Abstract
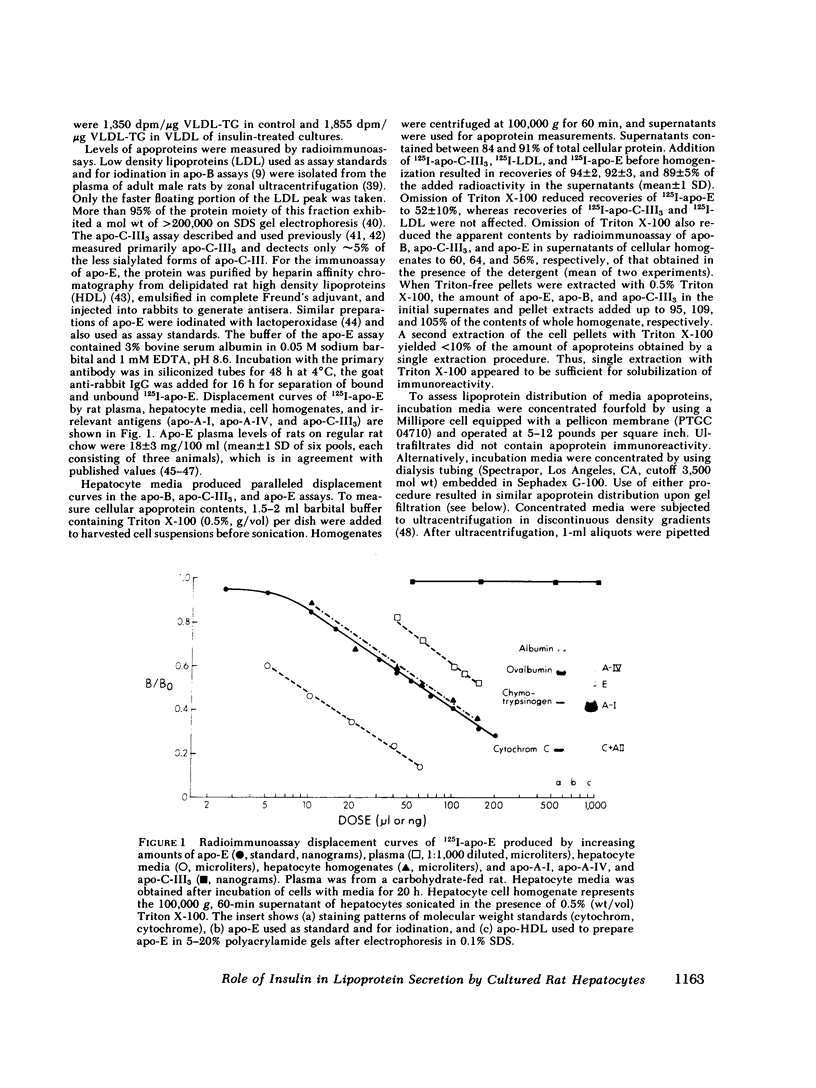
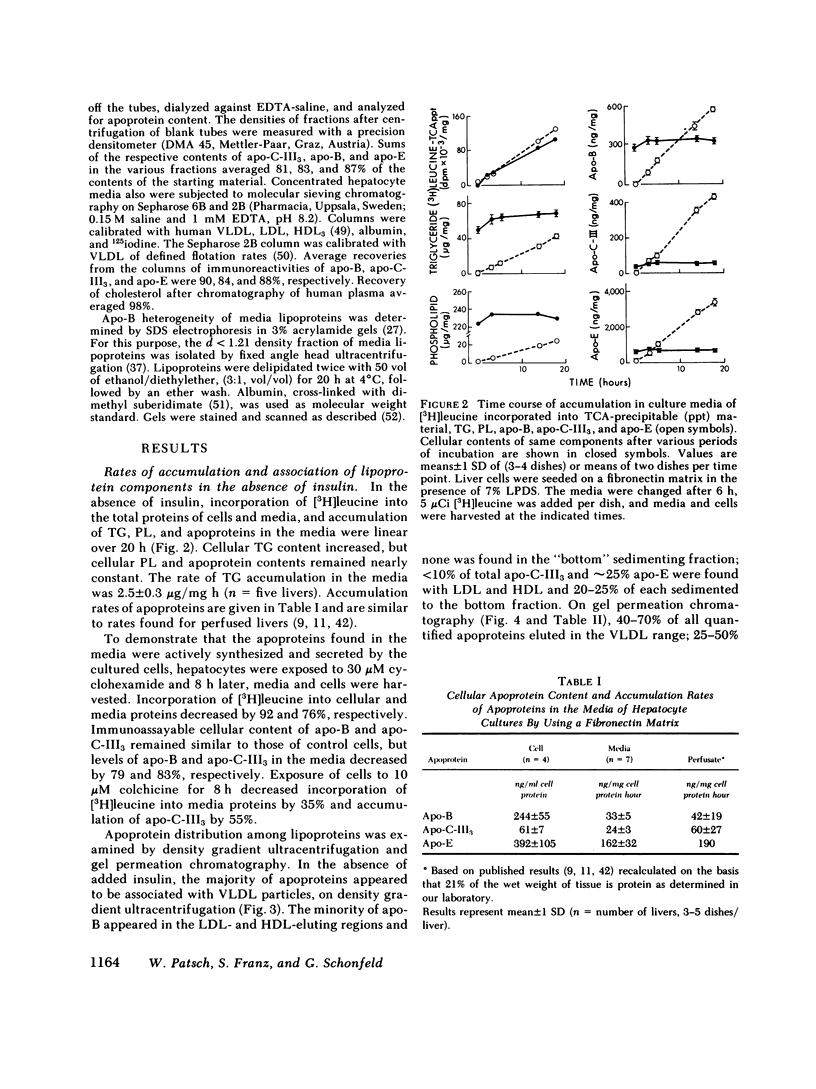
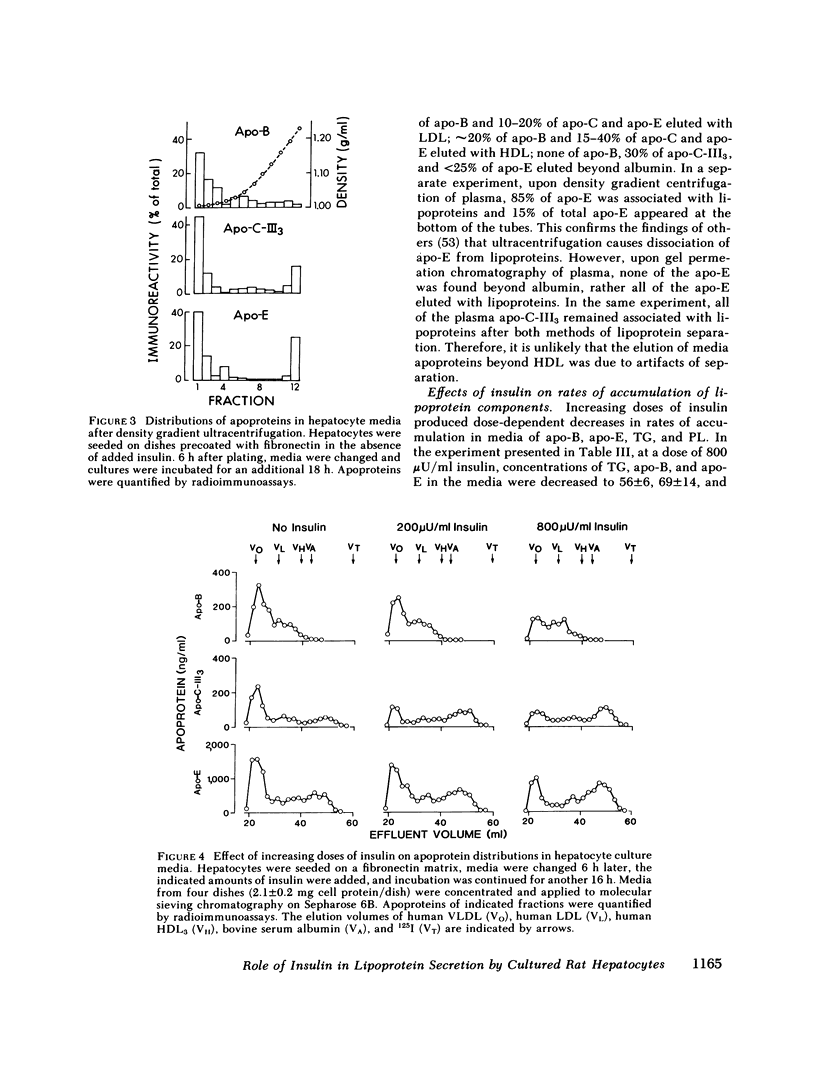
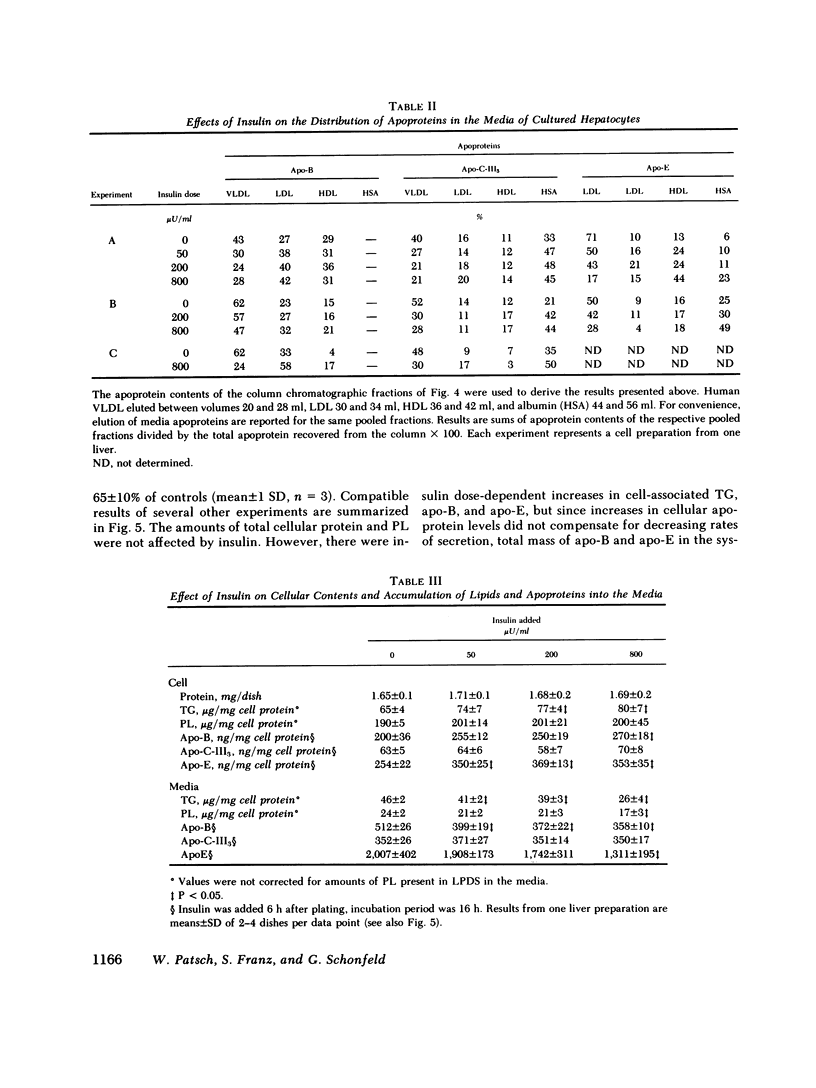
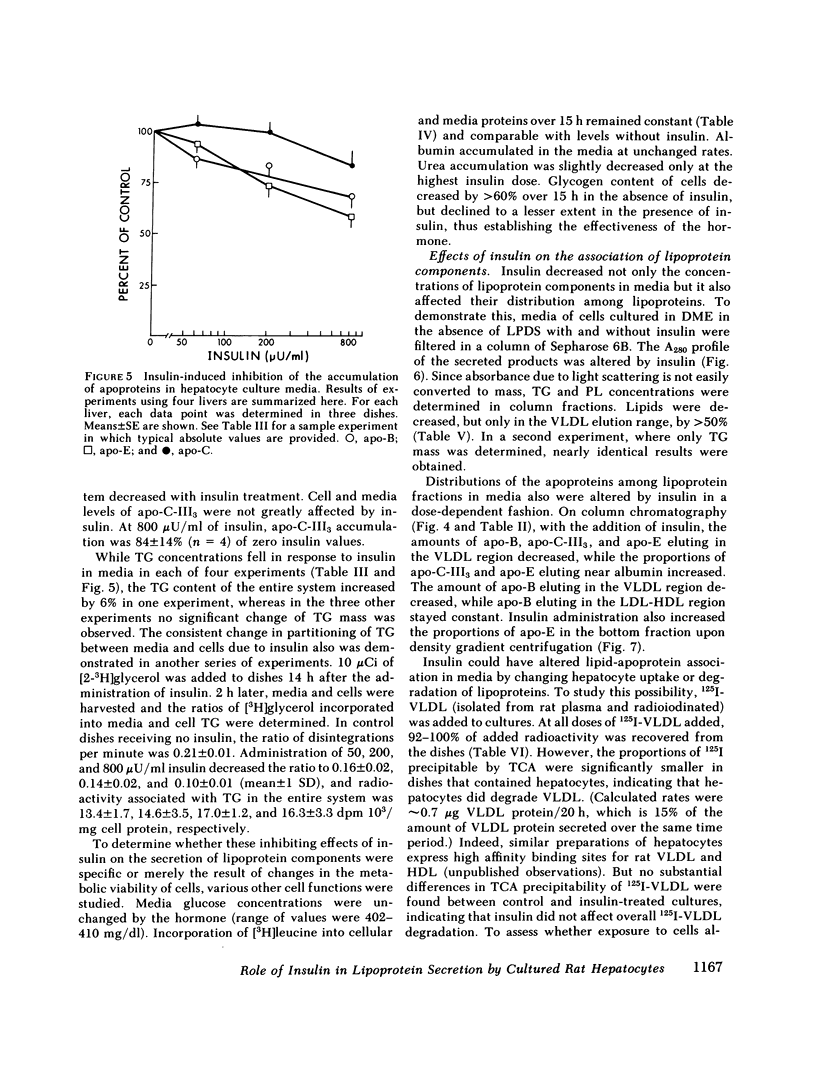
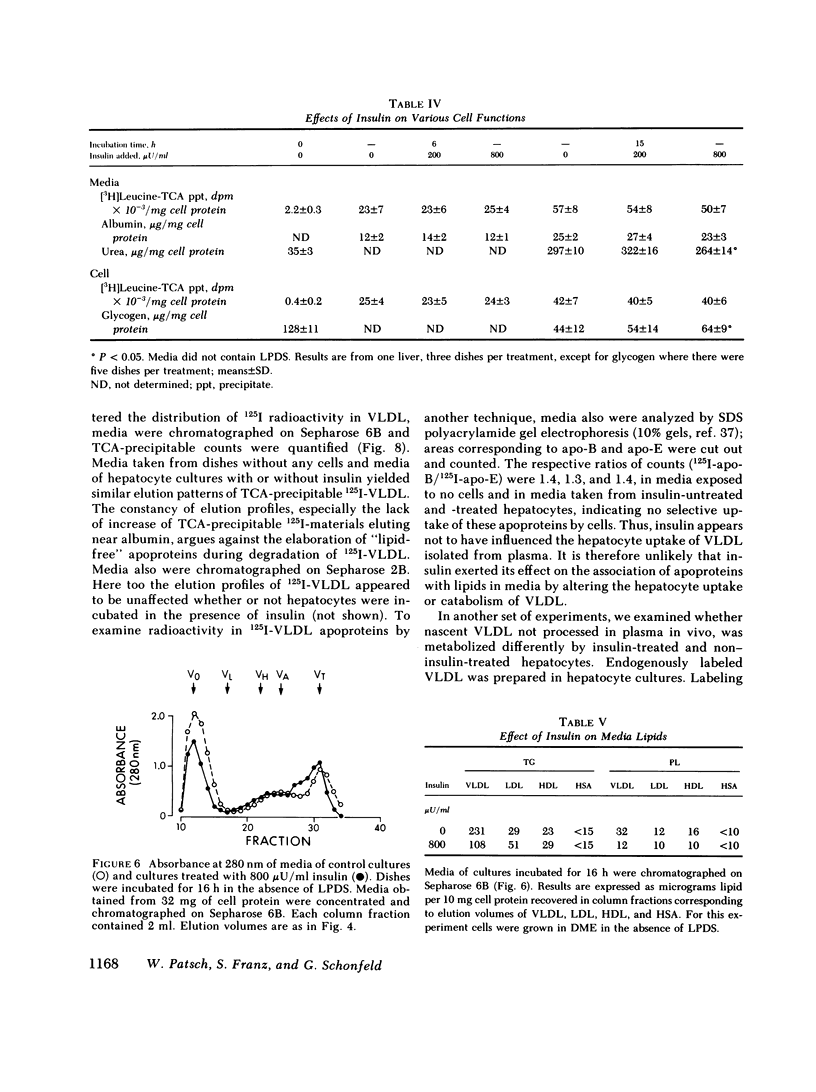
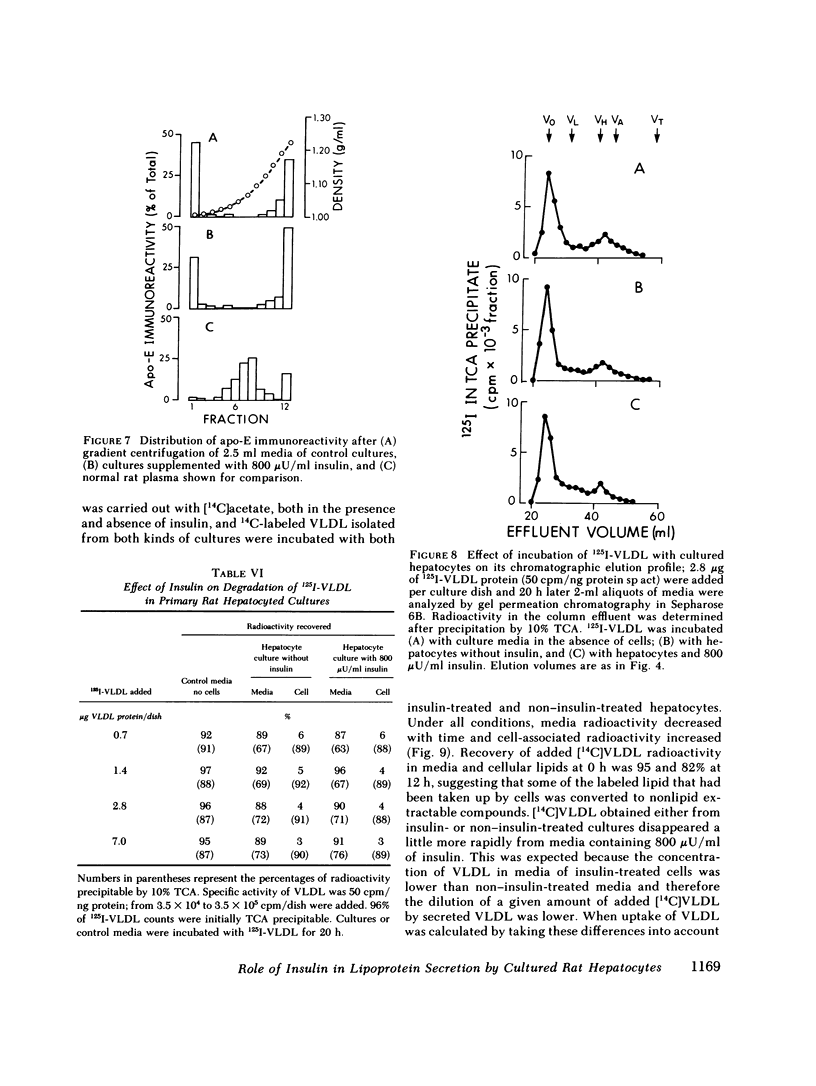
To study the effect of insulin on lipoprotein synthesis and secretion by the liver, apoprotein and lipid levels were measured in primary rat liver cell cultures grown on fibronectin-coated dishes. Triglycerides, phospholipids, apoprotein (apo) B, apo-E, and apo-C-III3 all accumulated in culture media linearly for periods up to 20 h. During incubations, cellular triglyceride contents increased slightly, while cellular apoprotein and phospholipid contents remained constant. In the absence of insulin, rates of accumulation in media of triglycerides, apo-B, apo-C-III3, and apo-E were 2.5 +/- 0.3 micrograms/mg and 33 +/- 5, 24 +/- 3, and 162 +/- 32 ng/mg cell protein per h, respectively. On gel permeation chromatography and density gradient ultracentrifugation, the majority of apoproteins in media were found to be associated with very low density lipoproteins (VLDL) and very little eluted or sedimented with albumin. Incubations in the presence of 50-800 microU/ml of insulin resulted in dose-dependent decreases of triglyceride, phospholipid, apo-B, and apo-E accumulation in the media, paralleled by increases in the cellular contents of these lipoprotein components. The inhibitory effects of insulin on secretion were reversible. Levels of apo-C-III3 and albumin were not affected by insulin. In addition to decreasing secretory rates, the proportion of apo-B, apo-E, and apo-C-III3 associated with VLDL also decreased after the addition of insulin. Concomitantly, the proportion of apo-B eluting with LDL and apo-C-III3, and apo-E eluting near albumin increased. Control experiments, in which exogenous 125I-VLDL or endogenously labeled [14C]VLDL were added to cultures, revealed that the insulin-induced differences in VLDL accumulation and the lipid association of media apoproteins were not due to differences in the processing of VLDL by cells cultured in the presence or absence of insulin. Therefore, it appears that insulin may inhibit the secretion of VLDL perhaps by reducing the intracellular association of lipids and apoproteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bell-Quint J., Forte T., Graham P. Synthesis of two forms of apolipoprotein B by cultured rat hepatocytes. Biochem Biophys Res Commun. 1981 Mar 31;99(2):700–706. doi: 10.1016/0006-291x(81)91800-3. [DOI] [PubMed] [Google Scholar]
- Bernaert D., Wanson J. C., Drochmans P., Popowski A. Effect of insulin on ultrastructure and glycogenesis in primary cultures of adult rat hepatocytes. J Cell Biol. 1977 Sep;74(3):878–900. doi: 10.1083/jcb.74.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M. Primary hepatocyte culture: substratum requirements and production of matrix components. Fed Proc. 1981 Aug;40(10):2469–2473. [PubMed] [Google Scholar]
- Blue M. L., Protter A. A., Williams D. L. Biosynthesis of apolipoprotein B in rooster kidney, intestine, and liver. J Biol Chem. 1980 Nov 10;255(21):10048–10051. [PubMed] [Google Scholar]
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Engelhorn S. C., Pangburn S. H., Weinstein D. B., Steinberg D. Very low density lipoprotein synthesis and secretion by cultured rat hepatocytes. J Biol Chem. 1979 Mar 25;254(6):2010–2016. [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovson J., Huang Y. O., Baker N., Kannan R. Apolipoprotein B is structurally and metabolically heterogeneous in the rat. Proc Natl Acad Sci U S A. 1981 Jan;78(1):157–161. doi: 10.1073/pnas.78.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fainaru M., Havel R. J., Imaizumi K. Radioimmunoassay of arginine-rich apolipoprotein of rat serum. Biochim Biophys Acta. 1977 Jan 25;490(1):144–155. doi: 10.1016/0005-2795(77)90114-3. [DOI] [PubMed] [Google Scholar]
- Felker T. E., Fainaru M., Hamilton R. L., Havel R. J. Secretion of the arginine-rich and A-I apolipoproteins by the isolated perfused rat liver. J Lipid Res. 1977 Jul;18(4):465–473. [PubMed] [Google Scholar]
- Foster L. B., Hochholzer J. M. A single-reagent manual method for directly determining urea nitrogen in serum. Clin Chem. 1971 Sep;17(9):921–925. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L., Berry M. N., Williams M. C., Severinghaus E. M. A simple and inexpensive membrane "lung" for small organ perfusion. J Lipid Res. 1974 Mar;15(2):182–186. [PubMed] [Google Scholar]
- Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J Clin Invest. 1976 Sep;58(3):667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayford J. T., Danney M. M., Thompson R. G. Triglyceride-integrated concentration: relationship to insulin-integrated concentration. Metabolism. 1979 Nov;28(11):1078–1085. doi: 10.1016/0026-0495(79)90145-8. [DOI] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Dishmon G., Fried M. Lipoprotein lipid transport by livers from normal and CCl-4-poisoned animals. Am J Physiol. 1965 Nov;209(5):1053–1060. doi: 10.1152/ajplegacy.1965.209.5.1053. [DOI] [PubMed] [Google Scholar]
- Johansson S., Kjellén L., Hök M., Timpl R. Substrate adhesion of rat hepatocytes: a comparison of laminin and fibronectin as attachment proteins. J Cell Biol. 1981 Jul;90(1):260–264. doi: 10.1083/jcb.90.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. P., Arky R. A. Effects of insulin on triglyceride and free fatty acid metabolism in man. Metabolism. 1965 Dec;14(12):1287–1293. doi: 10.1016/s0026-0495(65)80010-5. [DOI] [PubMed] [Google Scholar]
- KAY R. E., ENTENMAN C. The synthesis of "chylomicronlike" bodies and maintenance of normal blood sugar levels by the isolated, perfused rat liver. J Biol Chem. 1961 Apr;236:1006–1012. [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Lin-Lee Y. C., Tanaka Y., Lin C. T., Chan L. Effects of an atherogenic diet on apolipoprotein E biosynthesis in the rat. Biochemistry. 1981 Oct 27;20(22):6474–6480. doi: 10.1021/bi00525a028. [DOI] [PubMed] [Google Scholar]
- MCFARLANE A. S. Labelling of plasma proteins with radioactive iodine. Biochem J. 1956 Jan;62(1):135–143. doi: 10.1042/bj0620135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER L. L., BLY C. G., WATSON M. L., BALE W. F. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J Exp Med. 1951 Nov;94(5):431–453. doi: 10.1084/jem.94.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Holcombe K. S. Alterations of the plasma lipoproteins and apoproteins following cholesterol feeding in the rat. J Lipid Res. 1977 May;18(3):314–324. [PubMed] [Google Scholar]
- Marsh J. B. Apoproteins of the lipoproteins in a nonrecirculating perfusate of rat liver. J Lipid Res. 1976 Jan;17(1):85–89. [PubMed] [Google Scholar]
- Morrison M., Bayse G. S., Webster R. G. Use of lactoperoxidase catalyzed iodination in immunochemical studies. Immunochemistry. 1971 Mar;8(3):289–297. doi: 10.1016/0019-2791(71)90484-8. [DOI] [PubMed] [Google Scholar]
- Nakaya N., Chung B. H., Patsch J. R., Taunton O. D. Synthesis and release of low density lipoproteins by the isolated perfused pig liver. J Biol Chem. 1977 Nov 10;252(21):7530–7533. [PubMed] [Google Scholar]
- Olefsky J. M., Farquhar J. W., Reaven G. M. Reappraisal of the role of insulin in hypertriglyceridemia. Am J Med. 1974 Oct;57(4):551–560. doi: 10.1016/0002-9343(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Patsch J. R., Sailer S., Kostner G., Sandhofer F., Holasek A., Braunsteiner H. Separation of the main lipoprotein density classes from human plasma by rate-zonal ultracentrifugation. J Lipid Res. 1974 Jul;15(4):356–366. [PubMed] [Google Scholar]
- Patsch W., Kim K., Wiest W., Schonfeld G. Effects of sex hormones on rat lipoproteins. Endocrinology. 1980 Oct;107(4):1085–1094. doi: 10.1210/endo-107-4-1085. [DOI] [PubMed] [Google Scholar]
- Patsch W., Patsch J. R., Kostner G. M., Sailer S., Braunsteiner H. Isolation of subfractions of human very low density lipoproteins by zonal ultracentrifugation. J Biol Chem. 1978 Jul 25;253(14):4911–4915. [PubMed] [Google Scholar]
- Patsch W., Schonfeld G., Gotto A. M., Jr, Patsch J. R. Characterization of human high density lipoproteins by zonal ultracentrifugation. J Biol Chem. 1980 Apr 10;255(7):3178–3185. [PubMed] [Google Scholar]
- Patsch W., Schonfeld G. The degree of sialylation of ApoC-III is altered by diet. Diabetes. 1981 Jun;30(6):530–534. doi: 10.2337/diab.30.6.530. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Bell E., Alpers D. H. Intestinal apoproteins during fat absorption. J Clin Invest. 1978 Jun;61(6):1539–1550. doi: 10.1172/JCI109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld G., Grimme N., Alpers D. Detection of apolipoprotein C in human and rat enterocytes. J Cell Biol. 1980 Aug;86(2):562–567. doi: 10.1083/jcb.86.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld G., Patsch W., Pfleger B., Witztum J. L., Weidman S. W. Lipolysis produces changes in the immunoreactivity and cell reactivity of very low density lipoproteins. J Clin Invest. 1979 Nov;64(5):1288–1297. doi: 10.1172/JCI109584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne F. A., Quarfordt S. H. The interaction of heparin with an apoprotein of human very low density lipoprotein. J Clin Invest. 1977 Oct;60(4):944–950. doi: 10.1172/JCI108849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutar A. K., Myant N. B., Thompson G. R. Metabolism of apolipoprotein B-containing lipoproteins in familial hypercholesterolaemia: effects of plasma exchange. Atherosclerosis. 1979 Mar;32(3):315–325. doi: 10.1016/0021-9150(79)90175-8. [DOI] [PubMed] [Google Scholar]
- Stajner A., Sůva J. The determination of nonesterified fatty acids in blood serum using a stable cupric reagent. J Clin Chem Clin Biochem. 1977 Sep;15(9):513–514. doi: 10.1515/cclm.1977.15.1-12.513. [DOI] [PubMed] [Google Scholar]
- Tarlow D. M., Watkins P. A., Reed R. E., Miller R. S., Zwergel E. E., Lane M. D. Lipogenesis and the synthesis and secretion of very low density lipoprotein by avian liver cells in nonproliferating monolayer culture. Hormonal effects. J Cell Biol. 1977 May;73(2):332–353. doi: 10.1083/jcb.73.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972 Jan;126(2):295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch B. R., Dyal K., Fraser T. R. Increased lipid synthesis by liver slice in a superfusion system following raised glucose or insulin concentration. Diabetologia. 1972 Aug;8(4):267–273. doi: 10.1007/BF01225570. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whiting M. J., Edwards A. M. Measurement of cholic acid synthesis and secretion by isolated rat hepatocytes. J Lipid Res. 1979 Sep;20(7):914–918. [PubMed] [Google Scholar]
- Windmueller H. G., Levy R. I. Production of beta-lipoprotein by intestine in the rat. J Biol Chem. 1968 Sep 25;243(18):4878–4884. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Perfusion in situ with tritium oxide to measure hepatic lipogenesis and lipid secretion. Normal and orotic acid-fed rats. J Biol Chem. 1966 Jun 25;241(12):2891–2899. [PubMed] [Google Scholar]
- Witztum J. L., Schonfeld G. Carbohydrate diet-induced changes in very low density lipoprotein composition and structure. Diabetes. 1978 Dec;27(12):1215–1229. doi: 10.2337/diab.27.12.1215. [DOI] [PubMed] [Google Scholar]
- Wong L., Rubinstein D. The levels of apolipoprotein-E in hypercholesterolemic rat serum. Can J Biochem. 1978 Mar;56(3):161–166. doi: 10.1139/o78-028. [DOI] [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Relative contributions by liver and intestine to individual plasma apolipoproteins in the rat. J Biol Chem. 1979 Aug 10;254(15):7316–7322. [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Variant forms of plasma apolipoprotein B. Hepatic and intestinal biosynthesis and heterogeneous metabolism in the rat. J Biol Chem. 1981 Apr 25;256(8):3615–3618. [PubMed] [Google Scholar]
- Yedgar S., Weinstein D. B., Patsch W., Schonfeld G., Casanada F. E., Steinberg D. Viscosity of culture medium as a regulator of synthesis and secretion of very low density lipoproteins by cultured hepatocytes. J Biol Chem. 1982 Mar 10;257(5):2188–2192. [PubMed] [Google Scholar]