Abstract
Dendritic cells (DCs) play a pivotal role in the tumor microenvironment (TME), the latter of which is known to affect disease progression in many human malignancies. Infiltration by mature, active DCs into the tumors confers an increase in immune activation and recruitment of disease-fighting immune effector cells and pathways. DCs are the preferential target of infiltrating T cells. Tumor cells, however, have means of suppressing DC function or of altering the TME in such a way that immune suppressive DC are recruited. Advances in understanding these changes have led to promising developments in cancer therapeutic strategies targeting tumor-infiltrating DCs in order to subdue their immunosuppressive functions and enhance their immune stimulatory capacity.
INTRODUCTION
In recent years, it has become increasingly appreciated that the immune system's role in modulating malignancy is far more complex than anticipated. A number of studies have found correlations between the presence of infiltrating immune cells in the tumor microenvironment (TME) and prognosis of many cancers such as ovarian, renal cell, colorectal, and breast cancers (1). The immune component of the TME is comprised of predominantly CD4+ and CD8+ T cells, dendritic cells (DCs), macrophages, and regulatory T-cells (Tregs) (2). In general, T cell infiltration portends a better outcome (1, 3-5). One key example, published by Zhang, found that tumor-infiltrating T cells were observed in 55% of tumors obtained from advanced ovarian cancer patients. The 5-year overall survival rate for patients whose tumors contained tumor-infiltrating T cells was 38% in comparison to a 4.5% rate of survival for those whose tumors did not (1). In contrast, many other studies over the past decade have demonstrated that other subsets of adaptive immune cells are typically, but not always, associated with worse prognosis, seeming to promote tumorigenesis (6-9). For example, regulatory (CD4+/CD25+FOXP3+) T cells (Tregs) in ovarian cancer confer a significantly higher risk of death even when controlled for stage and degree of surgical reduction of disease (8). In addition to immune suppressive T cells, tumors by themselves are adept at preventing destructive capabilities of infiltrating anti-tumor immune effector cells. Tumors promote apoptosis and paralyze anti-tumor effector cells through the release of immune suppressive factors like nitric oxide (NO), IL-10, IL-6, arginase-I, vascular endothelial growth factor (VGEF), indoleamine 2, 3-dioxygenase (IDO), and TGF-β (10-14).
Also, suppressive cells of the innate arm of the immune system such as inflammation-induced myeloid derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) are known to be correlated with poor outcome and rapid disease progression (15-22). Although the negative roles of these innate immune suppressive cells in the TME are widely demonstrated, the role of others, such as DCs, has been subject to debate due to conflicting observations (23-26). DCs have an integral role in influencing the immune response, and are the subset of cells in the TME to which anti-tumor T cells are attracted, but they may alter their role from being immunostimulatory to immunosuppressive at different stages of cancer progression (27). The focus of this article is on tumor-infiltrating DCs (TIDCs). Here we will discuss their interaction with the progression or suppression of malignancy and we highlight the new directions for the therapeutic manipulation of such immunosuppressive DCs to tip the balance in favor of anti-tumor immunity.
DENDRITIC CELLS
Described in the early nineteenth century by Paul Langerhans and termed dendritic cells in 1973 by Ralph M. Steinman and Zanvil A. Cohn, DCs are key decision makers, determining whether or not the adaptive arm of the immune system should or should not be activated. Crucial as professional antigen presenting cells (APCs), they not only present antigens but also provide a multitude of other necessary signals (co-stimulatory molecules and cytokines) for T cell activation and differentiation, thereby shaping the immune response. DCs also interact with other immune cells, including natural killer (NK) cells and B cells (28, 29). Many subsets of DCs with unique and specific functions, morphology, and localization have been described (30). These include Langerhans cells, monocyte-derived DCs (CD14+ DCs), myeloid DCs and plasmacytoid DCs. Furthermore, each of these has different maturation states that add to the complexity. Identification of DCs, due to their heterogeneity, can be challenging and variable between studies, with some using single and others multiple surface cell markers. At least 3 subsets of splenic murine DCs have been described: CD11chighCD8α+CD11b-DEC205+ lymphoid, CD11chighCD8α−CD11b+DEC205+ myeloid, and CD11cintermediateCD8α+/−CD11b−B220+ Gr-1+ plasmacytoid DCs (31). Generally, myeloid DCs are thought to exhibit stimulatory effects, while lymphoid or plasmacytoid DCs are involved with regulation or tolerogenesis. However, there are reports demonstrating immunosuppressive activities on the part of myeloid DCs and antigen presentation by plasmacytoid DCs (32). DC lineage is not as well characterized in humans; they are thought to arise from a myeloid progenitor, shared with macrophages, called a macrophage-dendritic cell progenitor which in turn gives rise to common dendritic cell progenitors (CDP). CDP then gives risk to plasmacytoid DCs and pre-dendritic cells that are progenitors of 2 myeloid subsets. Human plasmacytoid DCs express surface markers CD123, CD303 or BDCA-2, and CD304 or BCDA-4. Human myeloid DCs are defined largely by CD1c/BDCA-1+ or CD141/BDCA-3+ expression. There are many conserved molecular pathways when comparing murine to human DCs, suggesting preservation of function (33).
DCs tend to congregate in blood and lymphoid tissues, although found widely in the body, as Langerhans cells in the skin and as intestinal DCs in the GI tract. The characteristics of human TIDCs have perhaps best been studied, to date, in cutaneous melanoma. Noted though, is the difficulty of clearly characterizing human TIDCs due to significant patient/population heterogeneity, relatively low frequency of TIDCs, and differences in use of markers to assess TIDC populations. In melanoma, TIDC frequency tends to be higher in the peri-tumoral area and those cells exhibit a more mature phenotype, while more immature TIDCs are found within the tumors. However, the relationship between TIDC location and clinical outcome is not well understood, as it depends on TIDC type and communication with other cells (34). In general, mature DCs have been considered immune stimulatory whereas immature ones suppressive and tolerogenic. However, based on their attributes such as activation status, maturity and polarization in TME, DCs functional plasticity is considered complicated (35-39). This makes it difficult to make any generalized inference about the roles of DCs in the tumor microenvironment.
TUMOR-INFILTRATING DCS
Tumor-infiltrating DCs have an immature and/or paralyzed phenotype
Tumor-infiltrating DCs (TIDCs) have been found in the TME in many different cancer types, such as breast, colorectal, lung, renal, head and neck, bladder, gastric, and ovarian (40). Not surprisingly, due to the complexity of phenotype as well as the methods of identification, DC infiltration into tumors has been reported to have an association with both good and poor prognosis in different tumor types. For example, in one study patients with colorectal carcinoma, with high numbers of TIDCs, exhibited both shorter disease-free and overall survival than those with low numbers (41). Conversely, TIDC presence has been correlated with regression of melanoma (32). Prognostic impact may be more related to a shift in TIDC phenotype, or the relative proportions of subtypes within the tumor infiltrate, rather than simply the degree of TIDC infiltration into the tumor (42). TIDCs tend to exhibit a phenotype of low co-stimulatory molecule expression (26, 37), blunted antigen cross-presentation (26), and expression of regulatory molecules and receptors (37, 43) and are usually associated with immunosuppression. Animal modeling studies have shown that the type, phenotype, and amount of TIDCs are dynamic over time and may influence disease progression significantly at different stages of tumor growth. For example, our laboratory has shown in an ID8 mouse model of ovarian cancer that as the cancer progresses from light to heavy tumor burden, the numbers of TIDCs increases. Nearly all of the DCs progressively upregulated immune suppressive molecules; this change is associated with a concomitant loss of T cell infiltration (37). Scarlett made similar observations in a spontaneous murine model of ovarian cancer. In that model, tumor progression was associated with increasingly dense immune infiltrates, which included DCs, macrophages, MDSCs and T cells. A turning point was observed, at which aggressive growth correlated with a switch from immune stimulatory DCs to immune suppressive DCs (38). Depletion of DCs in that model early in the course of disease led to increased tumor expansion and aggression while depletion later led to abrogation of tumor growth, suggesting that tumors eventually develop strategies to prevent DCs from sensing endogenous danger signals and responding with immune stimulation. While it is not possible to determine if this is relevant to human cancer, there are some indications in the literature. For example, Kocian found, in a study of colorectal cancer, that relapsed patients were more likely to have had fewer TIDCs in their primary tumors. Importantly, there was a distinct difference in the proportion of mature and immature (i.e. tolerogenic) TIDCs noted, with those experiencing disease recurrence having higher densities of immature TIDCs and lower densities of mature TIDCs in their primary lesions (44). A study of non-small cell lung cancer TIDCs demonstrated that there are three DC subsets, those with high, intermediate, and no CD11c expression. In particular, the intermediate CD11c expressing group appeared to have low levels of co-stimulatory molecules and high levels of immune inhibitory molecule PD-L1 (25). The TIDCs as a group exhibited poor response to toll-like receptor stimulation in terms of antigen presentation capability. In breast cancer, plasmacytoid dendritic cells within the primary tumor have been correlated with worse prognosis. When isolated from breast tumor biopsies from those with high mitotic index and triple-negative breast tumors (aggressive breast tumors), pDCs produced low amounts of interferon-α in comparison to pDCs isolated from patients’ blood. These cells also were shown to sustain FoxP3+ Treg expansion, potentially contributing to immune tolerance and worsened clinical outcomes (45). While not yet clear what specifically induces these shifts in DC subset distribution and/or phenotypic shift, release of cytokines or other factors into the TME are likely involved.
Local induction of paralysis of tumor-infiltrating DCs
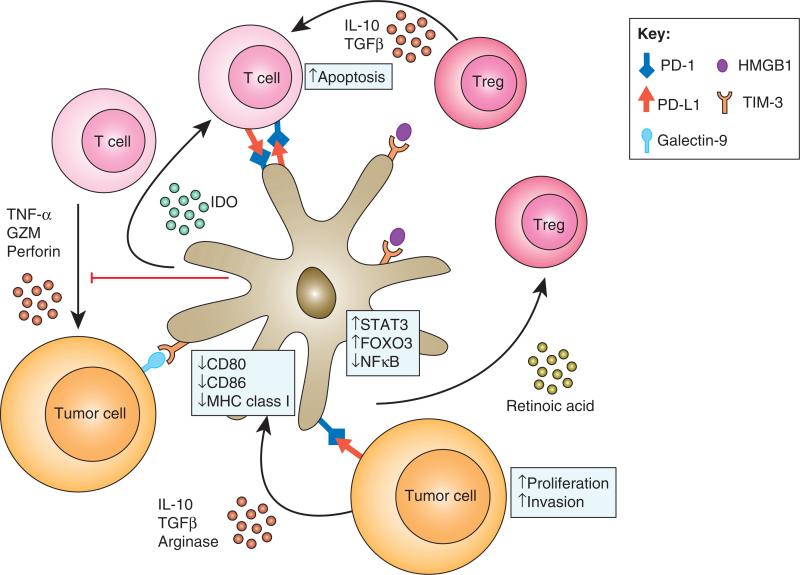
Tumor cells and the TME release factors that inhibit or reverse DC maturation and normal function. For example, Michielsen showed that conditioned media obtained from culturing human colorectal tumor explant tissue was high in VEGF and the chemokines CCL2, CXCL1, and CXCL5. Pre-treatment of DCs in vitro with this media resulted in inhibition of maturation (46). A dynamic network consisting of cell surface molecules and soluble factors regulates TIDC mediated tumor immune interactions in TME (Figure 1). Our group has observed that one way in which tumors paralyze DCs is through the induction of PD-1 expression (37). PD-1 and its ligands PD-L1 and PD-L2 constitute an important immune regulatory pathway which suppresses or impairs effective T, B, and myeloid cell responses both in the initiation (priming) and effector phases of the immune response. Our data shows that PD-1 expression on murine DCs is minimal in the early stages of tumor growth but as the disease progresses, nearly all TIDCs will eventually have high level of PD-1 (37, 47). Blockade of PD-1 on these TIDCs in vitro enhanced the production of immune stimulatory cytokines, increased NFκB activation in DCs, improved co-stimulatory molecule expression, and improve the ability of these DCs to activate T cells (37, 47). Thus, the paralyzed phenotype of TIDCs is likely reversible, at least pharmacologically. Another mechanism by which TIDCs are locked into an immune suppressive or non-immunogenic phenotype is through the upregulation of T-cell immunoglobulin and mucin domain 3 (TIM-3) at the cell surface. TIM-3 has been largely studied as a suppressive marker of Th1 type T cells and blockade of TIM-3 exacerbates various T cell mediated autoimmune diseases (48). More recently, its expression and role in modulating innate immune activation has become clearer (49). Chiba found that both murine and human tumors induce upregulation of TIM-3 on DC through multiple factors found in the TME such as IL-10, TGF-β1, VEGF-A, IDO and arginase (50). More importantly, they found that TIM-3 interacted with HMGB1 and prevented it from sensing tumor-derived nucleic acid danger signals in the TME. In addition to directly suppressing danger signal sensing, TIM-3 ligation with cross-linking antibody results in activation of Bruton's tyrosine kinase (BTK) and c-SRC resulting in the release of immune suppression factors by DCs that suppressed NFkB signaling (51).
Figure 1. TIDCs are central to tumor immune interactions in the TME.
DCs are recruited into the TME and induced to upregulate PD-1 and TIM-3. Interactions of PD-1 with PD-L1 in the TME blocks responsiveness to danger signals and prevents T cell activation through antigen presentation and co-stimulation. Danger signals are also reduced due to high TIM-3 expression, which binds HMGB1. T cells are preferentially drawn to TIDCs as they enter the TME. In addition to the lack of appropriate activating signals, T cell responses are blocked by engagement of PD-1 by PD-L1 on the DC surface. Tregs are also induced by the TIDCs to establish a tolerogenic environment.
Emerging evidence suggest that TIDCs have an impaired antigen presentation capacity. For example, using the B.16 mouse melanoma tumor model, Stoitzner showed that despite uptake of antigen TIDCs (CD11c sorted cells) cannot cross present the antigen to OVA-specific CD8+ OT I and CD4+ OT-II T cells (52). In unpublished data from our laboratory in which we used PD-1+ TIDCs from the ID8 mouse model of ovarian cancer, we observed that blockade of PD-1 on these PD-1+ TIDCs that are pulsed with ovalbumin enhanced their ability to activate OT-1 T cells. This suggests that PD-1 expressed on TIDCs might act as a switch that regulates antigen cross presentation capacity of TIDCs. On the other hand, Engeldardt showed that murine TIDCs (CD123-ve) ingest tumor-derived protein and present processed antigens to infiltrating tumor-specific T cells, even engaging in long-lived interactions with TIDCs, but that these interactions are non-productive (27). In addition to regulating antigen presentation capacity, tumors and/or TME are also known to play a role in modifying DC activity by inducing the upregulation of micro RNAs in these cells. Min showed that multiple miRNAs such as miR-16-1, miR-22, miR-155, and miR-503 are upregulated in DCs (BMDCs) by mouse ovarian cancer, melanoma, cervical, lung, and breast cancer cell lines. In the presence of tumor cells DCs undergo apoptosis at higher rates compared to DCs that were co-cultured with normal fibroblasts. Furthermore, it was observed that these miRNAs specifically targeted PI3K/AKT, MAPK and apoptotic signaling pathways in DCs (53).
Tumor-infiltrating DCs block adaptive immunit
Immature and paralyzed TIDCs suppress both innate and adaptive immune effectors using a wide variety of mechanisms (Figure 1). The ligand for PD-1, PD-L1, is constitutively expressed on activated DCs and its expression is further upregulated by the TME (54). Studies by Curiel and Krempski showed that blockade of PD-L1 specifically on murine TIDCs resulted in a better capability of TIDCs to stimulate T cell activation (37, 54). Krempski also showed that that the TIDCs co-express both PD-1 and PD-L1, suggesting the possibility that suppression of T cell activation may be mediated by either of the molecule's interactions with PD-L1 or PD-1 on the T cells, respectively (37). Using PD-L1 knockout T cells, Krempski went on to show that it was TIDC-bound PD-L1 that mediated direct immediate T cell suppression as assessed in mixed lymphocyte reaction, whereas DC-bound PD-1 paralyzed TIDCs rendered them unresponsive to danger signals and upregulated co-stimulatory molecules and cytokines (37). Thus, the suppressive nature of TIDCs mediated by PD-L1 appears to be constitutively present but is overcome by increased levels of co-stimulatory molecules and cytokines. This balance between inhibitory and activating potential is reminiscent of the killer phenotype of natural killer cells whose activity is a balance of several inhibitory and activating receptors (55).
Consistent with the idea that the immune activating potential of TIDCs is a balance between multiple inhibitory and activating molecules, DCs possess mechanisms other than just PD-L1 to block T cell activation. Liu reported that murine lung tumor cells release large amounts of PGE2 and TGFβ which results in the conversion of immune activating DCs into an immune suppressive DCs (CD11clowCD11bhighIalow) and these DCs suppress T cell responses mediated partially through upregulation of arginase I, which degrades arginine, an amino acid that T cells are unable to produce themselves and which is required for CD4 T cell proliferation (56). Higher arginase expression may also lead to higher levels of reactive oxygen intermediates (ROS) which blocks antigen-specific CD8 T cell responses (57). Indoleamine 2,3-dioxygenase (IDO) expression by TIDCs also plays an important role in mediating the suppression of adaptive immune responses (58). Tumor-derived PGE-2 induces IDO expression in TIDCs and these IDO+ TIDCs suppress CD8 T cell responses to antigens presented by these TIDCs themselves as well as those presented by third-party, non-suppressive DCs. TIDCs may also suppress adaptive immune responses indirectly. Indirect mechanisms include the induction of Tregs (by human pDCs) (59). Cytokines such as TGF-β, IL-10 and IL-2 induce DCs to stimulate Treg formation in vitro (60). Recently, it was described that these cytokines may act together with cosignaling/surface molecules such as inducible T-cell costimulator-ligand (ICOS-L), programmed death 1 ligand (PD-L1), CD80 and CD86 to induce DCs to stimulate Treg formation (59, 61-64).
The paralyzed, immune suppressive phenotype of TIDCs is regulated by hyperactivation of tumor-induced transcription factors
Also, TIDCs have been reported to overexpress several important regulatory genes which regulate their immune suppressive and/or paralyzed phenotype. The most prominently studied is the transcription factor STAT3, which has been found to be hyperactivated in DC following exposure to tumor derived factors (65, 66). STAT3 hyperactivation induces S100A9 protein which prevents full maturation of DCs thereby blocking their responsiveness to local danger signals (67). We have also found that IL-10, which is overexpressed in the TME of various tumors, can activate STAT3 and induce enhanced expression of PD-1 and PD-L1 on the dendritic cells (unpublished observation); hence rendering them ineffective. Human and murine prostate cancer TIDCs (pDCs and non-pDCs) have also been reported to overexpress another cell cycle associated transcription factor, FOXO3, whose overexpression upregulates IDO, arginase, and TGFβ while at the same time diminishing the expression of costimulatory molecules, release of inflammatory cytokines (43).
THERAPEUTIC MANIPULATION OF TUMOR-INFILTRATING DENDRITIC CELLS
Inroads have been made into the issue of how to modify TIDC effectively. Their central role in the maintenance of the innate and adaptive immune responses, as well as their ability to present antigen to effector cells, mark them as attractive targets for cancer treatment (68, 69). Over the past two decades, DCs have been used in vaccine models in both preclinical and clinical settings. However, an intriguing new area of potential therapeutic impact is the manipulation of TIDCs. As yet, this has not been translated into the clinical setting, but there are several promising pathways and targets that have been recently investigated.
Targeting the pathways that paralyze TIDCs appears to be a promising approach and certainly, one of the most attractive pathways is to specifically target the PD-1/PD-L1 inhibitory axis, particularly considering the rapid advances in the clinical development of biologics that block PD-1 and PD-L1 interactions (70). This may be particularly true with respect to advanced cancers in which PD-1 becomes highly expressed on TIDCs (37). Blocking the ligation of PD-1 on DCs as was discussed above restores danger responsiveness with increased T cell activation capabilities (37). Furthermore, recent data from our group has shown that PD-1 blockade specifically on murine TIDCs also leads to an increase in IL-7R expression by T cells resulting increased persistence in the tumor microenvironment (47). Further studies to validate the anti-PD-1 mediated rescue of immune stimulatory capacities of TIDCs will open up paths for combination therapies in the clinical setting, aimed at enhancement of anti-tumor immunity via the TIDC compartment of the TME. Anti-PD-1 antibody is under investigation and has shown some efficacy and safety in patients with different tumor types (71) and has recently been approved by FDA for use in patients with unresectable or metastatic melanoma if disease progresses following ipilimumab or in case of BRAF V600 mutation following BRAF inhibitor treatment. Similarly, therapeutic biologics targeting the immune suppressive molecule, TIM-3 are currently under development and showing great promise (72). Given the multiple cell types (e.g. TIDCs, T cells, macrophages) in the tumor that express both PD-1 and TIM-3, it will be necessary to carefully craft translational studies which enable a better understanding of the impact of these therapeutic modalities on the TIDCs. Alternatively, another potential modality is to block tumor produced factors that induce TIDC paralysis, such as IL-10, one of the key cytokines known to upregulate both TIM-3 and PD-1 on TIDC ((50) and unpublished observations). Vicari has shown that TIDCs (most of which were of immature phenotype) that were refractive to stimulation by a combination of LPS, anti-CD40, and IFNγ could be rescued by treatment with a combination of CpG and anti-IL-10R antibody both in vitro and in vivo, leading to better de novo IL-12 production and anti-tumor immune responses (73).
Other studies aimed at inducing the maturation of TIDCs have also been conducted. Saito showed that when tumor-bearing mice are treated with plasmids containing cDNA for Flt3L or intra-tumoral injection with adenoviral vector carrying IL-18 gene, the TIDCs expressed higher CD86 and exhibited better potential to activate T cells. Potent antitumor activity also developed in distant un-injected tumors from the treated mice (74). In addition to this, given the prominent role of miRNAs in maintaining the function of TIDCs, delivery of modulators of miRNAs may be of therapeutic benefit, although low bioavailability and cellular uptake are the challenges associated with the delivery of these types of agents. Taking advantage of the endocytic activity of DCs to deliver nanoparticles carrying oligonucleotide complexes, the activity of endogenous immune regulating miRNAs or transcription factors can be augmented or suppressed. Cubillos-Ruiz showed that such an approach can be used to deliver miRNA-155 which resulted in the subsequent skewing of TIDCs towards being immune stimulatory rather than immune suppressive, and eventually slowed ovarian cancer progression in a mouse model (75). A caveat however, is that some miRNAs have multiple roles, making them difficult targets. For example, miR-155 has also been shown to be associated with enhanced apoptosis of TIDCs and therefore therapeutic targeting may not have the desired effects (75). Luo found that incorporating STAT3 siRNA into a nanovaccine and immunizing mice intraperitoneally resulted in replacement of suppressive TIDCs in the TME with more immune stimulatory DCs indicating that the TIDC compartment in the TME is continuously being renewed (76).
Lastly, other interesting avenues for modification of TIDC behavior and phenotype are being explored. In one study of a murine model of lung cancer, a population of TIDCs was identified that increased in the tumor as the cancer progressed. Polarization of the TIDCs was found to be influenced by small rho GTPase signaling, which has been previously reported as integral to DC endocytosis, antigen processing/presentation, and motility. Rho GTPase inhibitor toxin B blocked the effect of paclitaxel in preventing tumor-induced shift towards regulatory DC's, offering a possible mechanism for tumor immunologic escape and a future point of intervention (77). Lactic acid production by tumor cells may be yet another means whereby cancer cells are able to induce immune suppressive TIDCs. For example, in spheroid cultures of tumor cell lines, melanoma and prostate carcinoma cells were noted to produce high levels of lactic acid. The addition of lactic acid to in vitro cultured DCs produced a phenotype similar to melanoma and prostate TIDCs (i.e. decreased IL-12 production and immature phenotype), and blockade of lactic acid reverted the phenotype to normal (78).
Targeting of these immune therapies to the tumor microenvironment in clinical practice will be a challenge given the complexity of immune regulation; there are several approaches under investigation by which these pathways may be utilized. The development of a systemic antibody affecting a tolerogenic pathway, such as an anti-PD-1 antibody or biologic targeting of TIM-3, is one such method and several agents are currently in development or under clinical investigation. Manipulation of immune tolerance and increased activation of dendritic cells in combination with development of a dendritic cell-based vaccine for cancer antigen presentation would be another approach, as well as intra-tumoral delivery of agents or TIDC modulating agents given in conjunction with chemotherapy to boost efficacy.
CONCLUSION
Over the past few years the concept of the TME in the setting of malignancy has become well developed and while many immune cells play integral roles in the TME it is increasingly clear that DC are central mediators. The complexities of their role have yet to be fully elucidated, but studies to date have yielded promising insights into their function within the TME and directions for DC-targeted cancer therapy. Evident now is the fact that TIDCs are impaired in a variety of ways, which lead these DCs to confer immune suppression rather than immune stimulation at the local TME. Pre-clinical studies demonstrate promising areas for therapeutic intervention, such as the use of chemotherapy drugs at lower, non-cytotoxic doses for immune modification, blocking the pathways that induce tumor-induced shifts to a suppressive or regulatory DC phenotype, and targeted activation of TIDCs. Understanding the mechanisms and players involved in inducing such impaired and immune suppressive DCs will open up avenues to explore therapies directed towards rescuing the immune stimulatory potential of TIDCs.
Acknowledgments
This work was supported by the Minnesota Ovarian Cancer Alliance, the Fred C. and Katherine B. Andersen Foundation, the Marsha Rivkin Foundation and the Mayo Clinic Ovarian Cancer SPORE (P50- CA136393, National Institutes of Health/National Cancer Institute).
REFERENCES
- 1.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 2.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25:2559–2572. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 4.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 5.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 7.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J. Cancer. Res. Clin. Oncol. 2010;136:1585–1595. doi: 10.1007/s00432-010-0816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 9.Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, Goode EL, Knutson KL. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One. 2013;8:e80063. doi: 10.1371/journal.pone.0080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gastman BR, Johnson DE, Whiteside TL, Rabinowich H. Tumor-induced apoptosis of T lymphocytes: elucidation of intracellular apoptotic events. Blood. 2000;95:2015–2023. [PubMed] [Google Scholar]
- 11.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin. Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Mocellin S, Wang E, Marincola FM. Cytokines and immune response in the tumor microenvironment. J. Immunother. 2001;24:392–407. [PubMed] [Google Scholar]
- 13.Botti C, Seregni E, Ferrari L, Martinetti A, Bombardieri E. Immunosuppressive factors: role in cancer development and progression. Int. J. Biol. Markers. 1998;13:51–69. doi: 10.1177/172460089801300201. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 15.Gorgun GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, Raje N, Munshi NC, Richardson PG, Anderson KC. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121:2975–2987. doi: 10.1182/blood-2012-08-448548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevko A, Umansky V. Myeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: thick as thieves. J. Cancer. 2013;4:3–11. doi: 10.7150/jca.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber AL, Munst A, Schlapbach C, Shafighi M, Kiermeir D, Husler R, Hunger RE. High expression of FOXP3 in primary melanoma is associated with tumour progression. Br. J. Dermatol. 2014;170:103–109. doi: 10.1111/bjd.12641. [DOI] [PubMed] [Google Scholar]
- 18.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F, Lamy T, Sonet A, Rousselet MC, Foussard C, Xerri L. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J. Clin. Oncol. 2008;26:440–446. doi: 10.1200/JCO.2007.12.8298. [DOI] [PubMed] [Google Scholar]
- 20.Ganjoo KN, Witten D, Patel M, Espinosa I, La T, Tibshirani R, van de Rijn M, Jacobs C, West RB. The prognostic value of tumor-associated macrophages in leiomyosarcoma: a single institution study. Am. J. Clin. Oncol. 2011;34:82–86. doi: 10.1097/coc.0b013e3181d26d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darrasse-Jeze G, Podsypanina K. How numbers, nature, and immune status of foxp3(+) regulatory T-cells shape the early immunological events in tumor development. Front. Immunol. 2013;4:292. doi: 10.3389/fimmu.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 23.Khazaie K, Blatner NR, Khan MW, Gounari F, Gounaris E, Dennis K, Bonertz A, Tsai FN, Strouch MJ, Cheon E, Phillips JD, Beckhove P, Bentrem DJ. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30:45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 24.Boissonnas A, Licata F, Poupel L, Jacquelin S, Fetler L, Krumeich S, Thery C, Amigorena S, Combadiere C. CD8+ tumor-infiltrating T cells are trapped in the tumor-dendritic cell network. Neoplasia. 2013;15:85–94. doi: 10.1593/neo.121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pacheco Y, Lebecque S. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J. Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 26.Harimoto H, Shimizu M, Nakagawa Y, Nakatsuka K, Wakabayashi A, Sakamoto C, Takahashi H. Inactivation of tumor-specific CD8(+) CTLs by tumor-infiltrating tolerogenic dendritic cells. Immunol. Cell. Biol. 2013;91:545–555. doi: 10.1038/icb.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, Egeblad M, Krummel MF. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21:402–417. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 29.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 30.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulendran B, T. H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets Curr. Opin. Immunol. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, S. G, Peivuan Z, Shurin MR. Dendritic Cells in the Cancer Microenvironment. J .Cancer. 2013;4:36–44. doi: 10.7150/jca.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chistiakov DA, S. I, Orekhov AN, Bobryshev YV. Myeloid dendritic cells: Development, functions, and role in atherosclerotic inflammation. Immunobiology. 2015 doi: 10.1016/j.imbio.2014.12.010. doi: 10.1016/j.imbio.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Klarquist JS, J. E. Melanoma-infiltrating dendritic cells: Limitations and opportunities of mouse models. Oncoimmunology. 2012;1:1584–1593. doi: 10.4161/onci.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 36.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 37.Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, Goode EL, Kalli KR, Knutson KL. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J. Immunol. 2011;186:6905–6913. doi: 10.4049/jimmunol.1100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, Fiering S, Conejo-Garcia JR. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J. Exp. Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol. Lett. 2010;127:77–84. doi: 10.1016/j.imlet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Karthaus N, Torensma R, Tel J. Deciphering the message broadcast by tumor-infiltrating dendritic cells. Am. J. Pathol. 2012;181:733–742. doi: 10.1016/j.ajpath.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Jochems C, S. J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp. Biol. Med. 2011;236:567–579. doi: 10.1258/ebm.2011.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang WJ, D. Y, Zhao X, Ma LY, Cao GW. Inflammation-related factors predicting prognosis of gastric cancer. World. J. Gastroenterol. 2014;20:4586–4596. doi: 10.3748/wjg.v20.i16.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, Ambs S, Yagita H, Hurwitz AA. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J. Clin. Invest. 2011;121:1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Kocian P, S. N, Drgac J, Cerna K, Hoch J, Kodet R, Bartunkova J, Spisek R, Fialova A. Tumor-infiltrating lymphocytes and dendritic cells in human colorectal cancer: their relationship to KRAS mutational status and disease recurrence. Hum. Immunol. 2011;72:1022–1028. doi: 10.1016/j.humimm.2011.07.312. [DOI] [PubMed] [Google Scholar]
- 45.Sisirak V, F. J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S, Durand I, Le Mercier I, Bajard A, Bachelot T, Puisieux A, Puisieux I, Blay JY, Ménétrier-Caux C, Caux C, Bendriss-Vermare N. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72:5188–5197. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- 46.Michielsen AJ, H. A, Marry J, Tosetto M, Cox F, Hyland JM, Sheahan KD, O'Donoghue DP, Mulcahy HE, Ryan EJ, O'Sullivan JN. Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer. PLoS One. 2011;6:e27944. doi: 10.1371/journal.pone.0027944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, Behrens MD, Knutson KL. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014;74:2974–2985. doi: 10.1158/0008-5472.CAN-13-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24:213–216. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front. Immunol. 2013;4:449. doi: 10.3389/fimmu.2013.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maurya N, Gujar R, Gupta M, Yadav V, Verma S, Sen P. Immunoregulation of dendritic cells by the receptor T cell Ig and mucin protein-3 via Bruton's tyrosine kinase and c-Src. J. Immunol. 2014;193:3417–3425. doi: 10.4049/jimmunol.1400395. [DOI] [PubMed] [Google Scholar]
- 52.Stoitzner P, Green LK, Jung JY, Price KM, Atarea H, Kivell B, Ronchese F. Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells. Cancer Immunol. Immunother. 2008;57:1665–1673. doi: 10.1007/s00262-008-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Min S, Liang X, Zhang M, Zhang Y, Mei S, Liu J, Liu J, Su X, Cao S, Zhong X, Li Y, Sun J, Liu Q, Jiang X, Che Y, Yang R. Multiple tumor-associated microRNAs modulate the survival and longevity of dendritic cells by targeting YWHAZ and Bcl2 signaling pathways. J. Immunol. 2013;190:2437–2446. doi: 10.4049/jimmunol.1202282. [DOI] [PubMed] [Google Scholar]
- 54.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 55.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J. Immunol. 2009;182:6207–6216. doi: 10.4049/jimmunol.0803926. [DOI] [PubMed] [Google Scholar]
- 57.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 58.Hargadon KM. Tumor-altered dendritic cell function: implications for anti-tumor immunity. Front. Immunol. 2013;4:192. doi: 10.3389/fimmu.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J. Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramos RN, de Moraes CJ, Zelante B, Barbuto JA. What are the molecules involved in regulatory T-cells induction by dendritic cells in cancer? Clin. Dev. Immunol. 2013;2013:806025. doi: 10.1155/2013/806025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conrad C, Gregorio J, Wang YH, Ito T, Meller S, Hanabuchi S, Anderson S, Atkinson N, Ramirez PT, Liu YJ, Freedman R, Gilliet M. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 2012;72:5240–5249. doi: 10.1158/0008-5472.CAN-12-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J. Immunol. 2004;172:2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc. Natl. Acad. Sci. U S A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukaya T, Takagi H, Sato Y, Sato K, Eizumi K, Taya H, Shin T, Chen L, Dong C, Azuma M, Yagita H, Malissen B, Sato K. Crucial roles of B7-H1 and B7-DC expressed on mesenteric lymph node dendritic cells in the generation of antigen-specific CD4+Foxp3+ regulatory T cells in the establishment of oral tolerance. Blood. 2010;116:2266–2276. doi: 10.1182/blood-2009-10-250472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 67.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 69.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 70.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin. Cancer Re.s. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Topalian SL, 1, H. F, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2014;2:393–398. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 73.Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, O'Garra A, Trinchieri G, Caux C. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saito T, Takayama T, Osaki T, Nagai S, Suzuki T, Sato M, Kuwano H, Tahara H. Combined mobilization and stimulation of tumor-infiltrating dendritic cells and natural killer cells with Flt3 ligand and IL-18 in vivo induces systemic antitumor immunity. Cancer Sci. 2008;99:2028–2036. doi: 10.1111/j.1349-7006.2008.00907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, Anadon-Arnillas J, Harwood NM, Korc M, Fiering SN, Sempere LF, Conejo-Garcia JR. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo Z, Wang C, Yi H, Li P, Pan H, Liu L, Cai L, Ma Y. Nanovaccine loaded with poly I:C and STAT3 siRNA robustly elicits anti-tumor immune responses through modulating tumor-associated dendritic cells in vivo. Biomaterials. 2015;38:50–60. doi: 10.1016/j.biomaterials.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 77.Zhong H, Gutkin DW, Han B, Ma Y, Keskinov AA, Shurin MR, Shurin GV. Origin and pharmacological modulation of tumor-associated regulatory dendritic cells. Int. J. Cancer. 2014;134:2633–2645. doi: 10.1002/ijc.28590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]