Abstract
Most lipid components of cell membranes are either neutral, like cholesterol, or zwitterionic, like phosphatidylcholine and sphingomyelin. Very few lipids, such as sphingosine, are cationic at physiological pH. These generally interact only transiently with the lipid bilayer, and their synthetic analogs are often designed to destabilize the membrane for drug or DNA delivery. However, anionic lipids are common in both eukaryotic and prokaryotic cell membranes. The net charge per anionic phospholipid ranges from −1 for the most abundant anionic lipids such has phosphatidylserine, to near −7 for phosphatidylinositol 3,4,5 trisphosphate, although the effective charge depends on many environmental factors. Anionic phospholipids and other negatively charged lipids such as lipopolysaccharides are not randomly distributed in the lipid bilayer, but are highly restricted to specific leaflets of the bilayer and to regions near transmembrane proteins or other organized structures within the plane of the membrane. This review highlights some recent evidence that counterions, in the form of monovalent or divalent metal ions, polyamines, or cationic protein domains, have a large influence of the lateral distribution of anionic lipids within the membrane, and that lateral demixing of anionic lipids has effects on membrane curvature and protein function that are important for biological control.
Biological membrane charge asymmetry
Biological membranes, in particular the plasma membrane of a cell, are composed largely of phospholipids. The primary function of a membrane is to separate what is outside from what is inside. In simple organisms, the plasma membrane is a partition between the complex, and sometimes delicate, molecules required for life from the harsh environment. In the case of a eukaryotic cell embedded in a multicellular organism, the plasma membrane mediates a precise balance of intracellular constituents – proteins, sugars, nucleic acids, and ions – from the extracellular milieu that has vastly different concentrations of these molecules. Cell membranes are much more than diffusion barriers; they catalyze reactions, serve as protein scaffolds, limit the flow of electrically charged solutes, and ultimately control the exchange of information between a cell and what surrounds it [4].
Cells devote up to 5% of their genome to synthesis of the lipids that are the primary ingredients of biological membranes [1]. When aggregated in droplets, lipids serve as energy reservoirs (often in the form of triacylglycerol or TAG) that can be catabolized to generate energy for the cell in the form of ATP. These droplets are also stores of fatty acids and sterols that can be used to generate other membrane components such as cholesterol. Degradation of lipids in the membrane by specialized proteins serves as a starting point for signaling cascades; when a lipid is cleaved in half, information extends across the membrane via the diffusion of hydrophobic lipid fragments and propagates into the cytosol via the diffusion of the polar lipid fragment. Modification of phospholipids that alter their charge, such as by phosphorylation of the head group, can initiate or prevent a cellular signaling cascade, and in one case, the so-called “futile cycle” of repeated phosphorylation and dephosphorylation of phosphoinositides may consume up to 7% of a cell’s energy in the form of ATP [5].
The inner leaflet and the outer leaflet have different compositions
There are hundreds of different lipid species [6] but in eukaryotic cells, the major lipid family is glycerophospholipids, which includes phosphatidylcholine (PtdCho), phosphatidylethanolamine (PtdEtn), phosphatidylserine (PtdSer), phosphatidic acid (PtdA), and phosphatidylinositol (PtdIns) and its phosphorylated derivatives (PtdInsP, PtdInsP2, and PtdInsP3; collectively PPIns). These molecules are typically named by their head group, which is attached to hydrocarbon acyl chains that can be either saturated or unsaturated (one or more double bonds) as shown in Figure 1. Up to 80 % of acyl chains contain a single double bond in mammalian cells and viruses [3].
Figure 1.
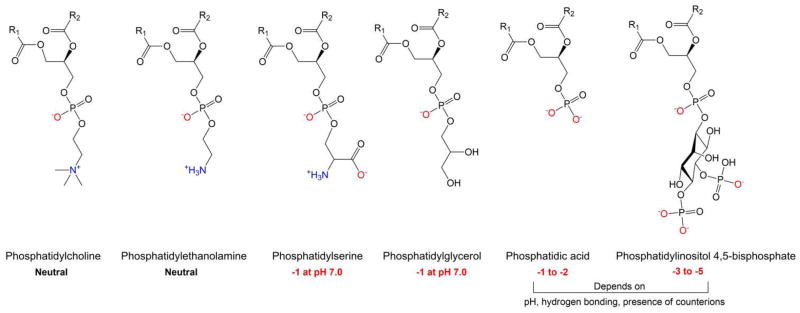
The structure of several common mammalian phospholipids that can be found in the plasma membrane along with their charge ranges under physiological conditions. The length and level of saturation in the hydrocarbon acyl chains, denoted R1 and R2, varies depending on head group, location in the cell, and other factors.
Recent advances in high-throughput mass spectrometry have enabled the determination of total lipid content within a cell. The lipids in different intracellular compartments and on either side of the plasma membrane are not the same; that is, there is an asymmetry in the distribution of lipid species across the two leaflets of the plasma membrane. Further, the ratio of lipid species changes greatly during maturation or after stimulation with growth factors and other cellular agonists [2, 3].
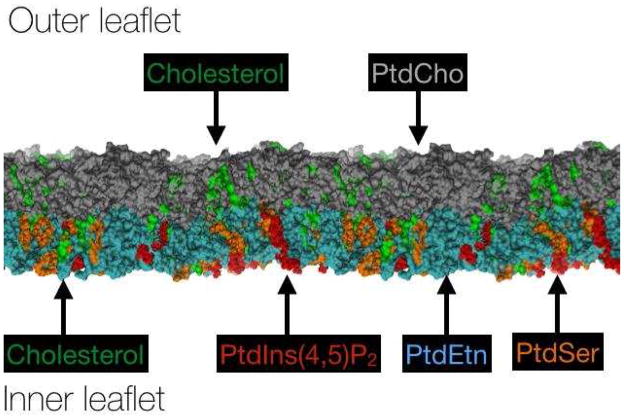
In general, the outer leaflet of the mammalian cell plasma membrane contains neutral and zwitterionic lipids whereas the inner leaflet contains a mix of neutral or zwitterionic lipids and the negatively charged species, PPIns (charges of −3, −4, or −5), PtdA (charge of −1 or −2), and PtdSer (charge of −1). A simplified representation of a eukaryotic cell membrane, demonstrating the distribution and relative amounts of common species is shown in Figure 2. Another negatively charged lipid, cardiolipin (CL; charge −1 or −2), is found on the inner mitochondrial membrane in eukaryotes and in the inner membrane of bacteria. Bilayer asymmetry is established by the trans-Golgi network, which sends lipids to the membrane in the form of vesicles, and then maintained by ATP-dependent proteins, dubbed “flippases” and “floppases” depending on the flip (outward) or flop (inward) direction, which sort and maintain certain lipids in each leaflet of the membrane bilayer [6]. An energy barrier of 80–220 kJ/mol must be surmounted to overcome the penalty of either transiently exposing the buried hydrophobic regions of a phospholipid to water or dehydrating the polar head group during a flip or flop [7]. Although the kinetics of lipid flipping have generally been thought to be extremely slow, on the order of days, more recent measurements suggest that the kinetics might in some cases be considerably faster, and the energy barrier much lower [7].
Figure 2.
A simplified representation of a typical mammalian membrane bilayer composition highlighting common lipid families. Cholesterol is shown on both sides of the bilayer, while the extracellular facing leaflet is shown containing PtdCho (grey); the cytosolic leaflet is shown containing negatively charged PtdSer (orange) and PtdIns(4,5)P2 (red) together with the zwitterionic PtdEtn (cyan). The total amount and ratio of lipid species in various cell types can be found in several sources, including [1–3].
Several candidate proteins with specific affinity for various lipid types have been suggested. Local production of a certain lipid species, such as by PI5K, a kinase that phosphorylates PtdIns(4)P to PtdIns(4,5)P2, can also result in enrichment of a lipid type in one leaflet. Treatment of red blood cells with extracellular calcium can result in an increase of intracellular calcium and subsequent loss of lipid asymmetry [8]. As many enzymes that modify phospholipids themselves depend on divalent cations, either as co-factors or to stabilize their structure, the relationship between counterions and phospholipids is complex.
Although the zwitterions PtdCho and PtdEtn are the most abundant phospholipids from yeast to mammals, the acidic phospholipids PtdSer, PPIns, PtdA, and sterols are a significant fraction of membrane bilayers, especially in the plasma membrane and Golgi apparatus [9]. Another acidic phospholipid, phosphatidylglycerol (PtdGly) that carries a charge of −1 due to a phosphodiester bond is abundant in bacteria, but generally scarce in mammalian cell membranes. However it can be found in extracellular pulmonary surfactant, where it aids in blocking viral attachment to host cells and can bind to Toll-like receptor 4, disrupting the interactions of lipopolysaccharides and other integral membrane proteins [10]. PtdGly is also found in plant cells, especially in the thylakoid membrane, and the amount of saturation in the acyl chains decreases with the plant’s resistance to chilling [11]. Cardiolipin (CL) is a dimer of two PtdGly lipids, linked through their glycerol headgroup moiety, and thus has four acyl chains. Increasing the level of CL in liposomal membranes increases the membrane-fluidizing effect of local anesthetics, with CL having a greater effect than PtdA, PtdGly, or PtdSer [12].
Just as the phospholipids in the plasma membrane are not evenly distributed in each leaflet, the phospholipids are not symmetrically distributed across the membrane of mitochondria [9]. Until recently, the membranes of bacteria were assumed to be a homogenous mixture of phospholipids without any clear domains or phase boundaries. However, the uneven distribution of lipid dyes, such as those conjugated to CL, suggest that the spatial arrangement of phospholipids in bacterial membranes is not random [13]. While the outer leaflet of the eukaryotic plasma membrane is actively maintained near charge neutrality, the outer leaflet of many bacterial membranes is negatively charged [14].
The fact that only the inner leaflet of eukaryotic cells usually contains the acidic phospholipids under physiological conditions results in a significant negative surface charge density. Mobile counterions in the cytoplasm are drawn to the membrane and interact with the negative lipids, sometimes forming a bond, reducing the overall electrostatic potential [15]. Non-specific, electrostatically driven adsorption of counterions to the charged lipid surface (forming the Stern layer) in some cases in combination with specific binding of multicationic solutes to unique anionic phospholipid species, can lead to significant changes in membrane structure including changes in membrane curvature and surface patterning. The concentration of Ca2+ in the Stern layer near the membrane can be more than ten times the bulk concentration in the cytosol [16], and there is likely competition between Ca2+ and other physiological divalent cations, such as Mg2+ [17]. The interplay among ions may result in competition for specific binding to anionic phospholipids that carry a valence of more than −2 or simple electrostatic attraction, usually to anionic phospholipids with a valence of −1. However, important differences remain between the effects of Ca2+ and Mg2+ and the concentration of counterions near a highly charged anionic membrane depends on both the valence and hydrated diameter of the cation [18, 19].
What drives patterning?
Lipid monolayers and membranes can form domains with divalent counterions
Just as acidic lipids are non-randomly distributed across the two leaflets of the bilayer, they are also increasingly recognized to be non-randomly distributed within the plane of the bilayer, especially at the interface of the plasma membrane and the cytosol. Formation of acidic lipid domains large enough to visualize by light microscopy that are stabilized by multivalent counterions have long been demonstrated using purified systems in vitro [20]. An important early fluorescence microscopy study showed that addition of Ca2+ but not Mg2+ to vesicles containing fluorescently labeled PtdSer or PtdA but not zwitterionic phospholipids lead to demixing of the acidic lipids within the membrane, that incorporation of cholesterol into the vesicle lowered the concentration of Ca2+ required for domain formation, and that similar domains were observed when fluorescent lipids were added to red cell ghost membranes [20]. Whether such domains appear or have functions in live cells has been more difficult to resolve in part because of difficulties in detecting sub-micron domains that could appear locally on a sub-cellular scale and on issues related to cholesterol-dependent lipid mixing (raft formation) in vivo. Recently, several lines of evidence strongly suggest that clusters or domains of acidic lipids form in cell membranes, that these domains are affected by fluxes in divalent metal ion or multivalent cation levels, and that they might act as transient platforms for signal transduction, cytoskeletal assembly, and vesicle trafficking. The most highly charged phospholipids, polyphosphoinositides (PPIns), are especially implicated in dynamic cell membrane domain formation.
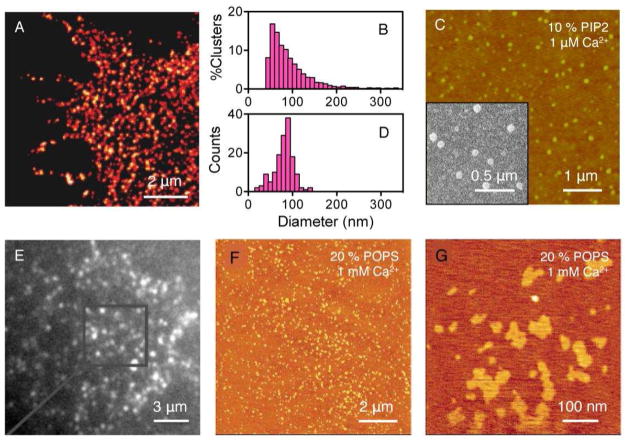
Recent studies show the formation of nanoscale PtdInsP2 clusters during critical PtdInsP2 -triggered cellular functions [21, 22]. Clustering of the SNAP receptor protein syntaxin-1A on the plasma membrane [23], which enables neuronal exocytosis, requires formation of lipid domains. Stimulated emission depletion (STED) microscopy revealed that PtdInsP2 in the plasma membrane of a PC12 cell forms nanoclusters with a relatively narrow size distribution centered at 73 nm in diameter (Figure 3 A&B) and a peak surface coverage of ~80% PtdInsP2 in these clusters. Similar size distributions of PtdInsP2 nanoclusters in a plasma membrane have also been reported using the same cell line but different imaging techniques [24]. Strikingly, PtdInsP2 clusters found in plasma membranes closely resemble the structures formed by purified PtdInsP2 and Ca2+ in purified mixed lipid monolayers (Figure 3 C&D) [22]. The cluster formation in either plasma or model membranes is not limited to PtdInsP2. PtdInsP3 is also found to present as clusters in an unroofed cell membrane when stained with mCherry-fused PH-GRP1 domains (Figure 3 E) [25]. Furthermore, Ca2+-induced formation of lipid clusters with similar sizes as those reported in Figure 3 A&C are found in supported lipid bilayers doped with 20 mol% PtdSer (POPS) (Figure 3 F&G) [26]. These results suggest that Ca2+-induced PtdInsP2 cluster formation is less likely to result from a unique binding of Ca2+ and PtdInsP2 than from a more general electrostatic interaction between soluble multivalent counterions and charged lipids within a membrane.
Figure 3.
The formation of PtdInsP2 clusters in both cell and model membranes. (A) Nanoscale-resolution STED image of a membrane sheet of PC12 cells immunostained with a monoclonal PtdInsP2 antibody and a secondary antibody labeled with Alexa Fluor 488. (C) The formation of PtdInsP2 clusters in supported lipid monolayers containing 10 mol% PtdInsP2 in background PtdCho (DOPC) at the presence of 1 μM Ca2+. Buffer: 10 mM HEPES, 5 mM DTT, pH 7.4 at room temperature. (B) & (D) The corresponding size distribution of PtdInsP2-rich clusters from images (A) & (C), respectively. (E) Fluorescence micrographs of a PC12 membrane sheet stained with PH-GRP1 genetically fused to mCherry which shows the clustering of PtdInsP3 in the plasma membrane. (F) SFM image of a PtdCho/PtdSer (POPC/POPS) (4:1) supported lipid bilayer prepared by vesicle spreading onto a mica surface. (G) Magnification of POPS-enriched microdomains. Image (A) and (B) are reprinted by permission from Macmillan Publishers Ltd: Nature 2011, 479, 552–555, copyright 2011. Image (C) and (D) are reprinted with permission from Journal of the American Chemical Society, 2012, 134, 3387–3395. Copyright 2012 American Chemical Society. Image (E) is reprinted with permission from Neuron 2013, 77, 1097–1108. Copyright 2013 Elsevier. Image (F) and (G) are adapted with permission from Biochemistry 2005, 44, 15296–15303. Copyright 2005 American Chemical Society.
The mechanisms of multivalent cation-induced perturbations in PtdInsP2 lateral structure depend on the lateral organization of PtdIndP2 in the absence of multivalent cations or proteins. The lateral organization of PtdInsP2 in model membranes in the absence of both multivalent cations and proteins has been proposed to depend on a balance of electrostatic repulsions and hydrogen bond attractions between highly charged PtdInsP2 head groups mediated by intervening water molecules [27]. The hypothesis that hydrogen bond networking plays a role in PtdInsP2 lateral structure is primarily supported by the fact that the phase transition temperature of PtdInsP2 is different from that of PtdCho (DPPC) determined by infrared spectroscopy from a binary lipid mixture, and such differences diminish as pH decreases [28, 29]. However, hydrogen bond networking by itself does not seem enough to trigger PtdInsP2 nanocluster formation. The absence of PtdInsP2 clusters in a binary fluidic PtdCho-containing bilayer in the absence of multivalent cations is inferred from a spectroscopic study since neither changes in fluorescence intensity nor anisotropy of NBD-PtdInsP2 due to clustering was observed in the pH range tested [30]. This conclusion is consistent with the results of a grazing incident X-ray scattering study in which PtdInsP2 was found to distribute homogeneously in PtdCho (DOPC)-containing bilayers unless the relative humidity was decreased below 90% [31].
The idea that divalent cations perturb the lateral structure of anionic lipids in the membrane was recognized in early observations that Ca2+ induces the phase segregation of PtdSer as detected by differential scanning calorimetry (DSC) [32] or electron spin resonance (ESR) [33]. A divalent cation-induced morphology change of PtdInsP2 was initially reported in micelles as revealed by light and electron microscopy [34]. The morphology of purified PtdInsP2 in aqueous suspension changes from 6 nm-diameter spherical micelles into striated fibrils composed of stacks of discoid micelles [34]. The differences among Mg2+, Ca2+, and Ba2+ in inducing PtdInsP2 aggregation are reflected in the diameter of the filaments, which are 19, 12 and 10 nm, respectively, and are correlated with the hydrated radii of the cations [35, 36]. More detailed small angle X-ray scattering suggest that Ca2+ induces structural changes of PtdInsP2 micelles from prolate micelles to disordered lamellae at Ca2+/PtdInsP2 = 0.70 and at pH 7.2. Such a phase transition can also be induced by Mg2+ at a higher concentration, but the transition is not as obvious [37]. PtdInsP2 clusters can also form in supported lipid bilayers containing PtdCho, at low pH (4.8) where the net charge of PtdInsP2 is −3, depending on buffer conditions with consequences for binding proteins [38].
In PtdSer membranes that also contain lecithin (PtdCho), the addition of as little as 1 mM Ca2+ (in 100 mM KCl buffer) induces broadening of the electron spin resonance (ESR) peaks, indicating that Ca2+ is capable of rapidly binding to PtdSer phospholipids and separating it from the lecithin molecules [39]. These effects, as the ones with PtdInsP2, are similar with Ba2+ or Sr2+, but not Mg2+; high concentrations of Mg2+ (> 50 mM) were found instead to increase the order of the membranes [40].
Ca2+-induced PtdInsP2 cluster formation in a lipid bilayer has been shown using giant unilamellar vesicles (GUVs) (Figure 4 A&B) [41]. This study suggests that Ca2+ and Mg2+ cluster PtdInsP2 at a concentration >25 μM for Ca2+ and >300 μM for Mg2+ and rupture vesicles at a concentration >300 μM for Ca2+ and >1 mM for Mg2+. PtdInsP2 demixing and PtdInsP2 clusters in GUVs have also been observed using Laurdan generalized polarization, where extremely small clusters (tens of molecules) have been inferred from the data [42]. Ca2+-induced PtdInsP2 clustering was also studied in a Langmuir lipid monolayer doped with fluorescent PtdInsP2 analogs by fluorescence microscopy [43]. The monolayer studies revealed that the formation of Ca2+-induced clusters coincides with a drop in surface pressure, and adding excess EDTA reverses both events. The formation and size distribution of Ca2+-induced PtdInsP2 clusters were investigated by transferring the monolayer onto coverslips so it could be examined more closely with fluorescence, atomic force, and electron microscopy. This approach was applied in investigating the phase diagram of Ca2+-induced PtdInsP2 cluster formation at various PtdInsP2 mole fractions and pH values [44].
Figure 4.
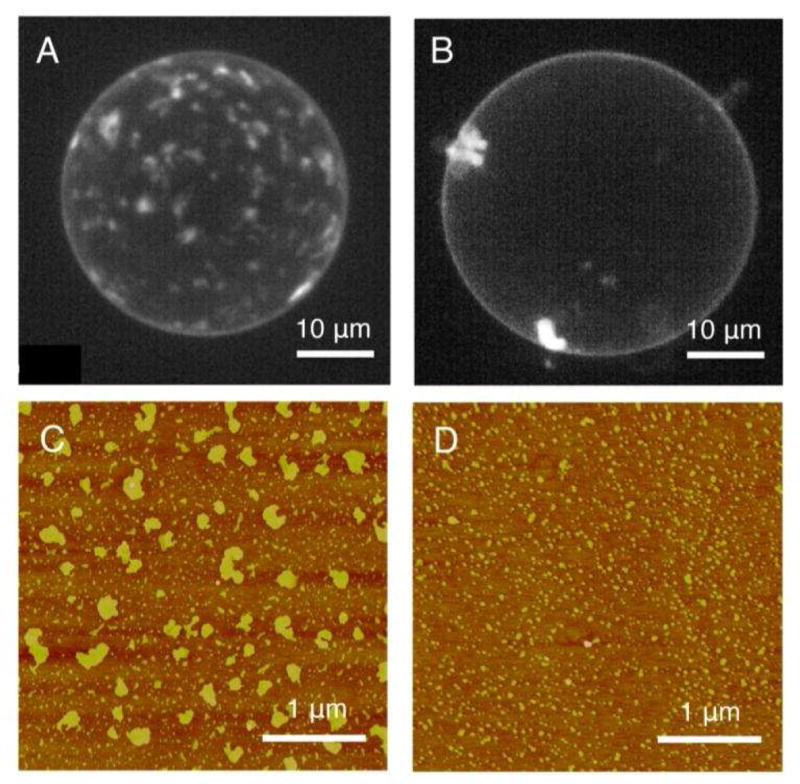
Ca2+ and Mg2+ induced cluster formation of PtdInsP2 in model membranes. Projections of a stack of images taken by confocal microscopy at different height of GUVs in the glucose buffer (A) with 90 μM Ca2+ ; (B) with 90 μM Mg2+. Lipid composition of the vesicle: DOPC/Chol/PtdInsP2/FL-PtdInsP2 = 80/15/4.9/0.1. AFM micrographs of divalent cation-induced PtdInsP2 cluster formation in supported lipid monolayers in the presence of (C) 1mM Ca2+ and (D) 1mM Mg2+ at a low ionic strength buffered at pH 7.4. Lipid composition of the monolayer: PtdInsP2/DOPC = 50/50. Image (A) and (B) are adapted with permission from Biophysical Journal, 2008, 95(9), 4348–4360, Copyright 2008 Elsevier. Image (C) and (D) are reprinted with permission from Journal of the American Chemical Society, 2012, 134, 3387–3395. Copyright 2012 American Chemical Society.
Replacing Ca2+ by Mg2+ changes the boundary of the phase diagram so that clusters form only at higher pH values and the clusters are much smaller in size. Representative atomic force microscope (AFM) images of Ca2+- and Mg2+-induced PtdInsP2 cluster formation are shown in Figure 4 C&D [17]. This observation is rationalized by a weaker electrostatic interaction due to a larger cationic size of Mg2+ compared to that of Ca2+. However, the hydrated shell of Mg2+ is only 4–15% larger compared to that of Ca2+ [35, 36, 45], suggesting that factors other than ionic diameter are responsible for the difference between interactions of Mg2+ and Ca2+ with PtdInsP2. Calorimetric studies show that the mixing enthalpy of Ca2+ with an aqueous PtdInsP2 dispersion is strongly endothermic, suggesting a Ca2+-induced dehydration of PtdInsP2 [46]. The endothermic change is attributed to the exclusion of water that penetrates deeply into the hydrophobic spaces between PtdInsP2 as a result of the neutralization effect of Ca2+ and therefore the decreased area per PtdInsP2 molecule [47]. The hydration level at the lipid-water interface in the presence of divalent cations has also been studied by attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) [17]. This result suggests that the loss of water also comes from the hydration shell of Ca2+ on binding to PtdInsP2 while Mg2+ remains hydrated. This proposed mechanism was further validated by MD simulations [48] and provides a feasible explanation for the weaker membrane condensation induced by Mg2+ compared to that induced by Ca2+. Similar differences in inducing PtdInsP2 cluster formation between Ca2+ and Mg2+ were reported in large unilamellar vesicles (LUVs). PtdInsP2 cluster formation in a lipid bilayer has also been studied by a steady-state spectroscopic method in which the degree of cluster formation was quantified by Förster resonance energy transfer (FRET), and the trend in inducing PtdInsP2 clustering in a bilayer membrane follows the order: Ca2+ ≫ Mg2+ > Zn2+.
Like PtdInsP2, PtdA has a strong response to Ca2+. In monolayers of PtdA, the addition of Ca2+ causes a drop in surface pressure [49], and the surface pressure is increased with increasing ionization of the phospholipid [50]. The charge of PtdA ranges from neutral to −2 as a function of pH, hydrogen bonds, the presence of divalent ions, and electrostatic correlation effects [24, 51], in the same manner that the charge of PtdInsP2 changes based on the same factors [52–54]. In mixed membranes containing PtdA and PtdCho, the addition of 10 mM Ca2+ caused ESR peak broadening [55], similar to the effect of Ca2+ on membranes containing PtdSer mixed with lecithin [39]. Inside the clusters, the average area per PtdA phospholipid is reduced and the acyl chains show increased ordering [55]. If the pH is lower than 5.5, where the net charge of PtdA is less negative, the ability of Ca2+ to induce phase segregation is decreased [39]. Moreover, the charge of PtdA has direct applications in cells; PtdA is capable of acting as a pH biosensor by altering the binding of a transcription factor, depending on head group protonation, and thus intracellular pH near the membrane [56, 57].
Polyamines such as spermine are the most abundant polyvalent cations in the cytoplasm of most cells, and their interactions with PtdInsP2 have been extensively studied [58–64]. In FRET studies it was shown that divalent metal ions have greater capacity to cluster PtdInsP2 than do polyamines with higher cationic charge, in contrast to the expectation if binding of counterions was purely electrostatic. The differences between polyamines and divalent metal ions are also reflected in their surface pressure effects on addition to PtdInsP2-containing monolayers. Polyamines with charges from +2 to +4 expand, rather than condense the membrane, in a charge-dependent manner [17].
Two fundamental differences between organic multivalent polyamines and inorganic divalent metal ions are thought to account for their difference when interacting with PtdInsP2: packing geometry and hydration energy. The positive charges of polyamines are distributed at fixed lengths along a conformationally flexible carbon chain [65], which might permit binding to linearly spaced phospholipids in a different geometry than the same number of individual Ca2+ or Mg2+ ions [44]. Another difference between organic amines and divalent metal ions is in their extent of hydration. 1H-NMR and other studies suggest that organic amines are less hydrophilic than divalent metal ions [66], in agreement with the finding that NH4+ has a much lower dehydration energy compared to that of Ca2+ [67].
The antagonistic effects of Ca2+ and polyamines when interacting with anionic membranes are evident in several distinct studies. For instance, Ca2+ promotes transmembrane lipid scrambling [68], which is antagonized by the addition of spermine, presumably by its interaction with PtdInsP2 that prevents the formation of Ca2+-PtdInsP2 complexes [62]. Other examples from the literature show that resealed erythrocyte ghosts are stabilized by polyamines in a concentration-dependent manner, which is reflected in an increased shear modulus [69] and an increased osmolysis barrier [70] of the membrane; in contrast, Ca2+ and Mg2+ at high concentration (millimolar), destabilize the membrane. The effects of polyamines and divalent metal ions on anionic membranes are not always antagonistic. The rate of Ca2+-induced vesicle fusion is significantly increased when the vesicles are pre-incubated with polyamines, although polyamines by themselves do not promote vesicle fusion in most cases [71, 72]. The complicated cross interaction between Ca2+ and polyamines on anionic membranes appears to warrant more investigation.
Charged block copolymers phase segregate in the presence of divalent counterions
Divalent ion-dependent domain formation of polyvalent anionic amphiphiles is not limited to phospholipids. Amphipathic block copolymers can under appropriate conditions also form bilayer vesicles called polymersomes [73–75], and when a polyanionic block copolymer such as poly(acrylic acid) poly(butadiene) (PAA-PBD) is mixed with a neutral copolymer like poly(ethylene oxide) poly(butadiene) (PEO-PBD) that has a similar hydrophobic block, the vesicles undergo reversible formation of domains when divalent metal ions such as Ca2+ or Cu2+ are added [76], as shown in Figure 5. The effects of divalent cations to cluster the charged polymers within the bilayer of the polymersomes are rapid and faster than the effects of raising pH to increase the net charge on the anionic copolymer, which presumably increases electrostatic repulsions that are not compensated by the divalent counterions and leading to fingering and destabilization of the polymer domains [77].
Figure 5.
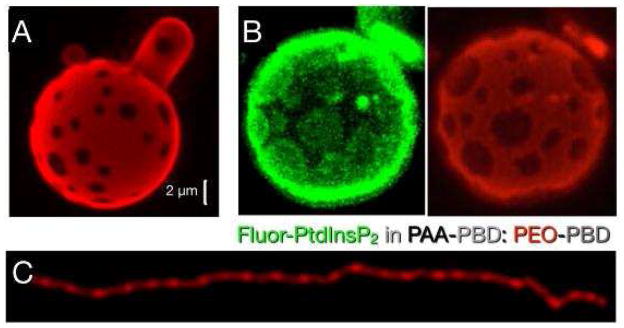
(A) The formation of domains in a GUV containing a negatively charged copolymer, poly(acrylic acid) poly(butadiene) (PAA-PBD), mixed with a neutral copolymer, poly(ethylene oxide) poly(butadiene) (PEO-PBD), after the addition of divalent metal ions. (B) Fluorescence images of a negatively phospholipid, PtdInsP2 (green domains), demixing with PAA-PDB from PEO-PDB (red background). (C) In rod-like micelles, the demixing happens in stripes that do not merge or coarsen, possibly due to the entropic cost of reducing the free volume of the counterions. Panels (A) through (C) are reprinted by permission from Macmillan Publishers Ltd: Nature Materials 2009, 8, 843–849, copyright 2009.
Remarkably, fluorescent anionic phospholipids such as BODIPY-FL PtdIns(4,5)P2 (molecular mass, Mw = 1514 g/mol) can also be incorporated into such polymersomes, and when Ca2+ is added, they demix together with the anionic block copolymer; (Mw = 10,050) [76] despite the large difference in size, as shown in Figure 5B
When anionic and neutral block polymers with a different balance of hydrophobic to hydrophilic block are mixed to form rodlike micelles, addition of Ca2+ can cause the charged blocks to demix into stripes (Figure 5C). Thermodynamic arguments based on the phase diagram describing the conditions when spots or stripes form suggest that the stability of the demixed state depends on a balance between the entropy of the counterions and the energy of electrostatic cross bridging [76, 77].
Antibacterial peptides can cluster anionic phospholipids
The idea that counterion-induced clustering of acidic lipids in mixed lipid bilayers has biological consequences has long been suggested by a variety of experiments. For example one early study showed that the ion transport rate through single gramicidin A channels within black lipid bilayers containing anionic phosphatidic acid and neutral lecithin (PtdCho) depended very strongly on the concentration of Ca2+ in a 1 to 10 μM range relevant to the biological context [78]. It is beyond the scope of this short review to discuss the vast literature reporting effects of multivalent cations on membranes containing anionic lipids, where lipid clustering effects are difficult to separate from specific ion binding effects, but one biologic problem in which consideration of counterion-driven demixing of lipids might have direct relevance to biologic function is the mechanism by which antimicrobial agents kill bacteria.
Antimicrobial peptides, the polycationic amphipathic peptides that are essential elements in innate immunity can form lateral domains with their anionic lipid targets [79–85], and this lipid clustering appears to be important for their toxic effects on prokaryotes. Antimicrobial agents such as cathelicidins or beta-defensins in mammals or squalamine and other cationic steroids in sharks and other organisms, are initially attracted to the negatively charged surfaces formed by lipopolysaccharides or lipoteichoic acid on the bacterial outer wall, but to kill their targets, antimicrobial agents must also breach the inner bacterial membrane, which is largely composed of phospholipids. For example, the smallest antibacterial fragment of the human cathelicidin peptide LL37 (KR-12) has the sequence KRIVQRIKDFLR [86] in which half of the residues are positive, with 1 negative charge and multiple hydrophobic residues. Unlike most full-length antimicrobial agents, which have a broad spectrum of activity against many bacterial strains, KR-12 is highly toxic to E. coli but not to S. aureus. A calorimetric and NMR study showed that KR-12 induces domains of anionic lipids to form in mixed lipid membranes. Even smaller peptides with 3 net positive charges can form clusters of the anionic lipid PtdGly in mixed membranes containing PtdEtn [82]. Clustering of anionic lipids is reported to be important for antibacterial function of numerous antimicrobial agents [79, 80]. The differential effect of KR-12 on E. coli and S. aureus is likely to be related to the fact that E. coli membrane contains both anionic and zwitterionic phospholipids, whereas the membrane of S. aureus is formed mainly by anionic lipids, and therefore KR-12 does not induce domains in it. Cationic antimicrobial factors also do not injure host eukaryotic cells at the concentrations at which they lyse bacteria, because the outer surface of eukaryotic cell membranes is conspicuously lacking anionic lipids and is largely composed of the zwitterions PtdCho and SM. The antimicrobial peptide arenicin-1 also causes ordering of acidic but not zwitterionic lipids in lipid monolayers, as detected by calorimetry, X-ray scattering and IR spectroscopy [85]. Anionic lipid clustering is not a universal feature of antimicrobial peptides, and a commonly studied antimicrobial peptide magainin does not cluster acidic lipids, possibly because of its lower positive surface charge density [83], but this peptide also penetrates into the hydrophobic membrane core [87]. A general mechanism for the membrane destabilizing effect of antimicrobial factors has not been unambiguously determined, and it is likely that multiple mechanisms exist. However, the general need to interact with multiple anionic lipids and the resulting lateral aggregation and induction of pores or local regions of high curvature [88] are physical consequences that are likely to have important biological effects. Electrostatic binding and anionic lipid clustering alone are generally insufficient to produce bacterial killing, and full length antimicrobial agents combine both the electrostatic effect with structures that penetrate into the hydrophobic region of the bilayers to generate curvature. Analysis of many such cationic amphipathic antimicrobial factors suggests that bacterial membrane destabilization is generated best by agents that create negative Gaussian curvature [88] by a combination of electrostatic and hydrophobic effects.
Polycationic protein domains organize anionic phospholipids
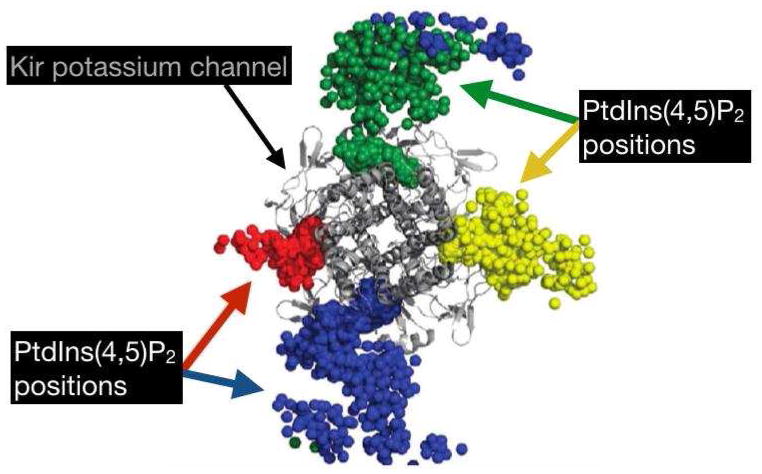
Clustering of acidic phospholipids can also occur due to the presence of proteins in or near the membrane bilayer. One class of proteins that is known to attract negatively charged phospholipids is the inwardly rectifying Kir potassium channels. PtdlnsP2 appears to be able to stabilize the open conformation of all known eukaryotic Kir channels, and form stable contacts with each subunit of the protein tetramer [83] (Figure 6). When PtdA is included in PtdCho membranes, an intermediate form of the nicotinic acetylcholine receptor, between the resting and desensitized state, is stabilized and PtdA is required for the receptor to adopt its native conformation [89]. Several other protein domains are able to bind negatively charged lipids with varying levels of selectivity. On their own, anionic phospholipids do not form structures with negative curvature, as this would cause their like-charged head groups to be packed more tightly, but neutralization by Ca2+ or positively charged peptides reduces or eliminates the energy barrier [14]. However, PtdGly phospholipids experience strong repulsion in low ionic strength liquids and can be seen to form structures with positive curvature that is eliminated in the presence of Na+ [90].
Figure 6.
The position of four individual PtdIns(4,5)P2 phospholipids interacting with a bacterial Kir channel over the course of a 0.5 us simulation, with each sphere of a single color indicating the position of a single PtdIns(4,5)P2 molecule at a given time step. Reprinted (adapted) with permission Stansfeld, P.J., et al., PIP(2)-binding site in Kir channels: definition by multiscale biomolecular simulations. Biochemistry, 2009. 48(46): p. 10926–33. Copyright 2009 American Chemical Society.
The PLCδ PH domain binds to Ptdlns(4,5)P2 with a Kd of 2 μM, effectively clamping the 4- and 5-phosphates with the positively charged amino groups of two lysines acting as counterions [91]. ENTH domains also bind Ptdlns(4,5)P2 using an exposed cluster of lysines that behave as counterions but do not strongly coordinate the phosphate groups of Ptdlns(4,5)P2 [91]. The epsin ENTH domain is specific for Ptdlns(4,5)P2 as confirmed by isothermal titration calorimetry; the affinity for lns(1,4,5)P3 is approximately 3.6 μM [92]. Several epsin ENTH domain are then able to induce curvature, collectively remodeling the membrane curvature [92, 93]. BAR domains are able to curve membrane bilayers if the electrostatic attraction energy is greater than the bending energy of the membrane, which may be about 20 kBT where kB is Boltzmann’s constant [94–96]. Several proteins have PH domains adjacent to BAR domains, and therefore these two methods could work in concert to produce local curvature or lateral inhomogeneity in the plasma membrane of cells [94].
The unstructured cationic peptide KKKKKRFSFKKSFKLSGFSFKKNKK derived from the abundant intracellular Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) is able to selectively bind Ptdlns(4,5)P2 in vesicles containing only 1% of the anionic phospholipid [91], even when another anionic lipid, PtdSer, is also present [97]. Theoretical calculations show that this clustering results in local enrichment of a phospholipid of valence −4 (e.g., Ptdlns(4,5)P2) by about 1000 times the bulk concentration [97]. As with antimicrobial peptides, the binding of the MARCKS peptide to membranes is not exclusively electrostatic because additional hydrophobic insertion of phenylalanine residues into the membrane is also important. A requirement for membrane insertion might be related to the finding that the MARCKS peptide binds anionic phospholipids vesicles of high curvature more strongly than chemically identical vesicles with a larger diameter (diameter of 30 nm versus 400 nm) [98], since highly curved vesicles are likely to have less lipid ordering. A relation between peptide sequences that bind selectively to Ptdlns(4,5)P2 and antimicrobial peptides is suggested by the finding that a peptide derived from the Ptdlns(4,5)P2 binding domain of gelsolin also exhibits antibacterial function equivalent to that of magainin, even though bacteria do not express Ptdlns(4,5)P2 [99].
Electrostatic ordering and membrane curvature at protein/lipid interfaces
In vivo the cell membrane is bent in several ways [108]. A difference in phospholipid composition between opposite leaflets may create local curvature. The degree to which the membrane bends is dependent on the size and charge of the opposing lipid head groups, as well as the degree of saturation of the acyl chains. Membranes can also be bent by forces arising from inside the cell, for example by the polymerization of actin filaments. Integral membrane proteins embedded in the phospholipid bilayer can bend the membrane through one or a combination of distinct methods. Some transmembrane proteins are known the bend the membrane based solely on their shape, acting as a wedge to induce local deformation due to an area asymmetry. Integral membrane proteins can also locally thicken (or thin) the membrane so that the hydrophobic regions of the phospholipids align with hydrophobic sections of the membrane protein. Additionally, some membrane proteins bend the plasma membrane by acting as a scaffold. The scaffolding creates curvature by conforming the phospholipid bilayer to the shape of the membrane protein; one face of these proteins typically carries a positive charge that is electrostatically attracted to the negative surface potential of the plasma membrane.
Energetics of bending anionic membranes
Physical descriptions of membrane elasticity have been used to inform the energetics of cell division and red blood cell shape [109, 110]. On length scales much larger than the thickness of the bilayer, the elastic energy of the membrane can be described by the Canham-Helfrich Hamiltonian [111], an energy functional that describes the elasticity of a fluid membrane according to
where H is the mean curvature of the surface, K is the Gaussian curvature or splay of the surface, and σ is the surface tension of the membrane. κ and κ́ are the bending rigidity and the saddle splay modulus of the membrane respectively. These are material properties of the membrane and vary with membrane composition. In vitro, the bending rigidity has been measured to be on the order of κ = 20 kBT [112] but also highly dependent on membrane composition. The saddle splay modulus is topologically invariant and coupled to shape and size fluctuations of lipid droplets [113] or the stalk energy of budding membranes [111, 114]. The topological invariance of the saddle splay modulus is derived from the Gauss-Bonnet theorem, which states that this energy term has constant magnitude over a constant topology. Its magnitude has been measured in simulations and experiments to be on the same scale as the bending rigidity in a few systems [115–117]. As these theories treat membranes as a two dimensional fluid, there is no bare surface tension. In reality, the surface tension of the cell membrane is proportional to the excess area present in either leaflet, which may vary locally in the cell membrane due to differences in membrane composition or the presence of membrane proteins.
The bending energy of a charged membrane is greater than that for a neutral membrane [118, 119] due to the presence of an electric double layer containing the counterions and then the coions, respectively. The electrostatic contribution to the bending energy depends on the induced osmotic potential, which in turn depends on the Debye length, the ion concentrations (number and valence), and the surface potential of the membrane [118]. The difference in bending energy between a flat, neutral membrane and GUVs containing greater than 15% PtdSer may be up to 10 kBT, measured through micromanipulation [119].
This mesoscale description of membrane elasticity defines a biophysical energetic penalty to deform the membrane on length scales larger than ~ 10 nm. Membrane remodeling processes are known to localize a higher concentration of membrane curvature inducing proteins to overcome this energy barrier for nucleation of buds or similar morphological changes. In the case of clathrin-mediated endocytosis many BAR and ENTH domain proteins are clustered to the region of the growing clathrin coat [120] and charged lipids, in turn, localize to the same loci. This curvature generation, in some combination with the clathrin coat backbone, is thought to overcome this barrier for budding. Several models in the literature have attempted to highlight the role of protein interactions is stabilizing curvilinear morphologies in cellular contexts [121, 122]. In the case of cell migration, the exocyst complex is known to promote nucleation of invadopodia; in particular, high concentrations of exo70 on the inner-surface of the plasma membrane create outward pointing tubules [123].
Modeling electrostatic systems
The average charge density of the inner leaflet of a membrane bilayer composed of 20% negatively charged monovalent lipids is about 1 electronic charge per 300 to 1000 Å2 (a single phospholipid may occupy anywhere from 35 to 100 Å2) [124]. The negative surface charge density produces a negative electrostatic potential that extends immediately adjacent to the membrane, into the cytosol. Mobile counterions in the cytosol are attracted to the negative electrostatic potential and reduce the surface charge density to approximately −30 to −60 mV (relative to bulk, where the electrostatic potential is fixed to be zero) [124], given that the counterions are monovalent and have an average concentration of 100 mM. The concentration of counterions at the surface of the membrane may be an order of magnitude greater than in the bulk phase (similarly, the concentration of coins may be an order of magnitude lower than bulk), and therefore, the local pH at the membrane interface may be 1 pH unit lower than in bulk [124, 125]. The distribution of ions away from the membrane can be predicted based on a number of theories. One popular theory, the Debye-Hückel expression for the electrostatic potential as a function of the distance from the membrane surface, falls off proportional to the exponential Debye length (which takes into account the permittivity, valence, and concentration of the counterions in the bulk phase); this is essentially a version of Coulomb’s law which includes an additional screening term due to the ionic strength of the aqueous phase. Regardless of which approximation is included, all of the theories must effectively balance the attraction due to electrostatics and randomly oriented diffusion, which will smear out the counterion cloud.
As discussed above, the attraction between Ca2+ and PtdSer is largely dominated by the electrostatic potential of the surface [16]. PtdA is also capable of binding to Ca2+ or Mg2+ [49], with Ca2+ exerting a greater effect on PtdA compared to Mg2+ [20, 101–104]. These quantities can be measured through the drop in surface pressure of a phospholipid monolayer at the air/liquid interface upon addition of counterions (generating a Langmuir isotherm). The drop in surface pressure occurs concomitantly with a change in the surface “texture” of the phospholipid film, corresponding to various levels of gelation. As the concentration of Ca2+ in the aqueous phase increases, the gel phase domains increase in size and irregularity; the gel phase domains have excess charge density and repel each other with such energy that they are capable of forming a Wigner lattice (the lowest energy spatial configuration of negatively charged particles in a positive electrostatic potential such that the Coulombic interactions dominate the kinetic energy) [49]. At pH 6, raising the Na+ concentration in the aqueous phase in contact with a PtdA monolayer increases the electrostatic forces, whereas Ca2+ is able to reduce them (Figure 7). This finding is consistent with the observation that Ca2+ is able to induce a strong condensing effect on charged monolayers containing PtdIns(4,5)P2 but Na+ is not and can even weakly increase the area of the monolayers containing PtdA [126] or PtdIns(4,5)P2 [27, 52]. The expanding effect of monovalent counterions is based on their augmentation of acidic lipid deprotonation, which leads to greater electrostatic repulsion in the plane of the membrane that more than compensates the increased screening effect of the counterions [52, 126].
Figure 7.
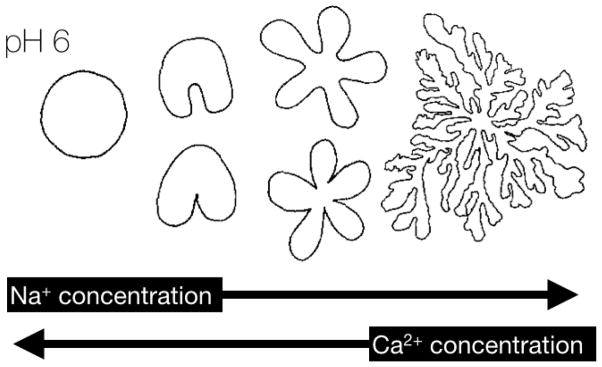
Domain shapes observed in phospholipid monolayers of PtdA. Increasing sodium concentration leads to more “chaotic” or dendritic” patterns whereas increasing calcium concerntration leads to more circular domain patterns. Reprinted (adapted) with permission Lösche, M. and H. Möhwald, Electrostatic interactions in phospholipid membranes: II. Influence of divalent ions on monolayer structure. Journal of Colloid and Interface Science, 1989. 131(1): p. 56–67. Copyright (1989) Elsevier B.V.
All-atom classical MD simulations of PtdSer membranes with either Ca2+ or Mg2+ show penetration of the divalent counterions into approximately the same plane as the PtdSer head group [127]. Both counterions become partially dehydrated, with anionic oxygen atoms from the PtdSer molecules present in the first solvation shell; in the case of Ca2+, three water oxygen atoms are replaced by four PtdSer oxygen atoms [127]. In the most dominant modes of binding to PtdSer, Ca2+ ions tend to be coordinated by both phosphate oxygen atoms and carboxyl oxygen atoms whereas Mg2+ ions are seen to be coordinated by either phosphate oxygen atoms or carboxyl oxygen atoms, but not both [127]. These effects are difficult to capture in theories that only consider purely electrostatic mechanisms of attraction between counterions and charged membranes.
A more detailed theoretical treatment of counterion adsorption can be accomplished by combining a Langmuir isotherm with the Gouy-Chapman theory, which predicts that the surface potential adjacent to a charged membrane falls more sharply than the Debye-Hückel theory for potentials larger than 25 mV [15]. If membrane bilayers contain less than 11% monovalent acidic phospholipids in a cluster, then the −25 mV electrostatic equipotential surface is roughly the shape of a dome centered around the negatively charged lipids and can be modeled accurately using Debye-Hückel theory using twice the total negative charge [88]. However, if the membrane contains between 11% and 25% acidic phospholipids, such as the mammalian plasma membrane, then the −25 mV electrostatic equipotential surface is roughly flat, following along the length of the inner leaflet of the membrane and agrees with Gouy-Chapman theory [88].
These theories have been used to compute the interaction energy between positively charged peptides, such as polylysine, and vesicles containing acidic phospholipids. Assuming the binding is primarily driven by electrostatics, an energy minimum occurs when the peptide is 2.5 Å away from the negatively charged lipids, such that there is a layer of water between the two [128].
It should however be recognized that mean-field theories may not present the complete picture in all scenarios. In a recent study, it was shown that divalent ion interaction with PtdIns(4,5)P2 can be coupled with electronic structure rearrangements leading to proton association/dissociation as well as intra-molecular proton transfer [48] and the chemical and electrostatic environment gets significantly perturbed owing to such rearrangements, which may have a direct bearing on clustering as well as protein recruitment.
The first molecular dynamics (MD) simulation of the interaction between the protein gelsolin and PtdInsP2 revealed that a small group of four PtdInsP2 molecules protruded from the membrane and subsequently were able to form hydrogen bonds with the protein [80]. Further work found that three PtdInsP2 molecules, when set to a charge of −5, were able to form long-lived hydrogen bonds with 13-mer of lysine over a 20 ns simulation, and this did not occur with PtdSer [81]. A 5-mer of lysine corresponding to the first five residues of the MARCKS peptide is capable of forming domains enriched in PtdSer and PtdInsP2 [129]. This effect can be modeled by assuming that domain formation minimizes a free energy that is composed solely of a mixing energy (between zwitterionic or neutral species and acidic phospholipids) and an electrostatic term, based on Gouy-Chapman theory. Mixed PtdCho and PtdSer bilayers were simulated in the presence of varying amounts of Ca2+ and varying concentration of PtdSer [130]. A single Ca2+ is capable of coordinating PtdSer and water in the stoichiometric ratio of 1:2:4, reduce the area per molecule, slightly increase the thickness of the bilayer, and contribute to dehydration of the membrane interface.
To investigate calcium-induced phase separation of PtdInsP2, numerical simulations of charged and neutral spheres were performed [44]. Anionic lipids were represented by negatively charged spheres, while zwitterionic and neutral lipids were represented by uncharged spheres; mobile counterions were modeled by smaller positively charged spheres. While the lipids were confined to a plane, the counterions were free to diffuse in three dimensions. Monovalent ions were not able to form domains or clusters, whereas divalent ions were able to induce cluster formation given the charge of the negative spheres (representing PtdInsP2) was less than or equal to −2. The ability to induce clusters decreases with increasing counterion size, also confirming the experimental finding that magnesium is weaker at forming clusters [17]. A more recent simulation employing quantum-level calculations demonstrates that physiological PtdInsP2 carries a charge of −4 and calcium is able to bind tightly, becoming partially dehydrated [48].
MD simulations have also confirmed that monolayers and bilayers of PtdA strongly interact with the divalent counterion Ba2+, which was used instead of the more physiological divalent counterion Ca2+, to aid in comparison with experimental data [51]. In monolayers of PtdA with dimyristoyl acyl chains (DMPA), Ba2+ counterions penetrate the surface of the membrane, judged relative to the position of the PtdA phosphate group. Each Ba2+ ion can interact with up to four DMPA molecules defined by the presence of an anionic DMPA oxygen atom in the first solvation shell of a Ba2+. Next to the Ba2+ counterions (the Stern layer) is a layer of negative Cl− coions forming a diffuse double layer. This effect is also seen qualitatively in simulations of PtdInsP2 (D.R.S. unpublished work). However, the total positive charge in the Stern layer (from the Ba2+ counterions) adjacent to DMPA molecules carrying a negative charge of −2, exceeds the negative charge of the membrane and thus, the net charge of the interface has become positive (overcharged or charge inversion). This result has been confirmed through X-ray reflectivity of PtdA monolayers in the presence of both divalent and trivalent counterions [100]. The X-ray reflectivity data also suggest, however, that there are more Ca2+ ions than Ba2+ ions per DMPA, owing to the very different electronic structure of the two ions.
Simulations of a coarse-grained model of PtdInsP2 interacting with Kir (see Figure 6) found stable contacts between a single PtdInsP2 residue and each subunit of the tetrameric protein [83]. For PH domains, which contain positively charged arginine and lysine to coordinate the phosphate groups of PtdInsP2 [131], the presence of PtdInsP2 in a PtdCho bilayer with mobile Na+ was demonstrated to increase the number of lipid-protein contacts [132]. A simulation of the N-terminal helices of the BAR (Bin, Amphiphysin, Rvs) domains showed coordination of protruding PtdInsP2 by positively charged amino acids of the protein, even in the presence of another anionic phospholipid, PtdSer.
Conclusions
Anionic phospholipids that carry a net charge that ranges from −1 to up to −5 or more are found on the inner plasma membrane in mammalian cells and in other intracellular membrane compartments. The distribution of anionic phospholipids is nonrandom; while proteins establish an asymmetric distribution of anionic phospholipids across the plasma membrane, physiological counterions in the form of monovalent and divalent metal ions, and polyamines organize the lateral structure of anionic phospholipids. Positively charged protein domains are nonspecifically attracted to regions of increased negative surface charge density and some protein domains specifically coordinate the phospholipid head groups with basic amino acids. These electrostatic effects may lead to the stabilization of small regions in the membrane where the concentration of anionic phospholipids is orders of magnitude higher than the bulk concentration. Models ranging from continuum mechanics to atomistic molecular dynamics simulations have been able to capture aspects of this process across a huge range of length and time scales, shedding light on the formation of lateral demixing of anionic phospholipids that might occur in biological systems and what factors might stabilize them.
Table 1.
A summary of selected counterion-mediated effects on negatively charged phospholipids and polyelectrolytes.
| Substrate | Charge | Counterion | Pattern formation? | References |
|---|---|---|---|---|
| PtdlnsP2 | −3 to −7 | Ca2+ | Clusters and domains | [17, 20, 22, 27, 34, 38, 41–44] |
| Mg2+ | Clusters at high concentration or high PtdlnsP2 charge | [17, 41, 43, 44] | ||
| Zn2+ | Weak ability to cluster | [17] | ||
| Polyamines | Weak or no ability to cluster | [17, 58–64] | ||
| Various protein domains | Clusters and co-localization in cells | [83, 91–93] | ||
| PtdA | −1 to −2 | Ca2+ | Phase demixing | [20, 24, 40, 50, 51, 55, 78, 100, 101] |
| Mg2+ | Increased ordering, increased phase transition temperature, and vesicle fusion at higher concentrations | [20, 101–104] | ||
| Polycationic amphipathic peptides | Proposed to cluster based on theoretical calculations | [81, 105–107] | ||
| PtdSer | −1 | Mg2+ | Increased ordering | [20, 39, 40] |
| Ca2+ | Phase demixing | [20, 39, 40] | ||
| PAA-PDB | Multivalent | Ca2+, Cu2+ | Vesicle fusion, striped patterning in micelles | [76, 77]. |
Acknowledgments
We acknowledge support from National Science Foundation grants DMR-1120901, CBET-1133267, and CBET-1244507, and National Institutes of Health grants NIH U01-EB016027, NIH DK-083592, and NIH T32-HL07954.
References
- 1.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nature reviews Molecular cell biology. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampaio JL, Gerl MJ, Klose C, Ejsing CS, Beug H, Simons K, et al. Membrane lipidome of an epithelial cell line. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1903–7. doi: 10.1073/pnas.1019267108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerl MJ, Sampaio JL, Urban S, Kalvodova L, Verbavatz JM, Binnington B, et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. The Journal of cell biology. 2012;196:213–21. doi: 10.1083/jcb.201108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronan JE. Bacterial membrane lipids: Where do we stand? Annual Review of Microbiology. 2003;57:203–24. doi: 10.1146/annurev.micro.57.030502.090851. [DOI] [PubMed] [Google Scholar]
- 5.Verhoeven AJM, Tysnes O-B, Aarbakke GM, Cook CA, Holmsen H. Turnover of the phosphomonoester groups of polyphospoinositol lipids in unstimulated human platelets. European Journal of Biochemistry. 1987;166:3–9. doi: 10.1111/j.1432-1033.1987.tb13475.x. [DOI] [PubMed] [Google Scholar]
- 6.Janmey PA, Kinnunen PK. Biophysical properties of lipids and dynamic membranes. Trends in cell biology. 2006;16:538–46. doi: 10.1016/j.tcb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Conboy JC. Direct measurement of the transbilayer movement of phospholipids by sum-frequency vibrational spectroscopy. Journal of the American Chemical Society. 2004;126:8376–7. doi: 10.1021/ja048245p. [DOI] [PubMed] [Google Scholar]
- 8.Bruckheimer EM, Schroit AJ. Membrane phospholipid asymmetry: host response to the externalization of phosphatidylserine. Journal of leukocyte biology. 1996;59:784–8. doi: 10.1002/jlb.59.6.784. [DOI] [PubMed] [Google Scholar]
- 9.Horvath SE, Gaum G. Lipids of the MItochondria. Progress in Lipid Research. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Numata M, Nagashima Y, Moore ML, Berry KZ, Chan M, Kandasamy P, et al. Phosphatidylglycerol provides short-term prophylaxis against respiratory syncytial virus infection. Journal of lipid research. 2013;54:2133–43. doi: 10.1194/jlr.M037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harwood JL. Plant Lipid Biosynthesis: Fundamentals and Agricultural Applications. Cambridge University Press; 1998. [Google Scholar]
- 12.Tsuchiya H, Ueno T, Mizogami M, Takakura K. Local anesthetics structure-dependently interact with anionic phospholipid membranes to modify the fluidity. Chemico-biological interactions. 2010;183:19–24. doi: 10.1016/j.cbi.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Kusaka J, Nishibori A, Hara H. Lipid domains in bacterial membranes. Molecular microbiology. 2006;61:1110–7. doi: 10.1111/j.1365-2958.2006.05317.x. [DOI] [PubMed] [Google Scholar]
- 14.Epand RM. Lipid polymorphism and protein-lipid interactions. Biochimica et biophysica acta. 1998;1376:353–68. doi: 10.1016/s0304-4157(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin S. The electrostatic properties of membranes. Annual review of biophysics and biophysical chemistry. 1989;18:113–36. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin S, Mulrine N, Gresalfi T, Vaio G, McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. The Journal of general physiology. 1981;77:445–73. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y-H, Collins A, Guo L, Smith-Dupont KB, Gai F, Svitkina T, et al. Divalent cation-induced cluster formation by polyphosphoinositides in model membranes. Journal of the American Chemical Society. 2012;134:3387–95. doi: 10.1021/ja208640t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapovalov VL, Ryskin ME, Konovalov OV, Hermelink A, Brezesinski G. Elemental analysis within the electrical double layer using total reflection X-ray fluorescence technique. The journal of physical chemistry B. 2007;111:3927–34. doi: 10.1021/jp066894c. [DOI] [PubMed] [Google Scholar]
- 19.Shapovalov VL, Brezesinski G. Breakdown of the Gouy-Chapman model for highly charged Langmuir monolayers: counterion size effect. The journal of physical chemistry B. 2006;110:10032–40. doi: 10.1021/jp056801b. [DOI] [PubMed] [Google Scholar]
- 20.Haverstick DM, Glaser M. Visualization of Ca2+-induced phospholipid domains. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:4475–9. doi: 10.1073/pnas.84.13.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honigmann A, van den Bogaart G, Iraheta E, Risselada HJ, Milovanovic D, Mueller V, et al. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nature Structural & Molecular Biology. 2013;20:679. doi: 10.1038/nsmb.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, et al. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 2011;479:552–5. doi: 10.1038/nature10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sieber JJ, Willig KI, Kutzner C, Gerding-Reimers C, Harke B, Donnert G, et al. Anatomy and dynamics of a supramolecular membrane protein cluster. Science. 2007;317:1072–6. doi: 10.1126/science.1141727. [DOI] [PubMed] [Google Scholar]
- 24.Wang WJ, Anderson NA, Travesset A, Vaknin D. Regulation of the Electric Charge in Phosphatidic Acid Domains. Journal of Physical Chemistry B. 2012;116:7213–20. doi: 10.1021/jp303840a. [DOI] [PubMed] [Google Scholar]
- 25.Khuong TM, Habets RLP, Kuenen S, Witkowska A, Kasprowicz J, Swerts J, et al. Synaptic PI(3,4,5)P-3 Is Required for Syntaxin1A Clustering and Neurotransmitter Release. Neuron. 2013;77:1097–108. doi: 10.1016/j.neuron.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Menke M, Gerke V, Steinem C. Phosphatidylserine membrane domain clustering induced by annexin A2/S100A10 heterotetramer. Biochemistry. 2005;44:15296–303. doi: 10.1021/bi051585i. [DOI] [PubMed] [Google Scholar]
- 27.Levental I, Cebers A, Janmey PA. Combined electrostatics and hydrogen bonding determine intermolecular interactions between polyphosphoinositides. Journal of the American Chemical Society. 2008;130:9025–30. doi: 10.1021/ja800948c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redfern DA, Gericke A. Domain formation in phosphatidylinositol monophosphate/phosphatidylcholine mixed vesicles. Biophysical Journal. 2004;86:2980–92. doi: 10.1016/S0006-3495(04)74348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redfern Da, Gericke A. pH-dependent domain formation in phosphatidylinositol polyphosphate/phosphatidylcholine mixed vesicles. Journal of lipid research. 2005;46:504–15. doi: 10.1194/jlr.M400367-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes F, Loura LMS, Fedorov A, Prieto M. Absence of clustering of phosphatidylinositol-(4,5)-bisphosphate in fluid phosphatidylcholine. Journal of lipid research. 2006;47:1521–5. doi: 10.1194/jlr.M600121-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh SK, Aeffner S, Salditt T. Effect of PIP2 on bilayer structure and phase behavior of DOPC: an X-ray scattering study. ChemPhyschem. 2011;12:2633–40. doi: 10.1002/cphc.201100154. [DOI] [PubMed] [Google Scholar]
- 32.Chapman D, Urbina J, Keough K. Biomembrane Phase Transitions : Studies Of Lipid-Water Systems Using Differential Scanning. Journal of Biological Chemistry. 1974;249:2512–21. [PubMed] [Google Scholar]
- 33.Ito T, Ohnishi S, Ishinaga M, Kito M. Synthesis of a New Phosphatidylserine Spin-Label and Calcium-Induced Lateral Phase Separation in Phosphatidylserine-Phosphatidylcholine Membranes. Biochemistry. 1975;14:3064–9. doi: 10.1021/bi00685a004. [DOI] [PubMed] [Google Scholar]
- 34.Flanagan La, Cunningham CC, Chen J, Prestwich GD, Kosik KS, Janmey Pa. The structure of divalent cation-induced aggregates of PIP2 and their alteration by gelsolin and tau. Biophysical journal. 1997;73:1440–7. doi: 10.1016/S0006-3495(97)78176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kielland J. Individual Activity Coefficients of Ions in Aqueous Solutions. Journal of the American Chemical Society. 1937;59:1675–8. [Google Scholar]
- 36.Volkov AG, Paula S, Deamer DW. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochemistry And Bioenergetics. 1997;42:153–60. [Google Scholar]
- 37.Hirai M, Takizawa T, Yabuki S, Nakata Y, Hirai T, Hayashi K. Salt-dependent phase behaviour of phosphatidylinositol 4,5-diphosphate-wter system. Journal of the Chemical Society Faraday transactions. 1996;92:1493–8. [Google Scholar]
- 38.Braunger JA, Kramer C, Morick D, Steinem C. Solid supported membranes doped with PIP2: influence of ionic strength and pH on bilayer formation and membrane organization. Langmuir : the ACS journal of surfaces and colloids. 2013;29:14204–13. doi: 10.1021/la402646k. [DOI] [PubMed] [Google Scholar]
- 39.Ohnishi S, Ito T. Clustering Of Lecithin Molecules In Phosphatidylserine Membranes Induced By Calcium-Ion Binding To Phosphatidylserine. Biochemical and Biophysical Research Communications. 1973;51:132–8. doi: 10.1016/0006-291x(73)90518-4. [DOI] [PubMed] [Google Scholar]
- 40.Ohnishi S, Ito T. Calcium-Induced Phase Separations In Phosphatidylserine-Phosphatidylcholine Membranes. Biochemistry. 1974;13:881–7. doi: 10.1021/bi00702a008. [DOI] [PubMed] [Google Scholar]
- 41.Blin G, Margeat E, Carvalho K, Royer CA, Roy C, Picart C. Quantitative analysis of the binding of ezrin to large unilamellar vesicles containing phosphatidylinositol 4,5 bisphosphate. Biophysical Journal. 2008;94:1021–33. doi: 10.1529/biophysj.107.110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvemini IL, Gau DM, Reid J, Bagatolli LA, Macmillan A, Moens PD. Low PIP2 molar fractions induce nanometer size clustering in giant unilamellar vesicles. Chem Phys Lipids. 2014;177:51–63. doi: 10.1016/j.chemphyslip.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Levental I, Christian DA, Wang Y-H, Madara JJ, Discher DE, Janmey PA. Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers. Biochemistry. 2009;48:8241–8. doi: 10.1021/bi9007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellenbroek WG, Wang YH, Christian DA, Discher DE, Janmey PA, Liu AJ. Divalent cation-dependent formation of electrostatic PIP2 clusters in lipid monolayers. Biophys J. 2011;101:2178–84. doi: 10.1016/j.bpj.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiriukhin MY, Collins KD. Dynamic hydration numbers for biologically important ions. Biophysical chemistry. 2002;99:155–68. doi: 10.1016/s0301-4622(02)00153-9. [DOI] [PubMed] [Google Scholar]
- 46.Takizawa T, Hayashi K, Yabuki S, Nakata Y. Microcalorimetric Studies Of Ca2+-Binding To Triphosphoinositide And Phosphatidylserine. Thermochimica Acta. 1988;123:247–53. [Google Scholar]
- 47.Takizawa T, Hayashi K, Mitomo H. Differential Scanning Calorimetry of the Triphosphoinositide Water-System. Thermochim Acta. 1991;183:313–21. [Google Scholar]
- 48.Slochower DR, Huwe PJ, Radhakrishnan R, Janmey PA. Quantum and all-atom molecular dynamics simulations of protonation and divalent ion binding to phosphatidylinositol 4,5-bisphosphate (PIP2) The journal of physical chemistry B. 2013;117:8322–9. doi: 10.1021/jp401414y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lösche M, Möhwald H. Electrostatic interactions in phospholipid membranes: II. Influence of divalent ions on monolayer structure. Journal of Colloid and Interface Science. 1989;131:56–67. [Google Scholar]
- 50.Patil GS, Dorman NJ, Cornwell DG. Effects of ionization and counterion binding on the surface areas of phosphatidic acids in monolayers. Journal of lipid research. 1979;20:663–8. [PubMed] [Google Scholar]
- 51.Faraudo J, Travesset A. Phosphatidic acid domains in membranes: Effect of divalent counterions. Biophysical Journal. 2007;92:2806–18. doi: 10.1529/biophysj.106.092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levental I, Janmey PA, Cēbers A. Electrostatic contribution to the surface pressure of charged monolayers containing polyphosphoinositides. Biophysical Journal. 2008;95:1199–205. doi: 10.1529/biophysj.107.126615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kooijman EE, Tieleman DP, Testerink C, Munnik T, Rijkers DTS, Burger KNJ, et al. An electrostatic/hydrogen bond switch as the basis for the specific interaction of phosphatidic acid with proteins. The Journal of biological chemistry. 2007;282:11356–64. doi: 10.1074/jbc.M609737200. [DOI] [PubMed] [Google Scholar]
- 54.Kooijman EE, King KE, Gangoda M, Gericke A. Ionization properties of phosphatidylinositol polyphosphates in mixed model membranes. Biochemistry. 2009;48:9360–71. doi: 10.1021/bi9008616. [DOI] [PubMed] [Google Scholar]
- 55.Ito T, Onishi S. Ca2+-induced lateral phase separations in phosphatidic acid-phosphatidylcholine membranes. Biochimica et biophysica acta. 1974;352:29–37. doi: 10.1016/0005-2736(74)90176-x. [DOI] [PubMed] [Google Scholar]
- 56.Young BP, Shin JJ, Orij R, Chao JT, Li SC, Guan XL, et al. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science. 2010;329:1085–8. doi: 10.1126/science.1191026. [DOI] [PubMed] [Google Scholar]
- 57.Shin JJ, Loewen CJ. Putting the pH into phosphatidic acid signaling. BMC Biol. 2011;9:85. doi: 10.1186/1741-7007-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung L, Kaloyanides G, McDaniel R, McLaughlin a, McLaughlin S. Interaction of gentamicin and spermine with bilayer membranes containing negatively charged phospholipids. Biochemistry. 1985;24:442–52. doi: 10.1021/bi00323a030. [DOI] [PubMed] [Google Scholar]
- 59.Coburn RF, Jones DH, Morgan CP, Baron CB, Cockcroft S. Spermine increases phosphatidylinositol 4,5-bisphosphate content in permeabilized and nonpermeabilized HL60 cells. Biochimica et biophysica acta. 2002;1584:20–30. doi: 10.1016/s1388-1981(02)00265-2. [DOI] [PubMed] [Google Scholar]
- 60.Coburn RF, Labelle EF, Baron CB. Polyamines, PI(4,5)P2, and actin polymerization. Journal of Cellular Physiology. 2006;209:405–12. doi: 10.1002/jcp.20729. [DOI] [PubMed] [Google Scholar]
- 61.Meers P, Hong K, Bentz J, Papahadjopoulos D. Spermine as a modulator of membrane fusion: interactions with acidic phospholipids. Biochemistry. 1986;25:3109–18. doi: 10.1021/bi00359a007. [DOI] [PubMed] [Google Scholar]
- 62.Sulpice JC, Moreau C, Devaux PF, Zachowski A, Giraud F. Antagonist effects of Ca2+ and spermine on phosphatidylinositol 4,5-bisphosphate-mediated transmembrane redistribution of phospholipids in large unilamellar vesicles and in erythrocytes. Biochemistry. 1996;35:13345–52. doi: 10.1021/bi960624a. [DOI] [PubMed] [Google Scholar]
- 63.Tadolini B, Varani E. Interaction of Spermine with Polyphosphoinositides Containing Liposomes and Myo-Inositol 1,4,5 Triphosphate. Biochemical and biophysical research communications. 1986;135:58–64. doi: 10.1016/0006-291x(86)90942-3. [DOI] [PubMed] [Google Scholar]
- 64.Toner M, Vaio G, McLaughlin A, McLaughlin S. Adsorption of Cations to Phosphatidylinositol 4,5-Bisphosphate. Biochemistry. 1988;27:7435–43. doi: 10.1021/bi00419a039. [DOI] [PubMed] [Google Scholar]
- 65.Schuber F. Influence of polyamines. Biochemical Journal. 1989;260:1–10. doi: 10.1042/bj2600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hauser H, Phillips MC, Barratt MD. Differences in the interaction of inorganic and organic (hydrophobic) cations with phosphatidylserine membranes. Biochimica et biophysica acta. 1975;413:341–53. doi: 10.1016/0005-2736(75)90120-0. [DOI] [PubMed] [Google Scholar]
- 67.Halim NA, Ibrahim ZA, Ahmad AB. Intercalation of water and guest molecules within Ca2+-Montmorillonite. Journal of Thermal Analysis and Calorimetry. 2010;102:983–8. [Google Scholar]
- 68.Sulpice JC, Zachowski A, Devaux PF, Giraud F. Requirement For Phosphatidylinositol 4,5-Bisphosphate In The Ca2+-Induced Phospholipid Redistribution In The Human Erythrocyte-Membrane. Journal of Biological Chemistry. 1994;269:6347–54. [PubMed] [Google Scholar]
- 69.Ballas SK, Mohandas N, Marton LJ, Shohet SB. Stabilization of erythrocyte membranes by polyamines. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:1942–6. doi: 10.1073/pnas.80.7.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mager J. The stabilizing effect of spermine and related polyamines and bacterial protoplasts. Biochim Biophys Acta. 1959;36:529–31. doi: 10.1016/0006-3002(59)90195-7. [DOI] [PubMed] [Google Scholar]
- 71.Hong K, Schuber F, Papahadjopoulos D. Polyamines. Biological modulators of membrane fusion. Biochimica et biophysica acta. 1983;732:469–72. doi: 10.1016/0005-2736(83)90064-0. [DOI] [PubMed] [Google Scholar]
- 72.Schuber F, Hong KL, Duzgunes N, Papahadjopoulos D. Polyamines As Modulators Of Membrane-Fusion - Aggregation And Fusion Of Liposomes. Biochemistry. 1983;22:6134–40. doi: 10.1021/bi00295a015. [DOI] [PubMed] [Google Scholar]
- 73.Discher BM, Won YY, Ege DS, Lee JCM, Bates FS, Discher DE, et al. Polymersomes: Tough vesicles made from diblock copolymers. Science. 1999;284:1143–6. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 74.Regenbrecht M, Akari S, Forster S, Mohwald H. Shape investigations of charged block copolymer micelles on chemically different surfaces by atomic force microscopy. Journal of Physical Chemistry B. 1999;103:6669–75. [Google Scholar]
- 75.Shen H, Eisenberg A. Control of Architecture in Block-Copolymer Vesicles We thank the Petroleum Research Fund, administered by the American Chemical Society, for the support of this work. Angewandte Chemie. 2000;39:3310–2. [PubMed] [Google Scholar]
- 76.Christian DA, Tian A, Ellenbroek WG, Levental I, Rajagopal K, Janmey PA, et al. Spotted vesicles, striped micelles and Janus assemblies induced by ligand binding. Nature materials. 2009;8:843–9. doi: 10.1038/nmat2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spinler K, Tian A, Christian DA, Pantano DA, Baumgart T, Discher DE. Dynamic domains in polymersomes: mixtures of polyanionic and neutral diblocks respond more rapidly to changes in calcium than to pH. Langmuir : the ACS journal of surfaces and colloids. 2013;29:7499–508. doi: 10.1021/la304602e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bucki R, Janmey PA, Vegners R, Giraud F, Sulpice J-C. Involvement of Phosphatidylinositol 4,5-Bisphosphate in Phosphatidylserine Exposure in Platelets: Use of a Permeant Phosphoinositide-Binding Peptide. Biochemistry. 2001;40:15752–61. doi: 10.1021/bi010899c. [DOI] [PubMed] [Google Scholar]
- 79.Czaplewski C, Pasenkiewicz-Gierula M, Ciarkowski J. Molecular dynamics of a vasopressin V2 receptor in a phospholipid bilayer membrane. J Recept Signal Transduct Res. 1999;19:355–67. doi: 10.3109/10799899909036657. [DOI] [PubMed] [Google Scholar]
- 80.Liepina I, Czaplewski C, Janmey P, Liwo A. Molecular dynamics study of a gelsolin-derived peptide binding to a lipid bilayer containing phosphatidylinositol 4,5-bisphosphate. Biopolymers. 2003;71:49–70. doi: 10.1002/bip.10375. [DOI] [PubMed] [Google Scholar]
- 81.Lorenz CD, Faraudo J, Travesset A. Hydrogen bonding and binding of polybasic residues with negatively charged mixed lipid monolayers. Langmuir : the ACS journal of surfaces and colloids. 2008;24:1654–8. doi: 10.1021/la703550t. [DOI] [PubMed] [Google Scholar]
- 82.Cuello LG, Jogini V, Cortes DM, Perozo E. Structural mechanism of C-type inactivation in K(+) channels. Nature. 2010;466:203–8. doi: 10.1038/nature09153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stansfeld PJ, Hopkinson R, Ashcroft FM, Sansom MS. PIP(2)-binding site in Kir channels: definition by multiscale biomolecular simulations. Biochemistry. 2009;48:10926–33. doi: 10.1021/bi9013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh DK, Rosenhouse-Dantsker A, Nichols CG, Enkvetchakul D, Levitan I. Direct regulation of prokaryotic Kir channel by cholesterol. The Journal of biological chemistry. 2009;284:30727–36. doi: 10.1074/jbc.M109.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Travkova OG, Andra J, Mohwald H, Brezesinski G. Influence of arenicin on phase transitions and ordering of lipids in 2D model membranes. Langmuir : the ACS journal of surfaces and colloids. 2013;29:12203–11. doi: 10.1021/la402340d. [DOI] [PubMed] [Google Scholar]
- 86.Bock CW, Kaufman A, Glusker JP. Coordination of Water to Magnesium Cations. Inorganic Chemistry. 1994;33:419–27. [Google Scholar]
- 87.Lazaridis T. Implicit solvent simulations of peptide interactions with anionic lipid membranes. Proteins-Structure Function and Bioinformatics. 2005;58:518–27. doi: 10.1002/prot.20358. [DOI] [PubMed] [Google Scholar]
- 88.Peitzsch RM, Eisenberg M, Sharp KA, McLaughlin S. Calculations of the electrostatic potential adjacent to model phospholipid bilayers. Biophys J. 1995;68:729–38. doi: 10.1016/S0006-3495(95)80253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baenziger JE, Morris M-L, Darsaut TE, Ryan SE. Effect of Membrane Lipid Composition on the Conformational Equilibria of the Nicotinic Acetylcholine Receptor. Journal of Biological Chemistry. 2000;275:777–84. doi: 10.1074/jbc.275.2.777. [DOI] [PubMed] [Google Scholar]
- 90.Epand RM, Hui S-W. Effect of electrostatic repulsion on the morphology and thermotropic transitions of anionic phospholipids. FEBS Letters. 1986;209:257–60. doi: 10.1016/0014-5793(86)81123-1. [DOI] [PubMed] [Google Scholar]
- 91.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annual review of biophysics and biomolecular structure. 2002;31:151–75. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 92.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–6. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 93.Lai C-L, Jao CC, Lyman E, Gallop JL, Peter BJ, McMahon HT, et al. Membrane Binding and Self-Association of the Epsin N-Terminal Homology Domain. Journal of Molecular Biology. 2012;423:800–17. doi: 10.1016/j.jmb.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zimmerberg J, McLaughlin S. Membrane curvature: How BAR domains bend bilayers. Curr Biol. 2004;14:R250–R2. doi: 10.1016/j.cub.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 95.Cui H, Ayton GS, Voth GA. Membrane binding by the endophilin N-BAR domain. Biophys J. 2009;97:2746–53. doi: 10.1016/j.bpj.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arkhipov A, Yin Y, Schulten K. Membrane-bending mechanism of amphiphysin N-BAR domains. Biophys J. 2009;97:2727–35. doi: 10.1016/j.bpj.2009.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gambhir A, Hangyas-Mihalyne G, Zaitseva I, Cafiso DS, Wang J, Murray D, et al. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys J. 2004;86:2188–207. doi: 10.1016/S0006-3495(04)74278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morton LA, Yang H, Saludes JP, Fiorini Z, Beninson L, Chapman ER, et al. MARCKS-ED Peptide as a Curvature and Lipid Sensor. ACS Chemical Biology. 2012;8:218–25. doi: 10.1021/cb300429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bucki R, Janmey PA. Interaction of the gelsolin-derived antibacterial PBP 10 peptide with lipid bilayers and cell membranes. Antimicrob Agents Chemother. 2006;50:2932–40. doi: 10.1128/AAC.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bu W, Flores K, Pleasants J, Vaknin D. Preferential affinity of calcium ions to charged phosphatidic acid surface from a mixed calcium/barium solution: X-ray reflectivity and fluorescence studies. Langmuir : the ACS journal of surfaces and colloids. 2009;25:1068–73. doi: 10.1021/la803161a. [DOI] [PubMed] [Google Scholar]
- 101.Jacobson K, Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975;14:152–61. doi: 10.1021/bi00672a026. [DOI] [PubMed] [Google Scholar]
- 102.Trauble H, Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:214–9. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papahadjopoulos D, Vail WJ, Pangborn WA, Poste G. Studies on membrane fusion. II. Induction of fusion in pure phospholipid membranes by calcium ions and other divalent metals. Biochimica et biophysica acta. 1976;448:265–83. doi: 10.1016/0005-2736(76)90241-8. [DOI] [PubMed] [Google Scholar]
- 104.Verkleij AJ, Demaagd R, Leunissenbijvelt J, Dekruijff B. Divalent-Cations and Chlorpromazine Can Induce Non-Bilayer Structures in Phosphatidic Acid-Containing Model Membranes. Biochimica et biophysica acta. 1982;684:255–62. doi: 10.1016/0005-2736(82)90014-1. [DOI] [PubMed] [Google Scholar]
- 105.Kooijman EE, Carter KM, van Laar EG, Chupin V, Burger KN, de Kruijff B. What makes the bioactive lipids phosphatidic acid and lysophosphatidic acid so special? Biochemistry. 2005;44:17007–15. doi: 10.1021/bi0518794. [DOI] [PubMed] [Google Scholar]
- 106.Testerink C, Munnik T. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005;10:368–75. doi: 10.1016/j.tplants.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 107.Tigyi G, Parrill AL. Molecular mechanisms of lysophosphatidic acid action. Prog Lipid Res. 2003;42:498–526. doi: 10.1016/s0163-7827(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 108.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–6. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 109.Watson MC, Penev ES, Welch PM, Brown FLH. Thermal fluctuations in shape, thickness, and molecular orientation in lipid bilayers. The Journal of Chemical Physics. 2011;135:244701. doi: 10.1063/1.3660673. [DOI] [PubMed] [Google Scholar]
- 110.Deuling HJ, Helfrich W. Red blood cell shapes as explained on the basis of curvature elasticity. Biophys J. 1976;16:861–8. doi: 10.1016/S0006-3495(76)85736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Zeitschrift fur Naturforschung Teil C: Biochemie, Biophysik, Biologie, Virologie. 1973;28:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 112.Song J, Waugh RE. Bending rigidity of SOPC membranes containing cholesterol. Biophysical Journal. 1993;64:1967–70. doi: 10.1016/S0006-3495(93)81566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Safran SA. Saddle-splay modulus and the stability of spherical microemulsions. Phys Rev A. 1991;43:2903–4. doi: 10.1103/physreva.43.2903. [DOI] [PubMed] [Google Scholar]
- 114.Kozlovsky Y, Efrat A, Siegel DP, Kozlov MM. Stalk phase formation: effects of dehydration and saddle splay modulus. Biophys J. 2004;87:2508–21. doi: 10.1529/biophysj.103.038075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu M, Briguglio JJ, Deserno M. Determining the Gaussian Curvature Modulus of Lipid Membranes in Simulations. Biophysical Journal. 2012;102:1403–10. doi: 10.1016/j.bpj.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baumgart T, Das S, Webb WW, Jenkins JT. Membrane elasticity in giant vesicles with fluid phase coexistence. Biophys J. 2005;89:1067–80. doi: 10.1529/biophysj.104.049692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Semrau S, Idema T, Holtzer L, Schmidt T, Storm C. Accurate determination of elastic parameters for multicomponent membranes. Physical review letters. 2008;100:088101. doi: 10.1103/PhysRevLett.100.088101. [DOI] [PubMed] [Google Scholar]
- 118.Winterhalter M, Helfrich W. Effect of surface charge on the curvature elasticity of membranes. The Journal of Physical Chemistry. 1988;92:6865–7. [Google Scholar]
- 119.Vitkova V, Genova J, Finogenova O, Mitov MD, Ermakov YA, Bivas I. Surface Charge Effect on the Bending Elasticity of Lipid Bilayers. Comptes REndus de l’Academie Bulgare des Sciences. 2004;57:11–25. [Google Scholar]
- 120.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature reviews Molecular cell biology. 2011;12:517–33. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 121.Liu J, Sun Y, Drubin DG, Oster GF. The mechanochemistry of endocytosis. PLoS biology. 2009;7:e1000204. doi: 10.1371/journal.pbio.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Agrawal NJ, Nukpezah J, Radhakrishnan R. Minimal mesoscale model for protein-mediated vesiculation in clathrin-dependent endocytosis. PLoS Comput Biol. 2010;6:e1000926. doi: 10.1371/journal.pcbi.1000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao Y, Liu J, Yang C, Capraro BR, Baumgart T, Bradley RP, et al. Exo70 generates membrane curvature for morphogenesis and cell migration. Developmental cell. 2013;26:266–78. doi: 10.1016/j.devcel.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McLaughlin S. Electrostatic Potentials and Membrane-Solution Interfaces. In: Bronner F, Kleinzeller A, editors. Curent Topics in Membranes & Transport. Academic Press; 1977. [Google Scholar]
- 125.Honig BH, Hubbell WL, Flewelling RF. Electrostatic interactions in membranes and proteins. Annual review of biophysics and biophysical chemistry. 1986;15:163–93. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- 126.Helm CA, Laxhuber L, Lösche M, Möhwald H. Electrostatic Interactions In Phospholipid-Membranes: I. Influence Of Mono-Valent Ions. Colloid and Polymer Science. 1986;264:46–55. [Google Scholar]
- 127.Martin-Molina A, Rodriguez-Beas C, Faraudo J. Effect of calcium and magnesium on phosphatidylserine membranes: experiments and all-atomic simulations. Biophys J. 2012;102:2095–103. doi: 10.1016/j.bpj.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ben-Tal N, Honig B, Peitzsch RM, Denisov G, McLaughlin S. Binding of small basic peptides to membranes containing acidic lipids: theoretical models and experimental results. Biophys J. 1996;71:561–75. doi: 10.1016/S0006-3495(96)79280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Denisov G, Wanaski S, Luan P, Glaser M, McLaughlin S. Binding of basic peptides to membranes produces lateral domains enriched in the acidic lipids phosphatidylserine and phosphatidylinositol 4,5-bisphosphate: an electrostatic model and experimental results. Biophys J. 1998;74:731–44. doi: 10.1016/S0006-3495(98)73998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vernier PT, Ziegler MJ, Dimova R. Calcium binding and head group dipole angle in phosphatidylserine-phosphatidylcholine bilayers. Langmuir : the ACS journal of surfaces and colloids. 2009;25:1020–7. doi: 10.1021/la8025057. [DOI] [PubMed] [Google Scholar]
- 131.Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochemical Society symposium. 2007;74:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Psachoulia E, Sansom MS. Interactions of the pleckstrin homology domain with phosphatidylinositol phosphate and membranes: characterization via molecular dynamics simulations. Biochemistry. 2008;47:4211–20. doi: 10.1021/bi702319k. [DOI] [PubMed] [Google Scholar]