Abstract
While modernization has dramatically increased lifespan, it has also witnessed the increasing prevalence of diseases such as obesity, hypertension and type 2 diabetes. Such chronic, acquired diseases result when normal physiologic control goes awry and may thus be viewed as failures of homeostasis. However, while nearly every process in human physiology relies on homeostatic mechanisms for stability, only some have demonstrated vulnerability to dysregulation. Additionally, chronic inflammation is a common accomplice of the diseases of homeostasis, yet the basis for this connection is not fully understood. Here we review the design of homeostatic systems and discuss universal features of control circuits that operate at the cellular, tissue and organismal levels. We suggest a framework for classification of homeostatic signals that is based on different classes of homeostatic variables they report on. Finally, we discuss how adaptability of homeostatic systems with adjustable set points creates vulnerability to dysregulation and disease. This framework highlights the fundamental parallels between homeostatic and inflammatory control mechanisms and provides a new perspective on the physiological origin of inflammation.
Changes in human ecology - including diet, physical activity, population density and microbial exposure - have dramatically shifted the spectrum of human diseases over the past century. Genes selected to protect from starvation, infections, injury, and predation may now, in the absence of some of these challenges, contribute to the increasing incidence of ‘modern human diseases’, including obesity, type 2 diabetes, atherosclerosis, autoimmunity, allergy, and certain psychiatric disorders. Plausible evolutionary explanations for the high prevalence of these diseases in industrialized countries include antagonistic pleiotropy (Williams, 1957) and the mismatch between modern environment and human evolutionary history (Gluckman et al., 2009; Stearns and Koella, 2008).
These modern human diseases seem to have two features in common: they involve disruption of homeostasis and they are nearly universally associated with chronic inflammation. Despite this well-documented connection between inflammation and diseases of homeostasis, the underlying evolutionary and mechanistic bases remain obscure. In most complex diseases, in contrast to rare Mendelian diseases, the pathological state has a normal, physiological counterpart. The etiology of modern human diseases may therefore point to the physiological rationale connecting inflammation and homeostasis.
Most physiological processes can only operate under a narrow range of conditions, which are maintained by specialized homeostatic mechanisms in the face of variations in the environment, and adjusted in response to changes in functional demands and biological priorities. Interestingly, only some of these processes are vulnerable to dysregulation and disease. For example, lipid and glucose metabolism can be derailed, leading to dyslipidemia, diabetes and obesity, while amino acid metabolism seems resistant to homeostatic dysregulation. Here we present a view that may help explain the differential susceptibility of physiological processes to diseases of homeostasis. We explore the fundamental connections between homeostasis and inflammation and discuss an evolutionary perspective on homeostatic diseases.
Homeostatic variables and control circuits
In the 19th century, Claude Bernard articulated the need to maintain a stable internal environment - milieu interieur - that would allow biological processes to proceed despite variations in the external environment (Bernard, 1878). Bernard’s concept was further explored, developed, and popularized by Walter Cannon, who coined the term “homeostasis” in describing how key physiological variables are maintained within a predefined range by feedback mechanisms (Cannon, 1929). His contemporary, Curt Richter, expanded the notion of homeostasis to include behavioral responses as an important mechanism by which homeostasis could be regulated in addition to the internal controls systems described by Bernard and Cannon (Moran and Schulkin, 2000; Richter, 1943). Nearly two decades after Cannon, James Hardy proposed a model in which homeostatic mechanisms maintain physiological variables within an acceptable range by comparing the actual value of the variable to a desired value or ‘set point,’ (Hardy, 1953).
Homeostasis is a unifying theme of modern physiology and much has been elucidated about molecular mechanisms of homeostatic control. However the term, being intuitively simple, is often used loosely. For the purpose of this discussion, it is important to introduce and review some key definitions and concepts initially developed in control theory and systems dynamics theory, but applicable to homeostatic control in biological systems (see Table 1 for glossary).
Table 1.
| Term | Definition | Examples |
|---|---|---|
| Stock | A system’s variable that represents quantity | Blood glucose concentration |
| Flow | A system’s variable that represents a process that changes the stock | Gluconeogenesis, glycogenolysis, glycolysis, gluconeogenesis, glucose transport |
| Regulated variable | A physiologic variable that is maintained at a stable level (near set point) by homeostatic circuit(s). Regulated variables are stocks | Blood glucose concentration |
| Controlled variable | A physiologic variable that is manipulated in order to maintain the regulated variable within desired range. Controlled variables are flows | Gluconeogenesis, glycogenolysis, glycolysis, gluconeogenesis, glucose transport |
| Set point | An optimal value of the regulated variable; divergence from set point value activates homeostatic control mechanisms | Normoglycemia (~5mM) |
| Error value |X-X’| | The difference between the set point and the actual value of the regulated variable | Difference between actual blood glucose concentration and normoglycemia |
| Controller | A component of the homeostatic circuit that monitors the value of regulated variable | Pancreatic α and β cells |
| Plant | An effector component of the homeostatic circuit that is activated by the Controller to change the value of regulated variable | Skeletal muscle, white adipose tissue, brown adipose tissue, liver |
| Controller gain | A characteristic of Controllers that determines the amount of signal produced in response to given error value |X-X’| | Amount of insulin produced by β-cells in response to a given blood glucose level |
| Gain tuning of Controller | A method to optimize Controller performance | Changing the amount of insulin produced in response to a given blood glucose level |
First, it is important to distinguish two types of variables that exist in homeostatic systems. The physiological variables that are maintained at a stable level, such as blood glucose or core body temperature, are called regulated variables. In contrast, controlled variables are activities, or rates, of the processes that contribute to the stability of regulated variables (Cabanac, 2006). For example, blood calcium concentration is a regulated variable, whereas the rate of urinary calcium excretion is a controlled variable that is manipulated in order to regulate blood calcium concentration. Multiple controlled variables typically contribute to the stability of a given regulated variable. Thus, in addition to calcium excretion in the kidney, the rates of intestinal calcium absorption and bone resorption are also controlled variables that contribute to the maintenance of stable blood calcium concentration. In the case of blood glucose concentration (a regulated variable), the controlled variables include the rates of intestinal and renal glucose transport, glycogenolysis, gluconeogenesis, glycolysis, glycogenesis and glucose transport from the blood into tissues. Thus, regulated variables refer to quantities, whereas controlled variables refer to processes, where process activity or rate is a variable. Put in systems dynamics terms, regulated variables are the stocks of the system, while controlled variables are the flows of the system: they either increase (in-flows) or decrease (out-flows) the value of the regulated variable (Figure 1). Notably, while all regulated variables are stocks, not all stocks are regulated variables. For example blood glucose is a regulated variable, whereas blood alcohol is not. Likewise, all controlled variables are flows, but not all flows are controlled variables. Thus heat loss through sweating is a controlled variable, while heat loss through conduction is not. Because these terminologies capture different aspects of system behavior we will use both during this discussion, to emphasize the relevant features of homeostasis.
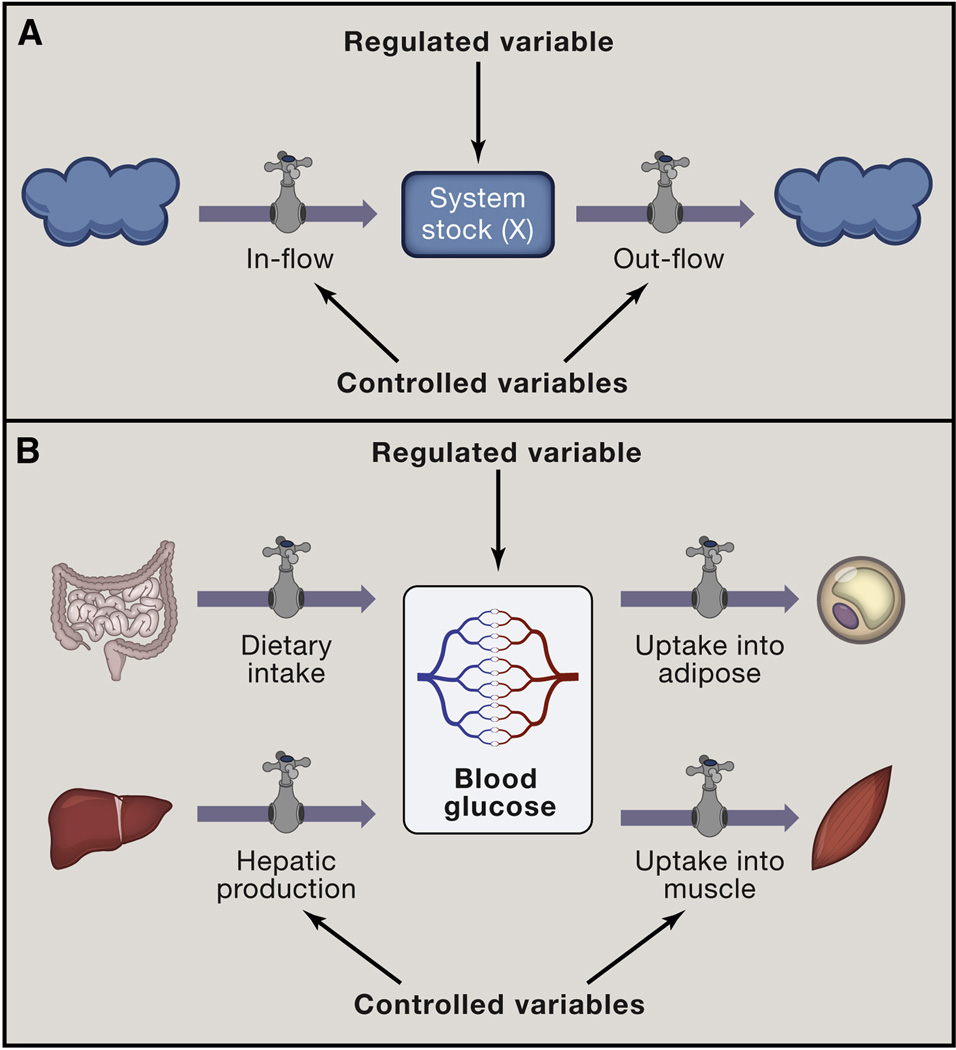
Figure 1. Stock and flow model of homeostasis.
(A) Stock and flow model highlights two types of variables in homeostasis: Stock is quantity of a regulated variable - a parameter that is maintained by homeostasis. Flows are the processes that change the value of the stock. Some, but not all flows are controlled variables and targets for homeostatic control signals (graphically represented here as dials). Clouds represent ‘sources’ and ‘sinks’ for regulated variable that are extrinsic to the homeostatic system.
(B) A physiologic example of stock and flow model: dietary glucose uptake, hepatic glucose production, or glucose uptake into adipose and muscle are flows that maintain the stock of blood glucose.
In order to be maintained within the desired range, the values of regulated variables must be continuously monitored and adjusted. Accordingly, all homeostatic systems have two essential components: Controllers and Plants. The Controllers monitor the value of the regulated variable (X), compare it to the reference value (or in Hardy’s terms, “set point”) (X’), and generate a signal that is proportional to the absolute value of the difference |X - X’| (the coefficient of proportionality is a characteristic known as the Controller’s gain) (Åström and Murray, 2008). This signal then acts on the Plant - the effector that creates flows into or out of the system – in order to bring the regulated variable closer to the reference value (Figure 2A). In a classic engineering example of a control system, the thermostat (Controller) compares the actual room temperature (regulated variable) to the desired room temperature (reference value, or set point). If actual room temperature is lower than the set point, a signal is generated and sent to the furnace (the Plant) to increase heat production (the flow) and raise room temperature towards the set point value. In physiology, the Controllers are typically endocrine cells and sensory neurons of the autonomic nervous system, lower brainstem (medulla), and hypothalamus (Hammel, 1968). They monitor deviations in regulated physiologic variables from their ‘set points’ and generate signals (hormones and neurotransmitters) that increase or decrease the flows created by various Plants (tissues and organs that can adjust these values) (Figure 2B). For example, pancreatic β-cells (Controller) produce insulin in response to an increase in blood glucose (regulated variable). Insulin acts on skeletal muscle, adipose tissue and liver (the Plants) to increase glucose uptake and utilization (out-flows) in muscle and fat and to inhibit gluconeogenesis (in-flow) in the liver, thereby reducing plasma glucose level (Figure 2C).
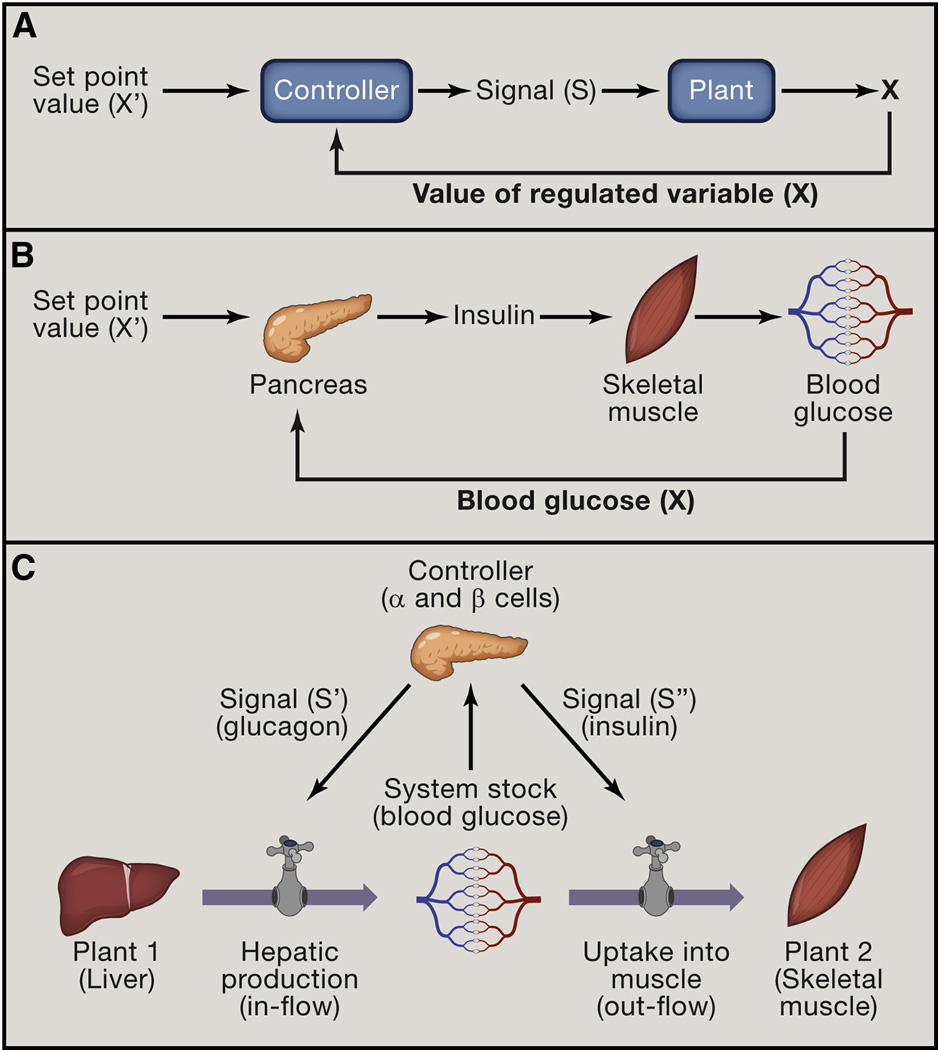
Figure 2. Homeostatic control circuit.
(A) Basic homeostatic control circuits have two essential components: Controllers and Plants. Controllers monitor the value of regulated variable (X) and compare it to the reference value (X’). In response to deviation of X from X’, Controllers generate a signal (S) that acts on Plants. Plants are the effectors of the homeostatic systems that change the value of the regulated variable.
(B) A physiologic example of control circuit: pancreatic beta cells act as Controller, sensing elevated blood glucose and producing insulin (signal S) to increase glucose uptake into skeletal muscle (Plant). In the simplest model, the output of the Controller (signal S) is proportional to the deviation of regulated variable from the reference value, |X-X’| . The proportionality constant is referred to as the gain.
(C) Combining stock and flow modeling with the basic control circuit provides a more complete model of homeostasis. The Controller monitors the value of the Stock and produces signals that act on Plants. Such signals cause Plants to modulate the flows that contribute to the Stock. In this example, glucose sensing by the pancreas (Controller), induces glucagon or insulin secretion (Signals S’ and S’’), which act on liver and muscle (Plants), to control glucose production and uptake, respectively (flows) and stabilize blood glucose (Stock)
Controllers and Plants are defined with respect to specific regulated variables. For example, pancreatic α- and β-cells are Controllers for blood glucose, but not for body temperature, whereas adipose tissue and liver are Plants for blood glucose, but not for blood calcium (where the relevant Plants are the kidney, intestine and bone). Additionally, most tissues and organs perform many functions and can therefore act as Plants for multiple regulated variables, depending on the requirements of the organism: because skeletal muscle can both consume glucose and generate heat during shivering thermogenesis, it can act as a Plant for both blood glucose and body temperature. Thus, Controllers are characterized by the regulated variables they monitor, while Plants are characterized by the controlled variables (activities of the flows) associated with them.
50 years after its inception, there is still disagreement over Hardy’s concept of ‘set point,’ which in his model was analogous to the reference value of engeneered systems. Some argue that regulated variables can reach steady state or ‘settling point’ without an external reference point (Wirtshafter and Davis, 1977). In stock and flow terms, the stock would not be regulated by comparison to a ‘set point,’ but simply reach a passive ‘settling point’ when in-flows and out-flows balance. In other words, one can think of set point as being either a predefined, or an emergent characteristic of a system. A full discussion of the strengths and limitations of these two models is beyond the scope of this article. However, the two models may not necessarily be mutually exclusive (Speakman et al., 2011). Regardless of whether a reference point is real or imaginary, the term ‘set point’, if nothing else, is a convenient shortcut by which to refer to the defended level of a regulated variable, and will be used herein for simplicity. For the sake of this discussion, it should not be thought of as equivalent to the external reference value in engeneered systems.
Homeostatic units
Homeostasis has been studied primarily with regard to systemically regulated variables such as plasma glucose level and core body temperature. However, many of the same variables are also homeostatically maintained at the level of individual cells within tissues. Such variables are referred to as System stocks when they are maintained at the systemic level and Plant stocks when they are maintained at the level of individual Plants. Thus, while blood glucose (System stock) is maintained by insulin, glucagon and catecholamines, glucose level in skeletal muscle (Plant stock) is simultaneously monitored by intracellular sensors and homeostatically maintained through regulated expression of glucose transporters and activity of metabolic pathways of glucose utilization (Herman and Kahn, 2006; Jensen et al., 2008). On the organismal level, pancreatic β-cells function as Controllers and skeletal muscle as Plants. Within individual myocytes, AMPK functions as a Controller (monitoring intracellular glucose level) and GLUT4 (a glucose transporter) functions as a Plant. The signal connecting Controllers to Plants in this case is the signaling pathway connecting AMPK to GLUT4 expression. Note that System stock and Plant stock are connected by a flow (e.g., glucose transport from blood into skeletal muscle by GLUT4) (Figure 3). GLUT4 expression and glucose flow can be controlled by both the system level Controller (in this case, by insulin) and by the tissue level Controller (in this case, by AMPK). In exercising muscle, for example, glucose and ATP depletion leads to AMPK activation, prompting insulin-independent glucose uptake (a tissue-level control) even when insulin-stimulated uptake might be suppressed (a system-level control) (Herman and Kahn, 2006; Russell et al., 1999). Conversely, when skeletal muscle energy stores are high, insulin-dependent glucose uptake is inhibited, as illustrated by insulin resistance that can be caused by fatty acid accumulation in the muscle (Samuel and Shulman, 2012) or by activity of the hexosamine biosynthetic pathway (Ruan et al., 2013).
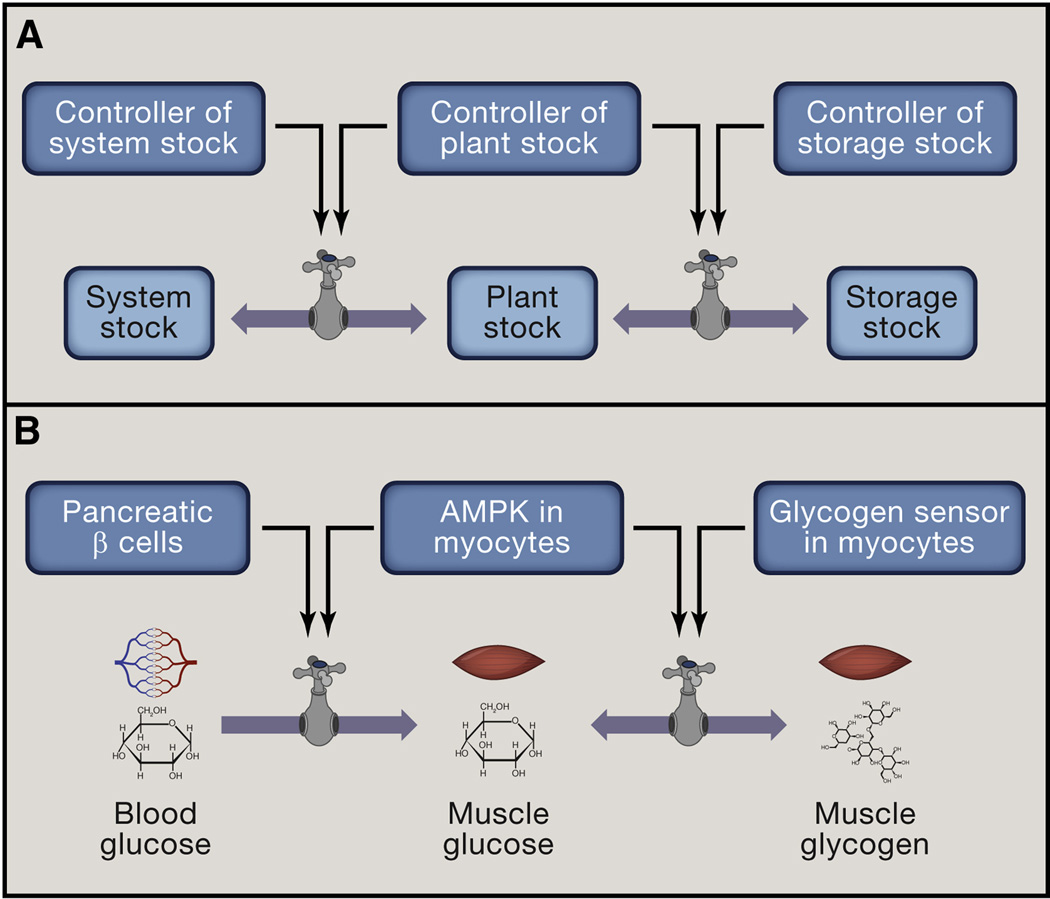
Figure 3. Homeostatic units.
(A) System stock, Plant stock and Storage stock each represent homeostatic units that are connected by flows. Each of the stocks is monitored by a specialized Controller, which regulates the flows into and out of the stock. Homeostatic system is thus hierarchically organized into ‘nested’ homeostatic units.
(B) Physiologic example of nested homeostatic units: System stock (blood glucose) is monitored by System Controller (pancreatic β-cells), Plant stock (glucose in skeletal muscle) is monitored by Plant specific Controller (e.g., AMPK) and Storage stock (muscle glycogen) is presumably monitored by a glycogen sensor, which is currently unknown. Each of the Controllers regulates the flows into and out of the corresponding stock.
Some Plant stocks have a special property: glycogen in the liver and muscle, triglycerides in the adipose tissue, and calcium phosphate in the bone are examples of Storage stocks. They buffer regulated variables (blood glucose, fatty acids, and calcium) from the variations in dietary intake or expenditure. The System stocks (e.g., blood glucose), Plant stocks (muscle glucose) and Storage stocks (muscle glycogen) are connected by in- and out-flows (glucose transport, glycogenolysis and glycogenesis), which are adjusted by hormones and neurotransmitters to maintain the System stock within a desired range (Figure 3). The relationship between regulated stocks and storage stocks is analogous to the relationship between pocket money and money in a bank account: they are connected by flows (deposits and withdrawals) and while the former is usually maintained within a relatively narrow range, the latter is not. Storage stocks exist for some regulated variables (glucose, fatty acids, vitamin A, calcium), but not for others (oxygen, sodium, potassium). Accordingly, the latter variables are more vulnerable to fluctuations in environmental availability.
As noted earlier, Plants are defined by the regulated variables they maintain. The notion of the Plant is only relevant with respect to a specific homeostatic circuit. When skeletal muscle is referred to as a Plant in glucose homeostasis, it is specifically its activities in glucose handling that are relevant. In that sense the terms ‘Plant’ and ‘Tissue’ are not equivalent. All tissues have their own homeostatic circuits that may or may not be related to their function as Plants or Controllers. Like any homeostatic systems, tissues have their own regulated and controlled variables. Oxygen and nutrient concentration, interstitial fluid volume, pH, osmolarity, cell number and cellular composition are all examples of regulated variables of tissue homeostasis (Chovatiya and Medzhitov, 2014). Cell proliferation, apoptosis and migration, lymphatic drainage and vascular permeability are examples of controlled variables. Typical Controllers include tissue resident macrophages, mast cells and somatosensory neurons, all of which monitor various regulated variables of tissue homeostasis. Finally, many cells within tissues (including vascular and lymphatic endothelium, stromal and parenchymal cells) can act as Plants, depending on the controlled variable (Chovatiya and Medzhitov, 2014).
As noted earlier, some regulated variables, for example, glucose, are homeostatically maintained as System stock (blood glucose), Plant stock (muscle glucose) and Storage stock (muscle glycogen). All three stocks are connected by flows. However, not all regulated variables are connected in this manner: for example, protein concentration in a cell and in plasma are both regulated variables, but they are not connected by flows; collagen stiffness/elasticity is a regulated variable of tissue homeostasis, but it does not even have a counterpart at cellular or organismal levels. When a regulated variable is maintained by homeostatic circuits at multiple levels that are connected by flows, the result is interdependent, ‘nested’ homeostatic units (Figure 3). This hierarchical organization of homeostasis provides buffering and flexibility in addressing systemic and tissue-specific physiologic needs and priorities.
Controllers as sensors of regulated variables
Controllers play a key role in homeostasis by monitoring the values of the regulated variables. There are two methods used by Controllers to perform this function. Some Controllers monitor the values of regulated variables through a flow that samples the System stock. As an example, β-cells monitor blood glucose level by transporting glucose through GLUT2 transporter and converting it by glucokinase into glucose-6-phosphate (G6P) to initiate glycolysis (Olson and Pessin, 1996). ATP generated by glycolysis then inhibits the ATP-sensitive potassium channel resulting in plasma membrane depolarization, calcium influx and insulin secretion (Newgard et al., 2002; Newgard and McGarry, 1995). The flow of glucose into β-cells has special features that enable glucose sensing: First, GLUT2 has a very high Km for glucose (15–20 mM) and only transports glucose when its level in the blood is high (Burant and Bell, 1992). Similarly, glucokinase has a low affinity for glucose compared to other hexokinases (Matschinsky, 1996). These properties make the β-cell sensitive to high plasma glucose level. Second, the flows into Controllers are not subject to inhibition by negative feedback, unlike the flows into Plants. Thus, glucokinase, unlike hexokinases, is not inhibited by G6P (Matschinsky, 1996); otherwise the amount of ATP generated by glycolysis would not be proportional to the amount of glucose transported into the β-cells.
An alternative means by which to monitor the system stock is through dedicated receptors. For example, sensory neurons typically use various gated channels and other sensors to monitor temperature (e.g., TRMP8 and TRPV1), pH (ASICS), oxygen (pO2 sensor in glomus cells of carotid body) and stretch sensors in baroreceptors (Krishtal, 2003; Montell, 2005; Prabhakar, 2000). Many metabolites, for example, fatty acids and ketones, can be monitored both directly by GPCRs (Briscoe et al., 2003; Oh et al., 2010) and through their flow into Controllers where they are metabolized.
Physiological priorities
As Cannon aptly noted when selecting the prefix homeo, or similar, rather than homo, same (Cannon, 1929), homeostatic variables are not maintained at a constant level, but rather within a certain range of values. Some physiological variables (e.g., plasma glucose) are tolerated over a relatively wide dynamic range, while others must remain within a narrow range (e.g., plasma calcium). Moreover, the same regulated variable can have a different acceptable dynamic range in different tissues: for example, the brain has low tolerance to deviations in many physiologic variables (including oxygen, glucose and temperature) while white adipose tissue is typically less demanding. Thus, the most sensitive tissues both define the limits of homeostatic range for the corresponding regulated variables and tend to be better protected from the fluctuations in these variables. For example, the brain is relatively insulated from the normal variation of blood glucose levels (ranging between 4 mM and 7 mM) due to the neuronal expression of the high affinity glucose transporter GLUT3, which has a low KM for glucose (~1mM) (Burant and Bell, 1992).
Homeostatic prioritization is also reflected in the contribution of the different Plants to the maintenance of the regulated variable. As eluded to earlier, a given regulated variable can be affected by multiple Plants. For example, blood glucose level can be affected by muscle, liver, adipose, kidney, and intestine through uptake, metabolism, and excretion. The relative contributions of different Plants to blood glucose level need to be coordinated to minimize fluctuation of the stock. Thus, increased glucose consumption by exercising skeletal muscle can be compensated for by decreased consumption by the adipose tissue and/or by increased gluconeogenesis by the liver. While all three Plants can affect the value of the regulated variable (in this case glucose), their relative contributions can change depending on their functional states and physiological priorities of the organism. The corollary to this feature is that increased flow burden is dynamically allocated between different Plants, which in turn necessitates communication between Plants to coordinate their contributions to systemic homeostasis, as we discuss next.
Homeostatic control signals
The classical view of homeostasis is that it is maintained by signals from the endocrine and autonomic nervous systems. Recent discoveries have extended this paradigm by demonstrating that signals produced by tissues and organs not historically thought of as endocrine organs - including adipose tissue, the intestine, the liver, the muscle, and the kidneys – also play critical roles in homeostatic control. Examples of these signals include the adipokines leptin (Friedman and Halaas, 1998), adiponectin (Yamauchi et al., 2001), and RBP4 (Yang et al., 2005); the hepatokine FGF21 (Fisher et al., 2011); the myokines IL-6 (Pedersen and Febbraio, 2012) and meteorin-like (Rao et al., 2014); and the gut hormones FGF15/19 (Potthoff et al., 2011), CCK (Gibbs et al., 1973) and GLP-1 (Holst, 2007). While the mechanisms of action of many of these signals are still being elucidated, one could argue that not all signals are equivalent in the type of information they communicate within a homeostatic circuit.
As discussed above, there are two types of variables in homeostasis: stocks and flows. The stocks can be further divided into System stocks (e.g., plasma glucose), Plant stocks (e.g., muscle glucose) and Storage stocks (e.g., muscle glycogen). We propose that each type of stock and flow is monitored and translated into a distinct class of homeostatic signals that reports on their value (Figure 4), giving rise to four classes of homeostatic signals:
Signals of the first class are produced by System Controllers and report on the value of the System stocks (Signal Sa in Figure 4). These are classical endocrine hormones and efferents of the autonomic nervous system that operate in negative feedback loops. Examples include insulin and glucagon reporting on plasma glucose level, or parathyroid hormone reporting on plasma calcium level.
Signals of the second class report the value of the Plant stocks (Signal Sb in Figure 4). Plant stocks are monitored by cell or tissue specific Controllers, such as AMPK, mTOR, HIF-1a, stretch receptors and many others. These sensors generate negative feedback signals that control the flows into Plant stocks in a cell or tissue autonomous manner (such as the example of insulin-independent glucose uptake in exercising muscle, described above). Additionally, Plants produce signals that control the flows in a systemic manner. Signals of this category include various myokines, such as IL-6 and meteorin-like (Pedersen and Febbraio, 2012; Rao et al., 2014), which appear to report on fuel depletion in muscle.
Signals of the third class report the value of Storage stocks (Signal Sc in Figure 4). For example, leptin reports on the available fat storage in adipose tissue, and therefore controls food intake (caloric inflow) and energy expenditure (caloric outflow) (Friedman and Halaas, 1998). Hepcidin, similarly, reports on the storage stock of iron in the reticuloendothelial system in order to inhibit dietary iron uptake and prevent iron overload (Nemeth et al., 2004). Signals reporting on available glycogen stores are not known but are likely to exist. Signals of this class also participate in negative feedback circuits.
Signals of the fourth class report the values of flows (Signal Sd in Figure 4). For example, the gut hormone, GLP-1, reports on dietary glucose inflow, and therefore anticipates rising systemic glucose stock (which is itself reported by insulin) (Holst, 2007). CCK and NAPEs (N-Acylphosphatidylethanolamines) similarly report on dietary fat inflow, and reduce appetite to suppress further inflow (Gibbs et al., 1973; Gillum et al., 2008). FGF21 is produced by hepatocytes during fasting (Badman et al., 2007; Inagaki et al., 2007) and potentially reports on flow of fatty acids from the adipocytes during lipolysis. FGF21 expression in the liver is induced by fatty acids through PPARα (Potthoff et al., 2012). One might speculate that while PPARγ sensing of fatty acids in adipose tissue is an indicator of the inflow into the fat storage stock (taking place during feeding-associated lipogenesis), PPARα sensing of fatty acids in the liver is an indicator of the outflow from the storage stock (taking place during fasting-induced lipolysis). One important feature of signals that report on flows is that they typically operate in a feed-forward fashion. Because a change in a flow is predictive of the subsequent change in the stock, the signal reporting on an increased inflow, for example, would be expected to increase the outflow and inhibit other inflows of the same stock. This is in contrast to signals that report on the System, Plant, and Storage stocks, which all operate in a feedback fashion to maintain the stock within an acceptable range.
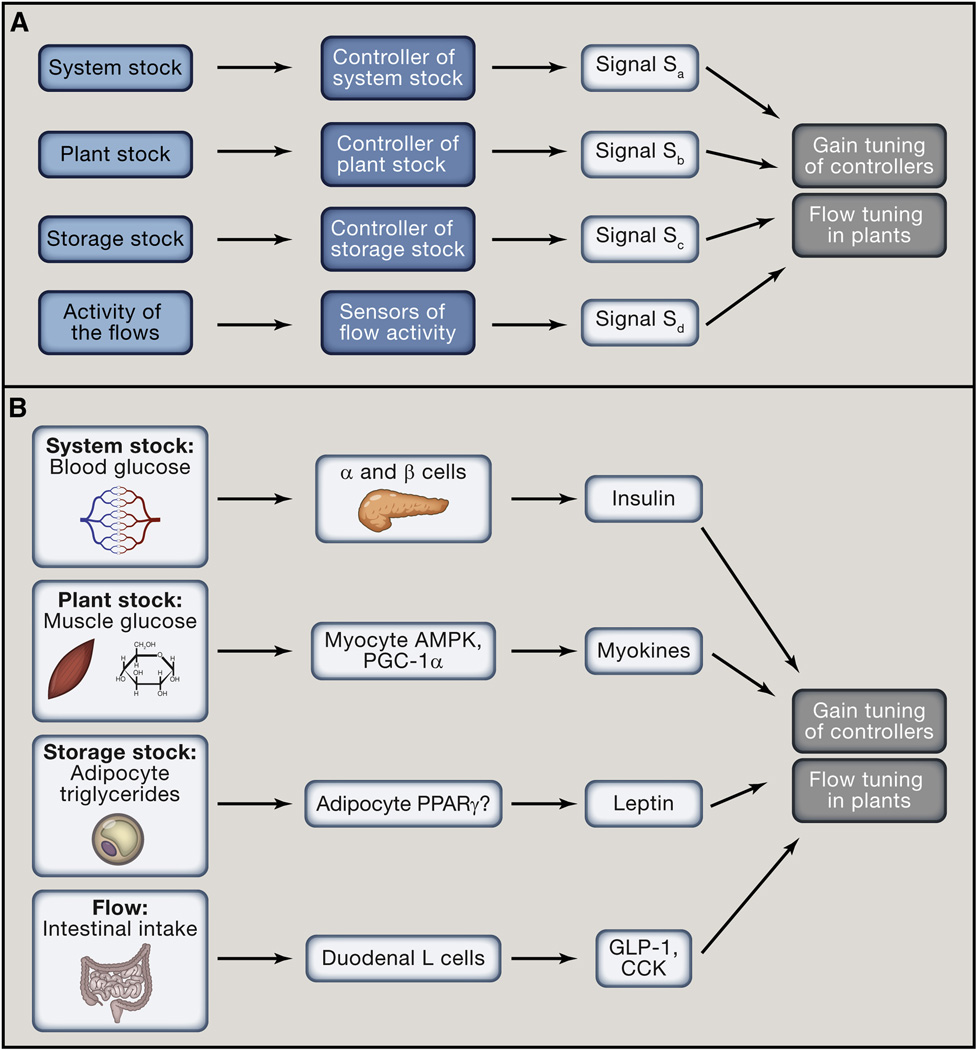
Figure 4. Four classes of signals control systemic homeostasis.
(A) Four classes of homeostatic signals report on values of four different types of variables: System stock (regulated variable), Plant stock, Storage stock and Flows. Each stock and the flows are monitored by dedicated Controllers and sensors. All four categories of homeostatic signals modulate gain tuning of Controllers and flow tuning in Plants. Signals that report on stocks operate in feed-back loops. Signals that report on flows operate in feed-forward loops.
(B) Signals reporting on the System stock (Sa) are classical endocrine hormones and efferents of the autonomic nervous system (e.g., insulin and glucagon). Signals reporting on Plant stocks (Sb) primarily operate in a cell or tissue autonomous manner (e.g., AMPK controlling GLUT4 expression), but may include signals acting systemically (e.g., AMPK controlling IL-6 expression in exercising muscle). Signals reporting on Storage stocks (Sc) indicate available resources (e.g., leptin reporting on fat stores). Finally, signals reporting on Flows (Sd) indicate anticipated changes in the System stock (e.g., GLP-1 reporting on incoming glucose). The examples are chosen to illustrate the point.
Monitoring the flows enables the system to minimize time delays that are unavoidable in negative feedback systems. Reacting to changing flows elicits an anticipatory response that makes the homeostatic system more robust to environmental fluctuations and helps to prevent dramatic changes in the stock. For example, intestinal glucose in-flow reporting by GLP-1 helps to prevent dramatic postprandial glucose spikes that would be unavoidable if only stock (blood glucose) reporting by insulin were available. Not every flow in the system needs to be monitored and reported as a signal. Presumably, only the flows that have a major impact on the system’s stock are monitored, particularly the flows that operate at the interface with the environment (for example, in the intestine, liver, kidney, lungs and skin).
The four categories of signals outlined above are defined by the homeostatic variables they report on. The effects of homeostatic signals fall into three categories: First, homeostatic signals directly regulate the flows of the system: for example, insulin suppresses hepatic gluconeogenesis. Second, homeostatic signals can change the sensitivity of the flows to another homeostatic signal: for example, placental hormones and glucocorticoids reduce the sensitivity of target tissues to insulin. Third, homeostatic signals can change the gains of the Controllers. For example, GLP-1 increases and leptin decreases the gain of the pancreatic β-cells – they change the amount of insulin produced in response to a given level of blood glucose. Thus, in addition to adjusting the flows of Plants, homeostatic signals can change the gains of Controllers.
In summary, a complex array of signals reporting on available stocks and flows allows Controllers to coordinate multiple Plants toward regulation of a homeostatic variable, while simultaneously balancing the needs and capabilities of individual Plants. Thus, application of the ‘stock and flow’ model provides a framework for functional classification of homeostatic signals and extends the traditional model of homeostasis, which is focused exclusively on Controller-to-Plant signals.
Adjustable set points and homeostatic adaptation
Homeostatic circuits can be broadly divided into two classes – those that have a single fixed set point and those with multiple or adjustable set points. The fixed set point circuits are characteristic of regulated variables that have a narrow dynamic range, such as arterial [pO2] or blood calcium concentration. Homeostatic systems with fixed set points are regulated solely by changing the flows, such as calcium resorption, excretion, storage and utilization. The adaptability of systems with a single set point is limited by the homeostatic range of the regulated variable; when the regulated variable deviates beyond the acceptable range (for example in extreme environments when the buffering capacity of the system is overwhelmed), the system can undergo catastrophic pathological changes. The failure of one homeostatic circuit may lead to a disruption of other connected circuits, resulting in particularly dangerous scenarios of cascading failures, as seen, for example, in sepsis.
In some cases, the changing environment or physiologic demands cannot be accommodated by homeostatic circuits with a fixed set point. In these cases, adjustable set points can be employed to maintain regulated variables within different dynamic ranges and enable more efficient adaptation to varying demands (Figure 5B–C). This ability to maintain conditions “at changing rather than similar levels or values” has been referred to as rheostasis (Mrosovsky, 1990).
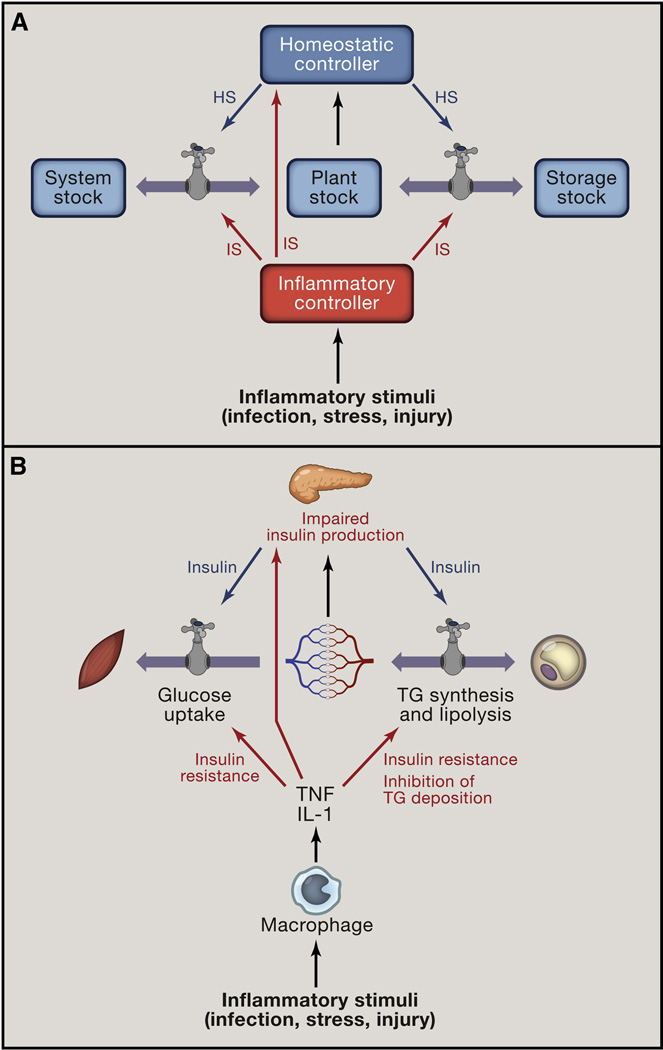
Figure 5. Inflammatory signals and homeostasis.
(A) Inflammatory signals (IS) act through the same control points (Plants flows and Controller gains) as homeostatic signals (HS). To illustrate the parallels between homeostatic and inflammatory signals, the source of inflammatory signal is referred to as Inflammatory Controller (e.g., macrophage), by analogy to Homeostatic Controller (e.g., endocrine pancreas).
(B) Macrophages produce TNF and IL-1 which act on the same flows as insulin, but in opposite direction: TNF and IL-1 induce insulin resistance and suppress lipid storage in adipose tissue by inhibiting lipoprotein lipase. In addition, these cytokines induce gain tuning of the pancreatic β-cells to reduce the amount of insulin produced in response to a given level of blood glucose. This effect is achieved in part by suppressing glucose flow into β-cells.
There are several examples of homeostasis with variable set points. Among the most obvious is fever, where the set point for core body temperature rises and is maintained at a higher level (as opposed to hyperthermia, where homeostatic mechanisms are engaged to return the temperature to the default set point). An extreme example of set point change is seen during hibernation: normally, ground squirrels exhibit an average daily body temperature near 37°C. During hibernation, however, their temperature may fall below 0°C and metabolic rate is dramatically suppressed (Barnes, 1989). This extreme physiologic switch is thought to permit adaptation to conditions of food scarcity that would be incompatible with life if the squirrels maintained their normal metabolic and temperature set points. Similarly, in human pregnancy, many physiologic parameters such as blood pressure, blood glucose, total body water and adiposity are dramatically altered in order to meet the needs of the fetus (King, 2000). These set point adjustments can occur even in a stable environment and reflect the adaptation to changing physiological priorities. Thus, a variety of environmental factors and changing physiological priorities, including seasonal and circadian changes, reproductive status (puberty and pregnancy), stress, nutrition, and infection, require homeostatic adaptations which in some cases appear to involve set point adjustments.
The change of the set points can occur in two different ways, depending on whether the set point-adjusting stimulus has to be continuously present to maintain a new set point value. The change of the body temperature set point during fever is induced by prostaglandin PGE2, which acts on thermoregulatory hypothalamic neurons (Romanovsky et al., 2005). As soon as inflammation subsides (or PGE2 production is blocked by COX2 inhibitors), the temperature set point changes back to the original value of 37°C. Thus, in this case, the continuous presence of PGE2 is required to maintain the altered set point for body temperature. The implication of this is that although all set points are defended, not all set points are equally stable: 37°C is the default set point for human body temperature, whereas set points induced by fever are not. As soon as the inducing stimulus subsides or is blocked, the system switches back from the induced set point to the default set point. This design feature provides a failsafe to prevent permanent and pathological shifts in the set point by requiring persistent stimulation. In contrast, the set point for human body weight appears to be maintained at multiple alternative stable states. The homeostatic systems that have alternative stable states without a default set point are particularly vulnerable to dysregulation, as we discuss next.
Set points and diseases of homeostasis
In contrast to circuits with fixed set points, which are generally robust to perturbations, homeostatic circuits with adjustable set points are vulnerable to dysregulation precisely because they are designed to be adjustable. For example, the adjustable set point for body weight and adiposity allows for adaptation to times of food abundance or scarcity, as well as to the accumulation of fuel stores to feed a growing fetus. However, in the setting of the modern environment, adjustable set points may have contributed to the current obesity epidemic (Speakman et al., 2011; Woods and Ramsay, 2007). If body adiposity had a fixed set point value, obesity would be impossible except for purely genetic reasons. In fact, most tissues other than visceral fat, have a single set point value for their size control as a function of body size, which is why they are not subject to homeostatic dysregulation. Like adiposity, glucose and lipid homeostasis are characterized by adjustable set points, while amino acid and purine/pyrimidine metabolism appear to have a single set point; accordingly, the former are vulnerable to homeostatic dysregulation while the latter are not.
One disease state particularly interesting from this perspective is insulin resistance. Insulin’s best-known function is to stimulate glucose uptake by skeletal muscle and adipose tissue, thereby reducing glycaemia. However, it is now appreciated that insulin has myriad effects, orchestrating a coordinated anabolic effort by liver, skeletal muscle and white adipose tissue to convert glucose and fatty acids into glycogen and triglycerides, respectively, to export these when necessary for storage in the appropriate organ, and to suppress the mobilization of stored fuels (Schenk et al., 2008; Shulman, 2011). In addition, insulin induces a trophic response in many cell types that promotes protein synthesis, and consequently cellular and tissue growth (Shulman, 2011). Interestingly, not all of these functions are reduced during the insulin resistant state (Brown and Goldstein, 2008), nor are all organs equally affected. Thus, insulin resistance is not equivalent to reducing the quantity of insulin in the blood, but rather is a method of physiologic set point adjustment that allows the organism to reallocate resources between different tissues.
Insulin sensitivity can be changed in many altered physiologic states. During pregnancy, critical illness, infection and stress, insulin responsiveness is diminished, presumably to allocate resources towards a growing fetus, tissue repair or the immune system, respectively (Odegaard and Chawla, 2013; Power and Schulkin, 2012; Watve and Yajnik, 2007). Conversely, insulin sensitivity is heightened during caloric restriction and weight loss, perhaps to increase anabolic efficiency.
Unfortunately, the adjustability of the insulin sensitivity set point also makes it vulnerable to disease. Insulin resistance is widely accepted as the pathological precursor for diabetes, a dangerous potential complication of obesity. Thus, the very mechanisms that evolved to make insulin receptor sensitivity adjustable also enable pathological insulin resistance. The same argument applies to other homeostatic systems with multiple set points that correspond to alternative stable states – they are vulnerable to dysregulation because they are designed to be adjustable.
As noted above, some homeostatic systems with multiple set points have a default set point value and any change of set point has to be actively maintained. Such systems, including control of body temperature, are generally less vulnerable to dysregulation because alternative set points are not stable.
Inflammation and homeostatic circuits
Inflammation is a protective response to extreme challenges to homeostasis, such as infection, tissue stress, and injury. Inflammatory signals - including cytokines, chemokines, biogenic amines and eicosanoids, induce myriad changes in diverse biological processes, ranging from local vascular responses to alterations of body temperature. Despite this complexity and diversity of functions, all the activities of inflammatory signals can be described in terms of their effects on homeostatic circuits: First, inflammatory signals can directly stimulate or inhibit the flows of various homeostatic systems. For example, TNF and IL-1β activate lipolysis, inhibit gluconeogenesis and increase vascular permeability to fluids and solutes, while IL-6 changes hepatic protein synthesis (Medzhitov, 2008). Second, in addition to directly affecting the flows, inflammatory signals can change the sensitivity of the Plants to homeostatic signals. For example, TNF makes liver, fat and skeletal muscle less sensitive to insulin (Hotamisligil et al., 1993; Weisberg et al., 2003). Third, inflammatory signals can change the gain of the Controllers. For example TNF and IL-1β suppress expression of GLUT2 and glucokinase in pancreatic β-cells, thus making them less sensitive to the blood glucose level (Park et al., 1999). Consequently, β-cells produce less insulin given the same amount of plasma glucose – an example of gain tuning of the Controller. As discussed above, homeostatic signals also operate by directly regulating flows, by changing sensitivity of Plants to other homeostatic signals, and by gain-tuning of Controllers. Thus homeostatic and inflammatory signals employ identical methods to change the same homeostatic variables (Figure 5).
Importantly, the inflammatory mediators are both antagonistic to and dominant over homeostatic signals. They are antagonistic because normal homeostasis is often incompatible with the goals of the inflammatory response, and the former has to be temporarily disengaged. Inflammatory signals are dominant because they have higher physiological priority as they orchestrate the protective response to life threatening insults of infection and injury. Thus, homeostatic control of body temperature (thermogenesis or sweating) is normally induced by changes in ambient temperature. However, acute inflammation overrides this control by raising the set point of body temperature, thereby inducing thermogenesis and fever regardless of ambient temperature. Likewise, acute inflammation-induced anorexia suppresses caloric intake regardless of the adiposity, circulating nutrient concentrations, or body weight.
It is increasingly appreciated that chronic inflammation is an important component of numerous disease states including obesity, type 2 diabetes, atherosclerosis, asthma, and neurodegenerative diseases. One potential mechanism by which inflammation may initiate or perpetuate disease is through set point changes. In obesity, for example, macrophages and other cells of the immune system infiltrate adipose tissue in response to the increased burden of lipid accumulation and adipocyte stress (Hotamisligil and Erbay, 2008; Weisberg et al., 2003). These cells produce inflammatory cytokines that are capable of shifting homeostatic set points in states of chronic inflammation, just as they do in acute inflammatory states. The rationale for transiently adjusting the insulin responsiveness in acute inflammation is presumed to be in shifting nutrient allocation from tissues that have lower priority during infection (adipose and skeletal muscle) towards the higher priority immune defenses (Hotamisligil and Erbay, 2008). In obesity, chronic inflammation may contribute to the shift of insulin sensitivity to an alternative set point.
Inflammation is a protective response that is engaged to defend and restore physiological functions when homeostatic mechanisms are insufficient. The inflammatory response can only achieve this goal by overriding or suppressing incompatible homeostatic controls. However, in its attempts to restore homeostasis, inflammation may enforce and propagate homeostatic set point changes that are detrimental and can result in chronic pathological states. This happens when a persistent change in the set point itself creates a problem sufficient to promote inflammation. For example, hyperglycemia can lead to glucose toxicity and tissue damage, which in turn can lead to secondary inflammation. Similarly, the abnormal accumulation of harmful lipid mediators (lipotoxicity) in adipocytes, liver, and muscle in obesity leads to cellular stress and tissue dysfunction, and consequently to inflammation (DeFronzo, 2010; Samuel and Shulman, 2012; Summers, 2006). Thus, a homeostatic perturbation initially induced by lipotoxicity may be further perpetuated by inflammation. In such scenarios, a vicious cycle can ensue that may explain the chronicity of some homeostatic diseases and their perpetuation by inflammation. Such a model is consistent with data demonstrating that inflammation is dispensable for the initial induction of insulin resistance, but contributes to maintaining and even worsening insulin resistance in states of chronic obesity (Oh et al., 2012).
Successful inflammatory response is followed by the resolution phase that restores homeostasis. However, because inflammation is induced by loss of homeostasis, but also intentionally disrupts incompatible homeostatic processes, the system has the potential to become locked in a state of a chronic inflammation that fails to resolve. The non-resolving inflammation may, in turn, account for the persistence of chronic diseases (Nathan and Ding, 2010; Serhan et al., 2007). It is therefore important to identify the mechanisms responsible for physiological shifts between alternative stable states of the homeostatic systems, as the same mechanisms could be employed therapeutically to reverse pathological states in chronic diseases of homeostasis.
Perspectives: Evolution, adaptation and disease
The concept of adaptability as vulnerability is pervasive in many forms of phenotypic variation, be they reversible (body weight) or irreversible (body height), continuous (reaction norms) or discontinuous (polyphenisms). Traits that are discontinuous are expressed through one of several alternative developmental pathways, a phenomenon known as phenotypic plasticity (Dewitt et al., 1998; Feinberg, 2007; Stearns and Koella, 2008). Such plasticity can allow for different phenotypes in the same organism, and can therefore afford greater adaptability. The choice of a particular developmental pathway is dictated by anticipation of certain environments where these pathways and associated traits would provide greater adaptation. However, if the environment is not as anticipated and the phenotypic choice is irreversible, maladapted phenotypes susceptible to disease may result (Dewitt et al., 1998; Feinberg, 2007; Stearns and Koella, 2008). Consequently, the mechanisms that afford greater adaptability can also create vulnerability to diseases (Bateson et al., 2004). Thus, phenotypic plasticity can be thought of as a developmental equivalent of homeostasis with alternative stable states dictated by adjustable set points.
The homeostatic capacity of an organism determines its ability to adapt to varying environments. Homeostatic systems with fixed set points are inflexible but resistant to dysregulation. If their buffering capacity is overwhelmed, the consequences are likely to be catastrophic, acute and transient, but rarely yielding chronic disease. Comparatively, homeostatic systems with adjustable set points provide a greater degree of adaptability, but are vulnerable to dysregulation and disease when the set points of the system are changed inappropriately, as often happens during chronic inflammation. Thus, the flexibility and adjustment of physiological and developmental characteristics, while providing a benefit of more efficient adaptation, are also responsible for the diseases of homeostasis. Treatment and prevention of diseases of homeostasis therefore will require a better understanding of the mechanisms responsible for the switch between developmental trajectories and homeostatic set points.
Summary
Here we present a framework that highlights the fundamental connections between homeostasis and inflammation. This framework is based on concepts previously developed in control theory and system dynamics theory. The key points of the framework are summarized below:
Homeostasis maintains essential parameters of the system within acceptable range. These parameters are regulated variables or stocks of the system. The processes that change or maintain these parameters are known as flows. The activity of the flow is a parameter known as controlled variable.
Homeostatic systems have two components: Controllers and Plants. Controllers monitor the stocks while Plants operate the flows.
If the value of regulated variable (X) differs from the set point value (X’), Controllers produce signals (S) that act on Plants to change the relevant flows.
Controller output is proportional to the error value |X-X’|. The coefficient of proportionality is a characteristic known as Controller’s gain.
Controllers can have a combination of different gains: proportional gain corresponds to the present error value, integral gain corresponds to the accumulated past error values, and differential gain corresponds to the anticipated future error value. The Controllers that have all three gains are known as PID (proportional, integral, differential) Controllers.
The gain of Controller can be tuned to change the setting of the system. In PID Controllers different gains can be tuned independently of each other to optimize system’s performance.
Homeostatic systems can have a single fixed set point, or multiple adjustable set points. The former are inflexible but robust to dysregulation. The latter are more adaptable but vulnerable to dysregulation. Chronic homeostatic diseases can result when the system becomes locked in an alternative stable state.
Plants have their own stocks. A special case of Plant stock is Storage stock. Storage stocks buffer the System stock from external fluctuations. System stock, Plant stock and Storage stock are connected by flows. Stocks connected by flows form nested homeostatic units, where each stock is regulated coordinately with other connected stocks.
- Homeostatic signals fall into four classes defined by the four types of homeostatic variables they report on: System stock, Plant stock, Storage stock and the flows. Each of these variables and the signals that report on them, provide different information about homeostatic system:
-
◦;System stock - information about the present value of regulated variable and its deviation from set point. Reported by classical endocrine hormones and efferents of the autonomic nervous system.
-
◦;Plant stock – information about the homeostatic capacity of individual Plants to maintain the System stock. Reported by non-endocrine tissue derived hormones.
-
◦;Storage stock - information about the amount of resources available to the system. Some storage stocks may reflect the accumulated past deviations of System stock from set point. Reported by hormones produced by tissues that serve as depots for regulated variables.
-
◦;Flows - information about the anticipated change in the System stock. Reported by hormones produced by tissues that operate flows with large impact on System stock.
-
◦;
Homeostatic signals affect two types of variables: Plant flows and Controller’s gains. In addition, the sensitivity of Controllers and Plants to homeostatic signals can also be regulated.
Signals that report on Storage stock tune the integral gain of Controllers, whereas signals that report on flows tune the differential gain of Controllers.
Inflammatory signals target the same control points as the homeostatic signals: these are Plant flows and Controller’s gains. In addition to directly affecting these parameters, inflammatory signals can modulate the sensitivity of Controllers and Plants to homeostatic signals.
Inflammatory response aims to restore homeostasis, but to achive this goal it has to suppresses incompatible lower priority homeostatic processes. Therefore, inflammatory signals are antagonistic to the incompatible homeostatic signals.
Inflammatory signals are dominant over homeostatic signals because they have higher priority. Physiological priorities determine the hierarchy of signals.
The parallels between homeostatic and inflammatory signals suggest the evolutionary origin of inflammation as a control system that complements the homeostatic control when the latter is insufficient.
Inflammation can change homeostatic settings of a system by changing Controller’s gains and by overriding homeostatic signals. Inflammation commonly accompanies homeostatic diseases associated with set point changes.
Acknowledgments
We thank Dr. Steven Stearns and members of the Medzhitov lab for discussions and critical reading of the manuscript. The work in the Medzhitov lab is supported by the Howard Hughes Medical Institute, Blavatnik Family Foundation, Else Kröner-Fresenius-Stiftung award and grants from the NIH. M.E.K. was supported by the MSTP program of Yale University Medical School.
References
- Åström KJ, Murray RM. Feedback systems: an introduction for scientists and engineers. Princeton: Princeton University Press; 2008. [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Barnes BM. Freeze avoidance in a mammal: body temperatures below 0 degree C in an Arctic hibernator. Science. 1989;244:1593–1595. doi: 10.1126/science.2740905. [DOI] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Bernard C. Paris: J. B. Baillière et fils; 1878. Leçons sur les phénomènes de la vie communs aux animaux et aux végétaux. [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. The Journal of biological chemistry. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell metabolism. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Burant CF, Bell GI. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry. 1992;31:10414–10420. doi: 10.1021/bi00157a032. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Adjustable set point: to honor Harold T. Hammel. J Appl Physiol. 2006;100:1338–1346. doi: 10.1152/japplphysiol.01021.2005. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Organization for physiological homeostasis. Physiological Reviews. 1929;9:399–431. [Google Scholar]
- Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Molecular cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt TJ, Sih A, Wilson DS. Costs and limits of phenotypic plasticity. Trends in ecology & evolution. 1998;13:77–81. doi: 10.1016/s0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, Kharitonenkov A, Spiegelman BM, Maratos-Flier E. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. Journal of comparative and physiological psychology. 1973;84:488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- Gillum MP, Zhang D, Zhang XM, Erion DM, Jamison RA, Choi C, Dong J, Shanabrough M, Duenas HR, Frederick DW, et al. N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 2008;135:813–824. doi: 10.1016/j.cell.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Beedle A, Hanson MA. Principles of evolutionary medicine. Oxford; New York: Oxford University Press; 2009. [Google Scholar]
- Hammel HT. Regulation of internal body temperature. Annual review of physiology. 1968;30:641–710. doi: 10.1146/annurev.ph.30.030168.003233. [DOI] [PubMed] [Google Scholar]
- Hardy JD. Control of heat loss and heat production in physiologic temperature regulation. Harvey lectures. 1953;49:242–270. [PubMed] [Google Scholar]
- Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. The Journal of clinical investigation. 2006;116:1767–1775. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nature reviews. Immunology. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell metabolism. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. American journal of physiology. Endocrinology and metabolism. 2008;295:E1287–E1297. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JC. Physiology of pregnancy and nutrient metabolism. The American journal of clinical nutrition. 2000;71:1218S–1225S. doi: 10.1093/ajcn/71.5.1218s. [DOI] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling molecules? Modulators? Trends in neurosciences. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Science's STKE : signal transduction knowledge environment. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Moran TH, Schulkin J. Curt Richter and regulatory physiology. American journal of physiology. Regulatory, integrative and comparative physiology. 2000;279:R357–R363. doi: 10.1152/ajpregu.2000.279.2.R357. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Rheostasis : the physiology of change. New York: Oxford University Press; 1990. [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Newgard CB, Lu D, Jensen MV, Schissler J, Boucher A, Burgess S, Sherry AD. Stimulus/secretion coupling factors in glucose-stimulated insulin secretion: insights gained from a multidisciplinary approach. Diabetes. 2002;51(Suppl 3):S389–S393. doi: 10.2337/diabetes.51.2007.s389. [DOI] [PubMed] [Google Scholar]
- Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annual review of biochemistry. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annual review of nutrition. 1996;16:235–256. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]
- Park C, Kim JR, Shim JK, Kang BS, Park YG, Nam KS, Lee YC, Kim CH. Inhibitory effects of streptozotocin, tumor necrosis factor-alpha, and interleukin-1beta on glucokinase activity in pancreatic islets and gene expression of GLUT2 and glucokinase. Archives of biochemistry and biophysics. 1999;362:217–224. doi: 10.1006/abbi.1998.1004. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature reviews. Endocrinology. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell metabolism. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes & development. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power ML, Schulkin J. Maternal obesity, metabolic disease, and allostatic load. Physiology & behavior. 2012;106:22–28. doi: 10.1016/j.physbeh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. (1985) [DOI] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP. Total self-regulatory functions in animals and human beings. Harvey Lecture Series. 1943;38:63–103. [Google Scholar]
- Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Frontiers in bioscience : a journal and virtual library. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Ruan HB, Singh JP, Li MD, Wu J, Yang X. Cracking the O-GlcNAc code in metabolism. Trends in endocrinology and metabolism: TEM. 2013;24:301–309. doi: 10.1016/j.tem.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. The Journal of clinical investigation. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GIaP, K F . Approach, W.F.a.B. Metabolism. In: Boron EL, editor. Medical Physiology: A Cellular and Molecular. Saunders: 2011. [Google Scholar]
- Speakman JR, Levitsky DA, Allison DB, Bray MS, de Castro JM, Clegg DJ, Clapham JC, Dulloo AG, Gruer L, Haw S, et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Disease models & mechanisms. 2011;4:733–745. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC, Koella JC. Evolution in health and disease. 2nd edn. Oxford ; New York: Oxford University Press; 2008. [Google Scholar]
- Summers SA. Ceramides in insulin resistance and lipotoxicity. Progress in lipid research. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Watve MG, Yajnik CS. Evolutionary origins of insulin resistance: a behavioral switch hypothesis. BMC evolutionary biology. 2007;7:61. doi: 10.1186/1471-2148-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, Satural Selection, and the Evolution of Senescence. Evolution; international journal of organic evolution. 1957;11:398–411. [Google Scholar]
- Wirtshafter D, Davis JD. Set points, settling points, and the control of body weight. Physiology & behavior. 1977;19:75–78. doi: 10.1016/0031-9384(77)90162-7. [DOI] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Homeostasis: beyond Curt Richter. Appetite. 2007;49:388–398. doi: 10.1016/j.appet.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature medicine. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]