Abstract
Hézode et al. recently reported the frequent occurrence of anemia and thrombocytopenia in the ANRS-CO20-CUPIC cohort of hepatitis C virus (HCV) cirrhotic experienced patients treated with pegylated-interferon (Peg-IFN), ribavirin (RBV), and telaprevir or boceprevir.1,2 Using frequent measurements of serum drug concentrations, hemoglobin, and platelet concentrations obtained in 15 patients of this cohort, we show how an on-treatment model-based approach could be used to individualize dose regimen and avoid the occurrence of RBV-induced anemia and Peg-IFN-induced thrombocytopenia.
In this commentary, we provide results from 15 HCV genotype 1 patients included in the MODCUPIC study, nine receiving telaprevir and six boceprevir. Twelve (80%) were men, with a median (min-max) age of 55 (44–64) years. Eleven patients received Peg-IFN-α2a (eight in telaprevir group, three in boceprevir group), three patients Peg-IFN-α2b (all in boceprevir group), and one patient in the telaprevir group did not receive any injection of Peg-IFN. The observed drug concentrations, the estimated steady-state trough serum concentrations, Css, and "effectiveness" EC50 for all drugs are available in Laouénan et al.3 Five patients received erythropoietin in supplementation (two in the telaprevir group, three in the boceprevir group) and hemoglobin level and platelet counts were censored afterwards.
RBV-induced anemia modeling
The median (min-max) baseline hemoglobin level was 15.1 g/dl (10.8–16.0) (15.4 g/dl (10.8–16.0) in the telaprevir group and 14.5 g/dl (12.6–15.8) in the boceprevir group, P = 0.3). The hemoglobin level decreased over time in all patients (Supplementary Figures S1 and S2, online) and was well captured by our model (Supplementary Figure S3). Adding the other drugs, Peg-IFN and protease inhibitors (PIs) did not improve the fit of the data (not shown). There was no significant effect of gender on model parameters. The model predicted that the concentration leading to a 50% blocking effectiveness of RBV in blocking hemoglobin production, IC50RBV, was equal to 7,090 ng/ml (Supplementary Table S1), leading to a median predicted hemoglobin level at steady state, Hbss, of 10.0 g/dl (7.8–11.8) (10.6 g/dl (7.8–11.6) in the telaprevir group and 9.1 g/dl (8.0–11.8) in the boceprevir group, P = 0.5). This corresponds to a median predicted change in hemoglobin level of 4.4 g/dl (2.1–6.6) (4.2 g/dl (2.1–5.5) in the telaprevir group and 4.9 g/dl (3.3-6.6) in the boceprevir group, P = 0.4). Figure 1a shows the relationship between individual predicted RBV concentration at steady state (CssRBV) and effect (blocking production of hemoglobin). For the six patients (40%) who had Hbss <10 g/dl (Table 1), the model predicted that a median RBV dose reduction of 373 mg/day (45–670) would be needed to avoid anemia corresponding to a median dose reduction of 31% (4–67).
Figure 1.
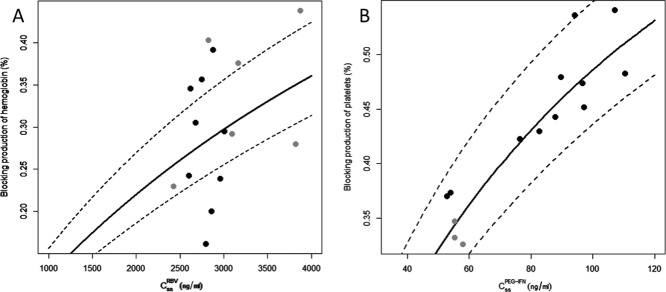
Relationship between: (a) RBV predicted trough serum concentration at steady state (Css) and predicted blocking production of hemoglobin (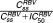 ) and (b) Peg-IFN predicted trough concentration at steady state (Css) and predicted blocking production of platelets (
) and (b) Peg-IFN predicted trough concentration at steady state (Css) and predicted blocking production of platelets (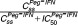 ). Nine patients in the telaprevir group in a (black) and six patients in the boceprevir group (gray). Eleven patients in the Peg-IFN-α2a group in b (black) and three patients in the Peg-IFN-α2b group (gray). The lines denote the predictions with the mean blocking production and the dotted lines denote 95% confidence interval computed with the standard errors predicted by the Fisher Information Matrix.
). Nine patients in the telaprevir group in a (black) and six patients in the boceprevir group (gray). Eleven patients in the Peg-IFN-α2a group in b (black) and three patients in the Peg-IFN-α2b group (gray). The lines denote the predictions with the mean blocking production and the dotted lines denote 95% confidence interval computed with the standard errors predicted by the Fisher Information Matrix.
Table 1.
Individual parameter estimates of the RBV-induced anemia model (Hb0, CssRBV, and Hbss) and proposed RBV dosage modifications for Hbss ≥10 g/dl and the Peg-IFN-induced thrombocytopenia model (PLT0, CssPeg-IFN, and PLTss) and proposed Peg-IFN dosage modifications for PLTss ≥50,000/mm3
| Patient | Treatment group PI | Treatment group Peg-IFN | RBV bid dose (mg/day) | Hb0 (g/dl) | CssRBV (ng/ml) | Hbss (g/dl) | Adjusted RBV dose for targeting Hbss ≥10 g/dl (mg/day) | Peg-IFN dose (μg/week) | PLT0 (/mm3) | CssPeg-IFN (ng/ml) | PLTss (/mm3) | Adjusted Peg-IFN dose for targeting PLTss ≥50,000/mm3 (μg/week) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Boceprevir | 2b | 1,000 | 15.5 | 2,827 | 9.2 | 807 | 100 | 162,060 | 55.3 | 108,301 | — |
| 2 | Boceprevir | 2a | 1,200 | 15.1 | 3,874 | 8.4 | 781 | 180 | 193,480 | 52.8 | 121,874 | — |
| 3 | Boceprevir | 2a | 1,200 | 14.4 | 3,162 | 9.0 | 874 | 180 | 105,610 | 54.0 | 66,173 | — |
| 4 | Boceprevir | 2b | 1,000 | 11.4 | 3,092 | 8.0 | 330 | 100 | 48,073* | 55.4 | 31,402 | — |
| 5 | Boceprevir | 2b | 1,200 | 15.4 | 2,428 | 11.8 | — | 100 | 88,981 | 57.9 | 59,999 | — |
| 6 | Boceprevir | 2a | 1,000 | 15.8 | 3,820 | 11.4 | — | 180 | 228,620 | 96.7 | 120,264 | — |
| 7 | Telaprevir | 2a | 1,000 | 12.8 | 2,875 | 7.8 | 439 | 135 | 123,540 | 107.1 | 56,680 | — |
| 8 | Telaprevir | 2a | 1,200 | 15.3 | 2,746 | 9.9 | 1,154 | 180 | 130,520 | 89.6 | 67,922 | — |
| 9 | Telaprevir | 2a | 1,000 | 14.2 | 2,602 | 10.7 | — | 180 | 76,232 | 82.6 | 43,486 | 125 |
| 10 | Telaprevir | 2a | 1,200 | 15.3 | 2,678 | 10.6 | — | 180 | 116,480 | 76.4 | 67,267 | — |
| 11 | Telaprevir | 2a | 1,000 | 14.2 | 3,008 | 10.0 | — | 180 | 165,530 | 110.4 | 85,613 | — |
| 12 | Telaprevir | 2a | 1,000 | 13.1 | 2,793 | 11.0 | — | 180 | 134,960 | 97.2 | 74,015 | — |
| 13 | Telaprevir | 2a | 1,200 | 15.2 | 2,958 | 11.6 | — | 180 | 72,758 | 87.8 | 40,545 | 103 |
| 14 | Telaprevir | — | 1,200 | 13.22 | 2,860 | 10.6 | — | |||||
| 15 | Telaprevir | 2a | 1,000 | 15.4 | 2,621 | 10.1 | — | 180 | 84,863 | 94.2 | 39,365 | 109 |
Hb0, baseline hemoglobin level; PLT0, baseline platelet counts; CssRBV, steady state trough ribavirin (RBV) plasma concentrations; CssPeg-IFN, steady state trough pegylated interferon (Peg-IFN) plasma concentrations; Hbss, hemoglobin level at steady state (in bold if <10 g/dl); PLTss, platelets count at steady state (in bold if <50,000/mm3).
One patient had a baseline platelet count <50,000/mm3.
Peg-IFN-induced thrombocytopenia modeling
Median baseline platelet counts were 125,500/mm3 (39,000–230,000) (126,000/mm3 (67,000–230,000) in Peg-IFN-α2a patients and 80,000/mm3 (39,000–161,000) in Peg-IFN-α2b patients, P = 0.4). The platelet counts decreased over time in all patients (Supplementary Figures S4 and S5) and could be well captured by our model (Supplementary Figure S6). Adding the other drugs (RBV and PIs) did not improve the fit of the data. The model predicted that the concentration leading to a 50% blocking effectiveness of Peg-IFN in blocking platelets production, IC50Peg-IFN, was equal to 104 ng/ml (Supplementary Table S1), leading to a median predicted platelet counts at steady state, PLTss, of 66,720/mm3 (31,400–121,900) (67,270/mm3 (39,370–121,900) in Peg-IFN-α2a patients and 60,000/mm3 (31,400–108,300) in Peg-IFN-α2b patients, P = 0.7), corresponding to a median predicted change in platelet counts of 51,490/mm3 (16,670–108,400) (60,940/mm3 (32,210–108,400) in the Peg-IFN-α2a group and 28,980/mm3 (16,670–53,760) in the Peg-IFN-α2b group, P = 0.09). Figure 1b shows the relationship between individual Peg-IFN concentration (CssPeg-IFN) and effect (blocking production of platelets). Four patients out of 14 (29%) had predicted PLTss <50,000/mm3 using the current dose or Peg-IFN, but one patient had a baseline PLT below 50,000/mm3 (Table 1). For these three patients the model predicted that a median Peg-IFN dose reduction of 37 μg/week (25–37) would be needed to avoid thrombocytopenia, corresponding to a median dose reduction of 60% (57–70).
Towards an approach integrating efficacy and toxicity
The increasing availability of highly effective treatment holds the promise that high rates of sustained virological response (SVR) with lower toxicity can be reached. However, the CUPIC study reporting an incidence of 34.1% and 17.0% for grade 2–4 anemia and grade 3–4 thrombocytopenia, respectively,1,2 should serve as a reminder that safety may remain an important concern in HCV treatment management, in particular in patients with advanced liver disease whose prevalence in real life is larger than in clinical trials.
Using a model to relate anemia and thrombocytopenia to RBV and Peg-IFN exposure, respectively, we predicted that a dose reduction of RBV and Peg-IFN in 40% and 30% of patients, respectively, would have been needed to avoid toxicity. This would correspond to a median dose reduction of 31% and 60% for RBV and Peg-IFN, respectively. These adjusted RBV and Peg-IFN doses are not practical to administer in actual clinical practice. But here we explore the feasibility and provide an order of magnitude of the amplitude of dose reduction to avoid the occurrence of toxicity.
Because both RBV and Peg-IFN have modest effectiveness against HCV, at least in the first weeks of treatment, where most viruses are sensitive to PIs,3 this dose reduction is unlikely to have a significant impact on the early viral kinetics. Consistent with this prediction, Poordad et al. showed in a randomized clinical trial that reduction in RBV dosage (up to 50% of the initial amount) throughout the course of triple therapy did not affect SVR rates.4
Clearly, the small sample size of our population is the main limitation of the study. The lack of statistical power may explain the lack of any significant effect of PI exposure on hemoglobin level and platelet count kinetics.5,6 It is well known that RBV causes mainly dose-dependent hemolytic anemia, leading to a reduction in the hemoglobin level,7 whereas Peg-IFN induces mainly suppression of hematopoiesis, leading to a reduction in platelet counts.8 This small population also constrained us to analyze the effects of RBV and Peg-IFN separately and independently and we could not evaluate the effect association between the two effects, such as the fact that the bone marrow suppressive effect of Peg-IFN may also contribute to the associated anemia.9
Both RBV and Peg-IFN concentrations were close to an inhibition effect in hemoglobin level and platelet counts, respectively, equal to 50%. Interestingly, we previously reported that Peg-IFN concentrations in this population also led to an antiviral effect in blocking viral production close to 50%.3 The fact that Peg-IFN concentrations led to comparable levels of efficacy and toxicity suggests that Peg-IFN has a particularly narrow therapeutic index and reinforces the interest of Peg-IFN therapeutic monitoring in this population. Of note, it should be acknowledged that the effects of RBV and Peg-IFN were described by turnover indirect models with a maximum inhibition of 100% and more data will be needed to evaluate this assumption.
In conclusion, by showing that the serum PK of RBV and Peg-IFN can be used to characterize the kinetics of hemoglobin level and platelet counts, respectively, this study suggests that individual monitoring of the drug concentrations may improve the management of anti-HCV therapy. This approach, combined with a viral kinetic model that can tease out the effect of each drug on the virologic response,3 holds the promise that integrated model-based approaches could optimize the trade-off between the efficacy and safety of triple therapy. However, this approach will need to be validated on large populations.
Patients and data
MODCUPIC is a substudy of the French ANRS-CO20-CUPIC cohort.3 From September 2011 to September 2012, patients chronically monoinfected with HCV genotype 1, compensated cirrhosis, nonresponders to a prior IFN-based therapy, and who started triple therapy were recruited. Telaprevir-based therapy included 12 weeks of telaprevir (750 mg tid) with Peg-IFN-α2a (180 μg/week) and RBV (1,000 or 1,200 mg/day, depending on body weight), then 36 weeks of Peg-IFN-α2a/RBV. Boceprevir-based therapy included 4 weeks (lead-in phase) of Peg-IFN-α2b (1.5 μg/kg/week) or Peg-IFN-α2a (180 μg/week) and RBV (800 or 1,400 mg/day, depending on body weight), then 44 weeks of Peg-IFN-α2b/RBV and boceprevir (800 mg tid).
Written informed consent was obtained before enrollment. The protocol was approved by the Ile-de-France IX Ethics Committee (Créteil, France).
Blood samples were collected post-PIs initiation at hours 0, 8, days 0, 1, 2, 3, and weeks 1, 2, 3, 4, 8, and 12. There were two additional visits during the boceprevir lead-in phase. Details of bioanalytical methods are available in Laouénan et al.3
Pharmacokinetic and pharmacodynamic model
We assumed that the changes in hemoglobin level and platelet counts were mainly driven by RBV and Peg-IFN concentrations, respectively.10 The effects of RBV and Peg-IFN were described by turnover indirect models assuming a maximum inhibition of 100% given by:
where Hb0 (PLT0) is the baseline level of hemoglobin (platelet counts), koutHb (koutPLT) is the rate constant of hemoglobin (platelets) elimination, "safety" IC50RBV (IC50Peg-IFN) is the half maximal effective RBV (Peg-IFN) concentration, and CRBV(t) (CPeg-IFN(t)) is the trough concentration predicted by a PK model previously developed.3 In brief, CRBV(t) and CPeg-IFN(t) were fitted using an exponential model to obtain the trough concentration at steady state CssRBV and CssPeg-IFN. We assumed a linear PK for both drugs.
Data analysis and parameter estimation
Wilcoxon tests were used to compare baseline hemoglobin level (boceprevir vs. telaprevir) and baseline platelet counts (Peg-IFN-α2a vs. -2b). Parameters (Hb0, PLT0, koutPLT, koutHb, IC50Peg-IFN, IC50RBV) were estimated using longitudinal data analyzed by nonlinear mixed-effect models with the Stochastic Approximation Expectation Minimization (SAEM) algorithm in MONOLIX v. 4.2 (http://www.lixoft.eu), assuming exponential random effects models and additive error models. The model codes and example datasets are provided in the Supplementary Materials online. Model evaluation was performed using goodness-of-fit plots, as well as the individual weighted residuals (IWRES) and the normalized prediction distribution errors (NPDE) over time. Wald tests on Hb0 and IC50RBV were used to assess the influence of gender for RBV-induced anemia. We also tested using the Bayesian information criteria whether the addition of other drug had an effect in each model.
Prediction of individual dosage regimen avoiding toxicity
Maximum a posteriori was used to obtain individual Empirical Bayesian Estimates (EBE). For each patient, from the EBEs, hemoglobin level and platelet counts at steady state, Hbss and PLTss, was obtained as:
where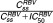 and
and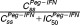 represent the blocking production of hemoglobin and platelets, respectively. Wilcoxon tests were used to compare Hbss and PLTss between treatment groups. If Hbss was predicted below 10 g/dl, the maximum dose of RBV leading to Hbss ≥10g/dl was calculated. If PLTss was predicted below 50,000/mm3, the maximum dose of Peg-IFN leading to PLTss ≥50,000/mm3 was calculated.
represent the blocking production of hemoglobin and platelets, respectively. Wilcoxon tests were used to compare Hbss and PLTss between treatment groups. If Hbss was predicted below 10 g/dl, the maximum dose of RBV leading to Hbss ≥10g/dl was calculated. If PLTss was predicted below 50,000/mm3, the maximum dose of Peg-IFN leading to PLTss ≥50,000/mm3 was calculated.
Acknowledgments
The study was sponsored and funded by the French National Agency for research on Aids and viral Hepatitis (ANRS) and in part by the Association Française pour l'Etude du Foie (AFEF). The authors thank Ventzislava Petrov Sanchez and Setty Allam (ANRS) and Cécile Dufour (Inserm UMR 1136, University Pierre et Marie Curie, Paris, France).
Conflict of Interest/Disclosure
JG has consulted with Gilead SC. FZ received speakers/consulting fees from Gilead SC, MSD, BMS, Janssen cilag, Abbvie, Boehringer Ingelheim. CH and JPB have been clinical investigators, speakers, and/or consultants for Abbvie, Boehringer Ingelheim, BMS, Gilead Sciences, Janssen, Merck Sharp & Dohme, and Roche. PM has been a clinical investigator, speaker, and/or consultant for Roche, Gilead, Vertex, Novartis, Janssen - Tibotec, MSD, Boehringer, Abbott, Pfizer, Alios BioPharma. GP has received travel grants, consultancy fees, honoraria, or study grants from various pharmaceutical companies, including Bristol-Myers-Squibb, Gilead SC, Janssen, Merck, ViiV Healthcare, and Splicos.
Author Contributions
CL, JG, and FM made the analysis and drafted the article; all authors provided the data; all authors read and approved the final article.
Supporting Information
Supplementary information accompanies this paper on the CPT: Pharmacometrics & Systems Pharmacology website (http://www.wileyonlinelibrary.com/psp4)
Supporting Information Table
Figure S1: Observed hemoglobin level (g/dl) over time. Nine patients in telaprevir group (black) and 6 patients in boceprevir group (grey).
Figure S2. Individual fits of the hemoglobin level Nine patients in telaprevir group (black curve) and 6 patients in boceprevir group (grey curve). Black crosses represent the observed hemoglobin level and curves were predicted by the model.
Figure S3. Goodness-of-fit of ribavirin-induced anemia model Residuals (weighted residuals calculated using individual predictions: IWRES and normalized prediction distribution errors: NPDE) versus time and versus individual predictions plots. Residuals seem to distribute homogenously around 0.
Figure S4: Observed platelets count (/mm3) over time. Eleven patients in Peg-IFN-α2a group (black) and 3 patients in Peg-IFN-α2b group (grey).
Figure S5. Individual fits of the platelets count Eleven patients in Peg-IFN-α2a group (black curve) and 3 patients in Peg-IFN-α2b group (grey curve). Black crosses represent the observed hemoglobin level and curves were predicted by the model.
Figure S6. Goodness-of-fit of the pegylated interferon-induced thrombocytopenia model Residuals (weighted residuals calculated using individual predictions: IWRES and normalized prediction distribution errors: NPDE) versus time and versus individual predictions plots. Residuals seem to distribute homogenously around 0.
Supporting Information
References
- Hézode C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J. Hepatol. 2013;59:434–441. doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- Hézode C, et al. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132–142. doi: 10.1053/j.gastro.2014.03.051. [DOI] [PubMed] [Google Scholar]
- Laouénan C, et al. Using pharmacokinetic and viral kinetic modeling to estimate the antiviral effectiveness of telaprevir, boceprevir, and pegylated interferon during triple therapy in treatment-experienced hepatitis C virus-infected cirrhotic patients. Antimicrob. Agents Chemother. 2014;58:5332–5341. doi: 10.1128/AAC.02611-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poordad F, et al. Effects of ribavirin dose reduction vs. erythropoietin for boceprevir-related anemia in patients with chronic hepatitis C virus genotype 1 infection––a randomized trial. Gastroenterology. 2013;145:1035–1044. doi: 10.1053/j.gastro.2013.07.051. [DOI] [PubMed] [Google Scholar]
- Bacon BR, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S, et al. Telaprevir for retreatment of HCV infection. N. Engl. J. Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- Morello J, Rodríguez-Novoa S, Jiménez-Nácher I. Soriano V. Usefulness of monitoring ribavirin plasma concentrations to improve treatment response in patients with chronic hepatitis C. J. Antimicrob. Chemother. 2008;62:1174–1180. doi: 10.1093/jac/dkn421. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Beppu T, Shirabe K, Maehara Y. Baba H. Management of thrombocytopenia due to liver cirrhosis: a review. World J. Gastroenterol. 2014;20:2595–2605. doi: 10.3748/wjg.v20.i10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi L, et al. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997–1004. doi: 10.1053/he.2000.5789. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS. Anemia in the treatment of hepatitis C virus infection. Clin. Infect. Dis. 2003;37(suppl. 4):S315–322. doi: 10.1086/376911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table
Figure S1: Observed hemoglobin level (g/dl) over time. Nine patients in telaprevir group (black) and 6 patients in boceprevir group (grey).
Figure S2. Individual fits of the hemoglobin level Nine patients in telaprevir group (black curve) and 6 patients in boceprevir group (grey curve). Black crosses represent the observed hemoglobin level and curves were predicted by the model.
Figure S3. Goodness-of-fit of ribavirin-induced anemia model Residuals (weighted residuals calculated using individual predictions: IWRES and normalized prediction distribution errors: NPDE) versus time and versus individual predictions plots. Residuals seem to distribute homogenously around 0.
Figure S4: Observed platelets count (/mm3) over time. Eleven patients in Peg-IFN-α2a group (black) and 3 patients in Peg-IFN-α2b group (grey).
Figure S5. Individual fits of the platelets count Eleven patients in Peg-IFN-α2a group (black curve) and 3 patients in Peg-IFN-α2b group (grey curve). Black crosses represent the observed hemoglobin level and curves were predicted by the model.
Figure S6. Goodness-of-fit of the pegylated interferon-induced thrombocytopenia model Residuals (weighted residuals calculated using individual predictions: IWRES and normalized prediction distribution errors: NPDE) versus time and versus individual predictions plots. Residuals seem to distribute homogenously around 0.
Supporting Information


