 |
| 11 | 11120981 | SS | C | 0.53 | −23.59 ~ −18.40 | 6.19 ~ 10.59 | 6.50 ~ 14.42 | Glyma11g15480 | NOT2A (AT1G07705) | 49 | |||
| 11 | 11121256 | SS | G | 0.51 | 4.40 ~ 18.40 | 6.11 ~ 9.91 | 5.96 ~ 16.85 | Glyma11g15480 | NOT2A (AT1G07705) | 49 | |||
| 11 | 11121307 | SS | T | 0.56 | −0.07 ~ 4.87 | 6.40 ~ 11.97 | 6.56 ~ 16.26 | Glyma11g15480 | NOT2A (AT1G07705) | 49 | |||
| 11 | 15362036 | 45.45(9.87) | −2.43(1.82) | 0.5656 | FT | A | 0.42 | −10.11 ~ −4.87 | 1.51 ~ 2.61 | 1.32 ~ 2.17 | Glyma11g18720 | OTLD1 (AT2G27350) | 46,47,48 |
| 12 | 5895633 | 37.23(8.08) | −3.73(3.71) | 0.7344 | FT | C | 0.53 | −12.96 ~ −5.21 | 7.63 ~ 8.65 | 6.16 ~ 7.00 | Glyma12g08150 Glyma12g08160 | ||
| 12 | 5895655 | 38.04(8.26) | −4.30(4.78) | 0.7830 | FT | A | 0.52 | −13.05 ~ −5.20 | 6.53 ~ 7.61 | 5.32 ~ 6.34 | Glyma12g08150 Glyma12g08160 | ||
| 12 | 5895785 | 38.74(8.41) | −4.19(4.55) | 0.7938 | FT | C | 0.53 | −4.54 ~ −1.30 | 8.14 ~ 9.33 | 6.44 ~ 7.32 | Glyma12g08150 Glyma12g08160 | ||
| 12 | 5957099 | 30.47(6.62) | −2.78(2.27) | 0.6017 | FT | A | 0.55 | −12.93 ~ −9.17 | 6.54 ~ 7.11 | 5.61 ~ 5.92 | Glyma12g08210 Glyma12g08230 | ||
| 15 | 39607166 | 32.10(6.97) | −3.89(3.40) | 0.6496 | FT | A | 0.48 | −14.97 ~ −10.93 | 1.11 ~ 1.74 | 1.06 ~ 1.54 | Glyma15g35080 | AREB3 (AT3G56850) | 38 |
| 16 | 30207175 | 30.67(6.66) | −3.47(3.28) | 0.5348 | SS | G | 0.35 | −7.48 ~ −3.97 | 6.99 | 8.49 | Glyma16g26030 Glyma16g26050 | ||
| 16 | 30207191 | SS | T | 0.44 | −10.29 ~ −8.41 | 6.47 ~ 8.05 | 6.02 ~ 9.97 | Glyma16g26030 Glyma16g26050 | |||||
| 16 | 30210113 | 30.90(6.71) | −3.74(3.74) | 0.6148 | SS | C | 0.37 | −2.13 ~ 0.84 | 7.25 ~ 10.22 | 7.24 ~ 12.01 | Glyma16g26030 Glyma16g26050 | ||
Four QTL were associated with flowering time (P-values of 4.68E-10 to 2.95E-7), and each QTL explained 5.32–7.32% of the total phenotypic variance. These QTL were positioned at 5895633, 5895655, 5895785, and 5957099 (bp) on chromosome 12 (Fig. 2a, b). The first three loci were associated with the first and full flowering times in 2010 to 2012, while the last locus was associated with the first flowering time in 2010 and 2012 and with the full flowering time in 2012. In addition, two loci at genomic positions 15362036 bp (P-values of 0.0064 to 0.0234) on chromosome 11 and 39607166 bp (P-values of 0.0183 to 0.0434) on chromosome 15 were associated with the flowering times at a 0.05 probability level in 2010 to 2012, except for the fact that the later locus was almost associated with the first flower at a 0.05 probability level in 2012 (P-value = 0.0640) (Fig. 2a, b).
A total of 8 QTL, each explaining 0.91–16.85% of the total phenotypic variance of seed size, were identified (P-values of 3.24E-15 to 7.76E-7). These QTL were mapped to genomic positions 10957940, 11100801, 11120981, 11121256, and 11121307 bp on chromosome 11 and 30207191, 30207175, and 30210113 bp on chromosome 16 (Fig. 2c–e). Among the five SNP loci on chromosome 11, the last four loci, which were close to the DR locus at 11111516 bp (~10 kb), were associated with the three seed size traits. The DR locus at 10957940 bp was associated with SW in 2008 to 2010 and with SL in 2009. Among the three loci on chromosome 16, one locus at genomic position 30207191 bp, which was close to the DR locus 30207175 bp, was correlated with SL and 100 SW in 2011 and 2012 and with SW in 2011. The DR locus at 30207175 bp was associated with 100 SW in 2012, and the DR locus at genomic position 30210113 bp was associated with SL and 100 SW in 2012. In summary, all four DR loci were associated with seed size traits.
Candidate DR genes for flowering time
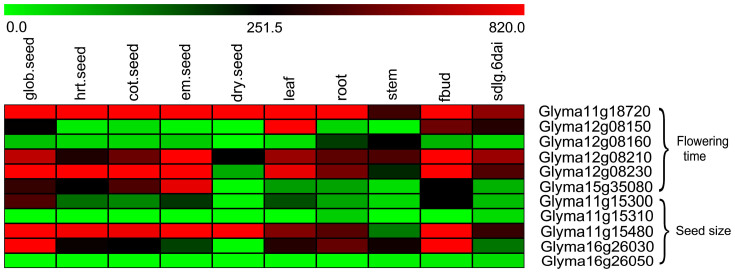
Candidate DR genes were selected if: 1) genes containing DR loci or genes in the adjacent regions to which DR loci were significantly associated with the flowering time or seed size, and 2) genes with high level of transcriptome expression. The locus at 15362036 bp on chromosome 11 was in the gene Glyma11g18720, which was homologous to the gene OTLD1 regulating FLC for flowering time and seed germination in Arabidopsis46,47,48 (Table 1, Fig. S2). The locus at 39607166 bp on chromosome 15 was in the gene Glyma15g35080 which regulated flowering time in soybean38. We also observed a relatively high expression of the Glyma11g18720 and Glyma15g35080 at specific stages of flowering time and seed development (Fig. 3).
Figure 3. Expression levels of candidate genes for soybean flowering time and seed size in various developmental stages or tissues.
The expression data were extracted from the soybean transcriptome data deposited at NCBI (http://www.ncbi.nlm.nih.gov/geo/).
A multiple linear regression analysis of each selected soybean gene (y) on Glyma11g18720 (x1) and Glyma05g28130 (x2) (homologous to FLC, Fig. S3) based on expression data, with the model y = b0 + b1x1 + b2x2 + b12x1x2 + ε, identified five significant flowering-time-related genes Glyma06g02470, Glyma08g02490, Glyma11g02110, Glyma15g17480, and Glyma02g11060 with P-values of 1.28E-5 to 7.81E-2 (Table 2). Of the five genes, Glyma06g02470 was homologous to the genes EEL and AREB338 related to flowering time in Arabidopsis (Fig. S4); Glyma08g02490 to the genes SPA1–SPA438 (Fig. S5); Glyma15g17480 to the genes FKF1, LKP2, and ZTL38 (Fig. S6); and Glyma02g11060 to the genes TEM2, RAV1, TEM1, and EDF338 (Fig. S7).
Table 2. Co-expressional genes of Glyma11g18720 and Glyma05g28130 for flowering time (FT) and Glyma11g15480 for seed size (SS) and homologous genes in Arabidopsis.
| Co-expressional analysis | Homologous gene in Arabidopsis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trait | Gene | F(P-value) | R2(%) | Gene | Similarity (%) | Notation | Functional description | Reference | Evolutionaryrelationship |
| FT | Glyma06g02470 | 3.77(7.81E-2) | 86.91 | AT3G56850; AT2G41070 | 23.2; 12.3 | AREB3; EEL | DNA binding/transcription factor | 38 | Figure S4 |
| Glyma08g02490; Glyma11g02110 | 6.42(2.66E-2) | 76.24;87.75 | AT2G46340; AT4G11110 | 35.4–61.7 | SPA1 ~ SPA4 | suppress photomorphogenesis, light signaling pathway | 38 | Figure S5 | |
| 14.33(3.83E-3) | AT3G15354; AT1G53090 | ||||||||
| Glyma15g17480 | 108.86(1.28E-5) | 98.20 | AT1G68050; AT2G18915 | 68.2–85.3 | FKF1, LKP2, ZTL | control flowering time by influencing the circadian clock period, light signaling pathway | 38 | Figure S6 | |
| AT5G57360 | |||||||||
| Glyma02g11060 | 13.29(4.66E-3) | 86.91 | AT1G68840; AT1G13260 | 53.9–61.6 | TEM2, RAV1 | repress flowering, light signaling pathway | 38 | Figure S7 | |
| AT1G25560; AT3G25730 | TEM1, EDF3 | ||||||||
| SS | Glyma01g41460 | 43.85(2.00E-4) | 84.57 | AT2G35670 | 29.4 | FIS2 | female gametophyte, endosperm | 50,51 | Figure S9 |
| Glyma05g26150; Glyma08g09090 | 12.09–82.46 | 66.17–91.16 | AT5G58230 | 63.0–92.5 | MSI1 | female gametophyte, embryo | 50,51 | Figure S10 | |
| Glyma11g09700; Glyma12g03700 | (<1.00E-4–8.40E-3) | ||||||||
| Glyma10g02690; Glyma02g17110 | 6.40–16.87 | 44.43–67.83 | AT3G20740 | 83.7–86.7 | FIE/FIS3 | female gametophyte, endosperm, embryo | 50,51 | Figure S11 | |
| Glyma13g36310 | (3.40E-3–3.53E-2) | ||||||||
| Glyma01g38150; Glyma11g07220 | 7.40(2.62E-2) | 48.07;46.66 | AT5G66750 | 76.2; 75.5 | DDM1 | chromatin remodeling factor; embryo, endosperm | 50,52 | Figure S12 | |
| 6.70(2.95E-2) | |||||||||
| Glyma06g18790 | 6.42(3.51E-2) | 44.51 | AT5G49160 | 73.5 | MET1 | methyl transferase; embryo, endosperm | 50,52 | Figure S13 | |
| Glyma11g05720; Glyma17g18640 | 9.85(1.38E-2) | 55.18;47.85 | AT4G36920 | 46.3; 49.5 | AP2 | AP2 domain transcription factor; Integument, endosperm, embryo | 53 | Figure S14 | |
| 7.34(2.67E-2) | |||||||||
| Glyma20g27320 | 5.37(4.90E-2) | 40.16 | AT2G24840 | 42.0 | AGL61 | MADS-box transcription factor; central cell, endosperm | 50 | Figure S15 | |
| Glyma07g35520; Glyma09g37000 | 35.83(3.00E-4) | 81.75;48.29 | AT4G25350 | 68.9; 64.8 | SHB1 | Transcription co-activator; embryo, endosperm | 54 | Figure S16 | |
| 7.47 (2.57E-2) | |||||||||
| Glyma20g29600 | 9.26(1.60E-2) | 53.66 | AT5G07280 | 73.9 | EXS/EMS1 | Leucine-rich repeat (LRR) receptor kinase; embryo, endosperm | 55 | Figure S17 | |
| Glyma04g37760; Glyma05g38540 | 6.88–10.96 | 46.24–57.80 | AT5G62000 | 70.1–72.1 | ARF2 | auxin-responsive element binding transcription factor; integument before pollination, embryo after pollination | 56 | Figure S18 | |
| Glyma06g17320; Glyma08g01100 | (1.07E-2–3.05E-2) | ||||||||
| Glyma19g36100 | 8.42(1.98E-2) | 51.28 | AT2G37260 | 61.8 | TTG2 | WRKY transcription factor; Integument | 57 | Figure S19 | |
| Glyma05g22970; Glyma17g17010 | 6.33(3.61E-2) | 44.16;51.39 | AT4G37750 | 60.5; 61.6 | ANT | AP2-like transcription factor; Integument before pollination, embryo after pollination | 58 | Figure S20 | |
| 8.46 (1.96E-2) | |||||||||
The genes Glyma12g08150 and Glyma12g08160 were adjacent to the domestication regions of 5895633 to 5895785 bp on chromosome 12, and the genes Glyma12g08210 and Glyma12g08230 were in close proximity to the DR locus at 5957099 bp on chromosome 12. The likely association of these genes with domestication was also reported by Chung et al.44. In addition, based on the soybean transcriptome data deposited at NCBI (http://www.ncbi.nlm.nih.gov/geo/), all the genes had relatively high expression levels in floral bud tissue (Fig. 3). Therefore, the above 10 genes may be associated with flowering time.
Candidate DR genes for seed size
The genes Glyma11g15300 and Glyma11g15310 were close to the DR locus at 10957940 bp on chromosome 11. Glyma11g15480 contained a DR locus at 11111516 bp on chromosome 11, and Glyma16g26030 and Glyma16g26050 were close to the domestication regions of 30207175 to 30210113 bp on chromosome 16. The three genes Glyma11g15300, Glyma11g15480 and Glyma16g26030 had relatively higher expression levels during seed development than did Glyma11g15310 and Glyma16g26050 (Fig. 3). The soybean gene Glyma11g15480 is homologous to the Arabidopsis gene NOT2A49 (Fig. S8) and was found to be associated with seed size in this study. A correlation analysis of Glyma11g15480 with each selected soybean gene based on the gene expression dataset showed that 24 genes were significantly correlated with Glyma11g15480 (P-values of 1.00E-4 to 4.90E-2) (Table 2). Among the 24 genes, 11 were homologous to five epigenetic regulation endosperm genes in Arabidopsis (FIS2, MSI1, FIE/FIS3, DDM1, and MET1)50,51,52 (Figures S9–S13); six were homologous to four endosperm development genes (AP2, AGL61, SHB1, and EMS1)50,53,54,55 (Figures S14–S17); five were homologous to two integument development genes (ARF2/MNT, and TTG2)56,57 (Figures S18–S19); and two genes were homologous to the embryo development gene (ANT)58 (Fig. S20). Therefore, the above 27 genes may be associated with seed size.
In addition, significant difference of allele frequencies for the SNPs in coding region of the above eight candidate DR genes in GWAS was observed between wild and cultivated soybeans (Tables S2, S3). Although the number of wild accessions was smaller than the number of cultivated soybeans, it is close to that reported in Chung et al.44 and Lam et al.59, and we adopted a stringent threshold of statistical significance in determining domestication loci (α = 4.72E-7 and FST > 0.45) and GWAS (α = 9.08E-7).
Some QTL (or genes) associated with DTs in this study were consistent with those reported previously (Table S4), for example, Glyma12g08150, Glyma12g08210 and Glyma12g08230 were reported as candidate domestication genes by Chung et al.44.
Conclusion
A total of 48 DR loci in the soybean genome were identified. Most of these loci were on chromosomes 11 and 15. Among these DR loci, 10 loci were associated with flowering time or seed size. Eight genes near the 10 loci were associated with the two traits. Among the eight genes, three genes Glyma11g18720, Glyma11g15480 and Glyma15g35080 were homologous to Arabidopsis genes, three known DR genes Glyma12g08150, Glyma12g08210 and Glyma12g08230 were linked to flowering time; and the other two DR genes Glyma11g15300 and Glyma16g26030 were reported for the first time. Glyma11g18720 and Glyma05g28130 were co-expressed with five genes homologous to flowering time genes in Arabidopsis, and Glyma11g15480 was co-expressed with 24 genes homologous to seed development genes in Arabidopsis. The method that integrates domestication analysis, GWAS and gene expression analysis is an efficient approach for identifying potentially useful DR genes.
Methods
Germplasm for genome-wide re-sequencing
The 286 soybean accessions used here included 14 wild, 153 landrace, and 119 bred accessions that were randomly selected from 6 geographic regions in China using a stratified random sampling method (Table S5). The seeds of the accessions were obtained from the National Centre for Soybean Improvement and Linyi Academy of Agricultural Sciences, and planted in three-row plots in a completely randomised design at the Jiangpu Experimental Station of Nanjing Agricultural University during 2008–2012. The plots were 1.5 m wide and 2 m long. A total of 20 seeds from five plants of the middle row in each plot were randomly selected for the measurement of the seed size traits, including SL and SW, using digital verniercalipers. The SL and SW for each accession were averaged based on 20 seeds and 100 SW for each replicate. The first and full flowering dates for each accession were recorded in the field during 2010 and 2012.
Genome-wide re-sequencing and sequence alignment
Approximately 0.3 g of fresh leaves obtained from each accession in 2012 was used to extract genomic DNA using the cetyltrimethylammonium bromide method. The DNA was digested with the EcoRI (G∧AATTC) enzyme. The RAD-seq libraries for sequencing were prepared according to the protocols described by Baird et al.60. We sequenced 50 bp at each end and used SolexaPipeline 1.0 for base call of 50-bp reads from the raw fluorescent images. To ensure quality, the raw sequence data were processed in two steps. In the first step, reads with incorrect adapter sequences were deleted, and any reads containing more than 50% low quality bases (quality value < = 5) were removed. In the second step, the remaining reads were demultiplexed according to the index of each sample. The sequences were subsequently aligned on the soybean reference genome Glyma1.1 (http://www.jgi.doe.gov) using the Burrows-Wheeler Alignment Tool (BWA)61. The base recalibration and determination of the SNP allele were performed using GATK62.
Detection of DR loci
The independence between each SNP and the evolutionary class (wild, landrace, and bred) was determined using the χ2 test63. The significant threshold α for each test was adjusted using the Bonferroni correction, that is, α = 0.05/106,013 = 4.72 × 10−7 (106,013 was the total number of SNPs identified). The SNPs significantly associated with evolutionary classes were used for the subsequent analysis.
A U-test at the 0.05 significance level was used to test the significance of the allelic differences between the wild (14) and cultivated (272) accessions for each candidate SNP.
For all the SNPs with significant differences in the U-test, the fixation index (FST) was calculated for the purpose of measuring population differentiation between the wild (14) and bred (119) classes using the software Genepop v4.264, and a FST threshold value of 0.45 from Lam et al.59 were adopted for the identification of DR loci.
Population structure analysis
The STRUCTURE 2.2 software was used to investigate the population structures based on all available accessions65. The number of subpopulations (K) was set from 2 to 732. In this study, the ΔK method of Evanno et al.66 was used to determine the optimal value of K. The Q matrix was calculated based on inferred K.
Genome-wide association study
A GWAS was performed using the general linear model in TASSEL V4.3 (www.maizegenetics.net/tassel) with the total average and population structure as covariates. The Manhattan plot of −log10P was generated using SAS software. As P-values from the software were not corrected for multiple test, the significance level α for each test was determined after Bonferroni correction, α = 0.05/55,052 = 9.08E-7 (55,052 was the number of SNPs with the concordance rate <99% by the TASSEL software). Elimination of the SNPs with concordance rate >99% was conducted by the TASSEL software.
Identification of soybean DR genes and homologous Arabidopsis genes
Many genes have been annotated with high confidence in the soybean genome26. The most likely functions of the genes have also been determined based on the annotation of the Arabidopsis homologs. The protein sequences of the annotated genes in soybean (Glyma1.1) and Arabidopsis (TAIR9 release) were downloaded from the Phytozome (www.phytozome.net) and The Arabidopsis Information Resource (TAIR) (www.arabidopsis.org) websites, respectively.
The Pfam gene annotations were downloaded from the Phytozome database version 9.0 (http://www.phytozome.net/). The annotations of the DR genes using BLASTX (e-value lE-4 or better) against NCBI's non-redundant database have been described at http://www.ncbi.nlm.nih.gov/. The top best hits from the species of interest were extracted for each gene.
Transcriptional activity and gene expression data analysis
The transcriptome sequences were obtained from the Gene Expression Omnibus database at http://www.ncbi.nlm.nih.gov/geo/ss (accession number GSE29163), and were used to identify the expression level of soybean gene during seed development and throughout the life cycle.
Author Contributions
Y.-M.Z. designed the project. L.Z., S.-B.W., Q.-C.G., J.W., G.-J.L., Y.-Q.L., J.Z., J.-Y.F., Y.N., L.Z., W.-L.R. and Y.-M.Z. performed the experiments and analyzed the data. J.J. and Z.W. conducted DNA sequencing of all the accessions and analyzed the genetic diversity. Y.-Q.L. provided partly soybean materials. L.Z. prepared figures and tables. Y.-M.Z., L.Z., Q.S. and J.M.D. wrote the manuscript text. All authors reviewed the manuscript.
Supplementary Material
Supplementary information
Acknowledgments
This work was supported by the National Key Basic Research Program of China (grant 2011CB109300), Fundamental Research Funds for the Central Universities (grants KJQN201414, KJQN201422, KYT201002 and KYZ201202-9), the National Natural Science Foundation of China (grants 31301229 and 31301004), a Specialised Research Fund for the Doctoral Program of Higher Education (grant 20120097110023), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Huazhong Agricultural University Scientific & Technological Self-innovation Foundation (Program No. 2014RC020). The authors declare that they have no conflict of interest.
References
- Wahl V. et al. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339, 704–707 (2013). [DOI] [PubMed] [Google Scholar]
- Adebisi M. A. et al. Influence of different seed size fractions on seed germination, seedling emergence and seed yield characters in tropical soybean (Glycine max L. Merrill). J Int Pharm Res 8, 26–33 (2013). [Google Scholar]
- Sun C. Q., Wang X. K., Li Z. C., Yoshimura A. & Iwata N. Comparison of the genetic diversity of common wild rice (Oryza rufipogon Griff.) and cultivated rice (O. sativa L.) using RFLP markers. Theor Appl Genet 102, 157–162 (2001). [Google Scholar]
- Li Y. H. et al. Genetic diversity in domesticated soybean (Glycine max) and its wild progenitor (Glycine soja) for simple sequence repeat and single-nucleotide polymorphism loci. New Phytol 188, 242–253 (2010). [DOI] [PubMed] [Google Scholar]
- Laidò G. et al. Genetic diversity and population structure of tetraploid wheats (Triticum turgidum L.) estimated by SSR, DArT and pedigree data. PLoS ONE 8, e67280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. K., Kulwal P. L. & Mir R. R. QTL mapping: methodology and applications in cereal breeding. In Gupta P. K. & Varshney R. K. (eds.), Cereal Genomics II pp319–340 (2013). [Google Scholar]
- Olsen K. M. & Wendel J. F. A bountiful harvest: genomic insights into crop domestication phenotypes. Annu Rev Plant Biol 64, 47–70 (2013). [DOI] [PubMed] [Google Scholar]
- Andersson L. & Georges M. Domestic-animal genomics: deciphering the genetics of complex traits. Nat Rev Genet 5, 202–212 (2004). [DOI] [PubMed] [Google Scholar]
- Doebley J., Stec A. & Gustus C. Teosinte branched1 and the origin of maize: evidence for epistasis and the evolution of dominance. Genetics 141, 333–346 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S. et al. An SNP caused loss of seed shattering during rice domestication. Science 312, 1392–1396 (2006). [DOI] [PubMed] [Google Scholar]
- Grisart B. et al. Positional candidate cloning of a QTL in dairy cattle: identification of a missense mutation in the bovine DGAT1 gene with major effect on milk yield and composition. Genome Res 12, 222–231 (2002). [DOI] [PubMed] [Google Scholar]
- Wang S. et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet 44, 950–594 (2012). [DOI] [PubMed] [Google Scholar]
- Simons K. J. et al. Molecular characterization of the major wheat domestication gene Q. Genetics 172, 547–555 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuda T. et al. Six-rowed barley originated from a mutation in a homeodomain-leucine zipper I-class homeobox gene. Proc Natl Acad Sci U S A 104, 1424–1429 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii J. et al. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science 253, 448–451 (1991). [DOI] [PubMed] [Google Scholar]
- Kim M. Y., Van K., Kang Y. J., Kim K. H. & Lee S. H. Tracing soybean domestication history: From nucleotide to genome. Breeding Sci 61, 445–452 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bactrian Camels Genome Sequencing and Analysis Consortium, Jirimutu. et al. Genome sequences of wild and domestic bactrian camels. Nat Commun 3, 1202 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z. et al. Two evolutionary histories in the genome of rice: the roles of domestication genes. PLoS Genet 7, e1002100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford M. B. et al. Comparative population genomics of maize domestication and improvement. Nat Genet 44, 808–811 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L. How selective sweeps in domestic animals provide new insight into biological mechanisms. J Intern Med 271, 1–14 (2012). [DOI] [PubMed] [Google Scholar]
- Lenser T. & Theißen G. Molecular mechanisms involved in convergent crop domestication. Trends Plant Sci 18, 704–714 (2013). [DOI] [PubMed] [Google Scholar]
- Millar C. D. & Lambert D. M. Towards a million-year-old genome. Nature 499, 34–35 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Morphological and genetic evidence for early Holocene cattle management in northeastern China. Nat Commun 4, 2755 (2013). [DOI] [PubMed] [Google Scholar]
- Moles A. T. et al. A brief history of seed size. Science 576, 307 (2005). [DOI] [PubMed] [Google Scholar]
- Carter T. E., Nelson J. R., Sneller C. H. & Cui Z. Genetic diversity in soybean. In Boerma H. R. & Specht J. E. (eds.) Soybeans: Improvement, Production and Uses, Am. Soc. of Agro, Madison, Wisconsin pp303–416 (2004). [Google Scholar]
- Schmutz J. et al. Genome sequence of the paleopolyploid soybean. Nature 463, 178–183 (2010). [DOI] [PubMed] [Google Scholar]
- Li Q.-G., Zhang L., Li C., Dunwell J. M. & Zhang Y.-M. Comparative genomics suggests that an ancestral polyploidy event leads to enhanced root nodule symbiosis in the Papilionoideae. Mol Biol Evol 30, 2602–2611 (2013). [DOI] [PubMed] [Google Scholar]
- Liu B. et al. QTL mapping of domestication-related traits in soybean (Glycine max). Ann Bot 100, 1027–1038 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H. Z. et al. Mapping quantitative trait loci for six seed shape traits in soybean. Henan Agriculture Science 45, 54–60 (2008). [Google Scholar]
- Xu Y. et al. Mapping quantitative trait loci for seed size traits in soybean (Glycine max L. Merr.). Theor Appl Genet 122, 581–594 (2011). [DOI] [PubMed] [Google Scholar]
- Hu Z. et al. Determination of the genetic architecture of seed size and shape via linkage and association analysis in soybean (Glycine max L. Merr.). Genetica 141, 247–254 (2013). [DOI] [PubMed] [Google Scholar]
- Xie F.-T. et al. Fine mapping of quantitative trait loci for seed size traits in soybean. Mol Breeding online, 10.1007/s11032-014-0171-7 (2014). [DOI] [Google Scholar]
- Niu Y. et al. Association mapping for seed size and shape traits in soybean cultivars. Mol Breeding 31, 785–794 (2013). [Google Scholar]
- Liu B. et al. Genetic redundancy in soybean photoresponses associated with duplication of the phytochrome A gene. Genetics 180, 995–1007 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S. et al. Map-based cloning of the gene associated with the soybean maturity locus E3. Genetics 182, 1251–1262 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S. et al. A map-based cloning strategy employing a residual heterozygous line reveals that the GIGANTEA gene is involved in soybean maturity and flowering. Genetics 188, 395–407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z. et al. Positional cloning and characterization reveal the molecular basis for soybean maturity locus E1 that regulates photoperiodic flowering. Proc Natl Acad Sci U S A 109, 2155–2164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. H., Wong C. E. & Singh M. B. Comparative genomic analysis of soybean flowering genes. PLoS ONE 7, e38250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z. et al. Artificial selection for determinate growth habit in soybean. Proc Natl Acad Sci U S A 107, 8563–8568 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. W. & Nelson R. L. Genetic variation and relationships among cultivated, wild, and semiwild soybean. Crop Sci 44, 316–325 (2004). [Google Scholar]
- Kim M. Y. et al. Whole-genome sequencing and intensive analysis of the undomesticated soybean (Glycine soja Sieb. and Zucc.) genome. Proc Natl Acad Sci U S A 107, 22032–22037 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupar R. M. Into the wild: The soybean genome meets its undomesticated relative. Proc Natl Acad Sci U S A 107, 21947–21948 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. Y., Shin J. H., Kang Y. J., Shim S. R. & Lee S. H. Divergence of flowering genes in soybean. J. Biosciences 37, 857–870 (2012). [DOI] [PubMed] [Google Scholar]
- Chung W. H. et al. Population structure and domestication revealed by high-depth resequencing of Korean cultivated and wild soybean genomes. DNA Res 21, 153–167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. H. et al. Molecular footprints of domestication and improvement in soybean revealed by whole genome re-sequencing. BMC Genomics 14, 579 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky A., Zaltsman A., Lacroix B. & Citovsky V. Involvement of KDM1C histone demethylase-OTLD1 otubain-like histone deubiquitinase complexes in plant gene repression. Proc Natl Acad Sci U S A 108, 11157–11162 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Y. Chromatin modification in plants. In Wendel J. F. et al. (eds), Plant Genome Diversity Volume I 15, 237–255 (2012). [Google Scholar]
- Stekhoven D. J. et al. Causal stability ranking. Bioinformatics 28, 2819–2823 (2012). [DOI] [PubMed] [Google Scholar]
- Wang L. et al. NOT2 proteins promote polymerase II–dependent transcription and interact with multiple micro RNA biogenesis factors in Arabidopsis. Plant Cell 25, 715–727 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Shantharaj D., Kang X. & Ni M. Transcriptional and hormonal signaling control of Arabidopsis seed development. Curr Opin Plant Biol 13, 611–620 (2010). [DOI] [PubMed] [Google Scholar]
- Gao M. J., Gropp G., Wei S., Hegedus D. D. & Lydiate D. J. Combinatorial networks regulating seed development and seed filling. In: Sato K. I. ed, Embryogenesis. Rijeka, Croatia: InTech; ISBN 979-953-307-439-8 (2012). [Google Scholar]
- Xiao W. et al. Regulation of seed size by hypomethylation of maternal and paternal genomes. Plant Physiol 142, 1160–1168 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto M. A., Floyd S. K., Fischer R. L., Goldberg R. B. & Harada J. J. Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis. Sex Plant Reprod 22, 277–289 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. et al. SHORT HYPOCOTYL UNDER BLUE1 associates with MINISEED3 and HAIKU2 promoters in vivo to regulate Arabidopsis seed development. Plant Cell 21, 106–117 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales C., Bhatt A. M., Scott R. & Dickinson H. EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr Biol 12, 1718–1727 (2002). [DOI] [PubMed] [Google Scholar]
- Schruff M. C. et al. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133, 251–261 (2006). [DOI] [PubMed] [Google Scholar]
- Garcia D., Fitz Gerald J. N. & Berger F. Maternal control of integument cell elongation and zygotic control of endosperm growth are coordinated to determine seed size in Arabidopsis. Plant Cell 17, 52–60 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y. & Fischer R. L. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci U S A 97, 942–947 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H. M. et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat Genet 42, 1053–1059 (2010). [DOI] [PubMed] [Google Scholar]
- Baird N. A. et al. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE 3, e3376 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20, 1297–1303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q. et al. Complete resequencing of 40 genomes reveals domestication events and genes in silkworm (Bombyx). Science 326, 433–436 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F. Genepop'007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resource 8, 103–106 (2008). [DOI] [PubMed] [Google Scholar]
- Falush D., Stephens M. & Pritchard J. K. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7, 574–578 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanno G., Regnaut S. & Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14, 2611–2620 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information