Abstract
The importance of sleep for cognition in young adults is well established, but the role of habitual sleep behavior in cognition across the adult lifespan remains unknown. We examined the relationship between sleep continuity and total sleep time assessed with a sleep detection device and cognitive performance using a battery of tasks in young (n = 59, mean age = 23.05) and older (n = 53, mean age = 62.68) adults. Across age groups, higher sleep continuity was associated with better cognitive performance. In the younger group, higher sleep continuity was associated with better working memory and inhibitory control. In the older group, higher sleep continuity was associated with better inhibitory control, memory recall, and verbal fluency. Very short and very long total sleep time was associated with poorer working memory and verbal fluency, specifically in the younger group. Total sleep time was not associated with cognitive performance in any domains for the older group. These findings reveal that sleep continuity is important for executive function in both young and older adults, but total sleep time may be more important for cognition in young adults.
Introduction
Sleep deprivation and restriction negatively impact cognition in young adults (Goel, Rao, Durmer, & Dinges, 2009). Less is known about whether normal variation in sleep quality and quantity affects daytime cognitive function, especially in older adults. Age-related changes in sleep are common with advancing age, especially in total sleep time (TST), sleep continuity (i.e. lower sleep efficiency (SE) and greater wake time after sleep onset (WASO)) and slow-wave sleep (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). Given that both sleep and cognitive function decline with advancing age, sleep may play an important role in the extent to which older adults exhibit cognitive deficits as well as the types of deficits displayed (Mander et al., 2013; Scullin, 2012; Wilckens, Erickson, & Wheeler, 2012). The contribution of these age-related changes in sleep to cognition has recently been investigated in relation to age-related memory decline, particularly sleep-dependent memory consolidation (Mander, et al., 2013; Pace-Schott & Spencer, 2011; Scullin, 2012). These studies demonstrate a relationship between slow-wave sleep and memory that is weakened with aging. It is possible that neural synchrony during slow-wave sleep enhances connectivity within memory networks, and that this process deteriorates with aging (Mander, et al., 2013; Scullin, 2012). Similar mechanisms may underlie sleep benefits to other cognitive functions.
Certain aspects of sleep, such as slow-wave sleep, appear to have preferential benefits to prefrontal cortex (PFC) function which may in turn benefit cognitive processes dependent on the PFC (Goel, et al., 2009; Muzur, Pace-Schott, & Hobson, 2002; Wilckens, et al., 2012). This view would hypothesize that cognitive processes supported by PFC-associated networks would be the most sensitive to individual differences in sleep, especially executive functions. Such cognitive processes include working memory, inhibition, and controlled memory processes. Accordingly, cognitive processes less supported by the PFC, such as processing speed (Baldo & Shimamura, 2002) may be less affected by sleep (Wilckens, et al., 2012). Interestingly, it is executive processes that tend to show the greatest age-related deficits (Buckner, 2004), but whether sleep plays a role in age-related executive deficits remains unknown.
Little attention has been paid to the role of sleep changes in cognitive aging partly due to findings that older adults are resilient to sleep deprivation in terms of vigilance and response speed (Duffy, Willson, Wang, & Czeisler, 2009; Philip et al., 2004). These paradoxical results however, could be attributable to the type of task used, according to one study comparing higher order decision making abilities in young and middle-aged adults (Killgore, Balkin, & Wesensten, 2006). On the other hand, age-related resilience to sleep loss does not address the question of whether age-related changes in different aspects of sleep contribute to individual differences in cognitive performance among older adults (Wilckens, et al., 2012). Deficits in executive function may be particularly prevalent in older adults with poor sleep.
A handful of studies have identified no relationship between sleep and cognition in healthy older adults (Crenshaw & Edinger, 1999; Szelenberger & Niemcewicz, 2000; Yaffe et al., 2011), making it unclear whether sleep is important for cognitive performance in older adults. Conversely, tasks of executive function appear to be particularly sensitive to sleep loss (Jones & Harrison, 2001) and a few studies assessing executive abilities in older adults have found positive relationships between sleep and cognition (Anderson & Horne, 2003; Blackwell et al., 2006; Miyata et al., 2013; Nebes, Buysse, Halligan, Houck, & Monk, 2009), but the sleep and cognitive domain may be critical. Certain aspects of sleep may be more important particularly for executive functioning. Nonetheless, none of the above studies have directly compared the relationship between sleep and cognition in older adults to young adults. Thus, it remains unclear whether sleep-cognition relationships differ between young and older adults and whether these relationships differ according to the sleep and cognitive domain.
The present study used a wide range of cognitive tasks, including paper-and-pencil neuropsychological tasks and computer-based cognitive tasks to test the specificity of the relationship between sleep and cognition to executive functions and to determine whether this relationship is moderated by age. Using a sleep detection device to objectively assess sleep continuity (WASO) and TST, we hypothesized that greater sleep continuity (i.e. lower WASO) would be associated with better performance on measures of executive function (working memory and inhibition). Further, we hypothesized that the relationship between sleep continuity and executive function would be independent of age. Given that very short and very long sleep durations and TST are often associated with negative health outcomes (Goldman et al., 2007; Grandner & Kripke, 2004; Hall et al., 2008), we expected that the relationship between TST and executive function would be reflected in an inverted U-shaped function whereby very short and very long TST would be associated with poorer cognitive function.
Method
Participants
Participants (n = 112) were community dwelling volunteers. Inclusion criteria pertinent to the present data included having at least 4 days of sleep data from a sleep detection device, and normal or corrected-to-normal vision. All participants had mini mental state exam (MMSE) scores ≥ 23. A liberal MMSE exclusion was used to maximize variability in cognitive performance across domains in relation to sleep. Exclusion criteria included a self-reported diagnosis of depression, current psychiatric medication use, dependence on drugs or alcohol, or a diagnosis of a neurodegenerative disease.
Fifty-nine participants were young adults between ages 21-30 and 53 participants were middle age to older adults between ages 55-77. Participants were paid $10 per hour for participation in the experiment and $50 for wearing the sleep detection device for one week. Participants provided informed consent as required by the University of Pittsburgh Institutional Review Board. Demographic information, MMSE scores, and sleep averages for young and older participants are displayed in Table 1.
Table 1.
Demographics, MMSE, and sleep measures for young and older adults
| Young | Older | |||
|---|---|---|---|---|
| mean | SD | mean | SD | |
| Age | 23.05 | 2.42 | 62.68 | 6.079 |
| Education | 16.09 | 1.65 | 15.22 | 3.05 |
| MMSE | 29.08 | 1.38 | 28.42 | 1.48 |
| WASO | 49.21 | 26.68 | 65.04 | 48.29 |
| TST (minutes) | 382.51 | 59.80 | 355.83 | 67.76 |
Measures
Cognitive Domains and Data Reduction
Cognition was assessed with the following domains based on a priori hypotheses that executive functions and controlled memory abilities would be most strongly related to sleep measures (working memory, inhibition, and recall). Table 2 indicates whether the tasks described below were paper-and-pencil-based or computer-based. Participants also completed a task-switching study, the results of which are reported elsewhere (Wilckens, Woo, Erickson, & Wheeler, 2014).
Table 2.
Paper-and-pencil and computer-based tasks by domain
| Domain | Paper-and-Pencil | Computer |
|---|---|---|
| Working Memory | Sternberg | |
| N-back | ||
| Processing Speed | Trails A | |
| Digit Symbol Substitution | ||
| Verbal Fluency | Categorical and Lexical Fluency (Animal and F) | |
| NART | ||
| Inhibition | Flanker | |
| Stroop | ||
| Recall | CERAD |
To reduce the probability of false positives from multiple comparisons, all of the cognitive task conditions were transformed into z scores and averaged to create 5 domains of interest (working memory, inhibition, verbal fluency, processing speed, and recall). To account for processing speed and general age-related slowing in each of the executive domains described below, we calculated “response time (RT) costs” by subtracting RTs associated with the easier task condition from RTs associated with the more difficult condition. In each domain, higher scores indicate better performance (higher accuracy and lower RT costs).
Working Memory
Working memory was assessed with computerized versions of the Sternberg working memory task and N-back task. In the Sternberg task, participants viewed 2 and 5-letter strings in upper case letters. After a 3000 ms delay, participants saw a lower case letter and were asked to judge whether the lower case letter matched one of the previous upper case letters. In the N-back task, participants viewed a series of letters appearing one at a time on the screen. Participants were required in one condition to identify whether the letter currently on the screen was the same as the previous letter (1-back) or, in the other condition, the same as the letter two previous (2-back). In these 2 computerized tasks, the dependent variables were RT on correct trials and accuracy. Two and 5 letter accuracy, 5-letter RT costs, 2-back accuracy, 2-back RT costs were included in the working memory domain.
Inhibition
Inhibition was assessed with computerized versions of the Stroop task and Flanker task. In the Stroop task, participants viewed words one at a time on the screen in blue, red, or green font colors and were asked to judge the font color of the word by responding with a button press. There were congruent trials (i.e. the word “red” in a red font) and incongruent trials (i.e. the word “blue” in a red font), and neutral trials (i.e. the word “table” in a red font). In the Flanker task, participants viewed 5 arrows (“flankers”) on the screen on a given trial (< < < < <) and were asked to judge the direction the center arrow was pointing by responding with a button press. This task included congruent trials (< < < < <) and incongruent trials (< < > < <). For both tasks, percent interference was assessed for RT, which was calculated as (incongruent mean-congruent mean)/congruent mean multiplied by 100%. Flanker and Stroop interference and incongruent accuracy were included in the Inhibition domain. One young participant did not complete the Flanker or Stroop task. Therefore, 111 participants were included in analyses with Inhibition.
Verbal Fluency and Proficiency
Verbal fluency and proficiency was assessed with the National Adult Reading Test (NART) and Categorical and Lexical Fluency tasks. For the NART, participants were given a list of words that should be pronounced differently from how they are spelled or “sounded out,” thus prior knowledge about the pronunciation of the word is required. Participants were asked to read the list of words aloud. Accuracy was scored by the experimenter. Number of correctly pronounced words was the dependent variable. In the categorical fluency task, participants were given a category (animal, fruit, and vegetable) and were asked to name as many words that fit in that category as possible in 60 seconds. In the lexical fluency task, participants were given a letter (F, A, and S) and were asked to name as many words that begin with the probe letter as possible in 60 seconds. Number of words named was the dependent variable. The first trial from the categorical and lexical fluency tasks, and NART accuracy were included in the Verbal Fluency domain.
Processing speed
Processing speed was assessed with Trails A and the digit symbol substitution subset of the Wechsler Adult Intelligence Scale III (Wechsler, 1997). In Trails A, the participant is asked to draw lines connecting encircled numbers distributed throughout a sheet of paper in sequential order 1-25. Time to connect all the numbers correctly was the dependent variable. In the digit symbol substitution task participants were given a worksheet that displayed a series of symbols that corresponded to the numbers 1-9. Below the symbol-number key was an array of numbers above blank boxes. The test required participants to write as many symbols as possible in 60 seconds within the box below each number. Number of symbols correctly completed was the dependent variable. Raw score performance for Trails A and digit symbol substitution were included in the Processing Speed domain.
Recall
Recall was assessed with delayed recall from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Memory test (Morris et al., 1989). Participants were asked to first read a list of 10 words aloud and then immediately say out loud as many words as they could remember from the list. They alternated between reading the list and attempting to immediately recall 3 times. Following an unrelated verbal fluency task that lasted approximately 10 minutes, participants were asked to perform a delayed recall task and name as many words from the list as they could remember. The number of words correctly recalled in the delayed recall condition was the dependent variable for the recall factor.
Physiological data collection
A sleep detection device (SenseWear® armband) was used to assess participants’ sleep. Participants wore the armband for one week between the two experiment sessions. The device estimated every 60 seconds whether participants were active, lying down, or asleep based on body axis, heat flux, activity, galvanic skin response, body temperature, and near body temperature (Sunseri et al., 2009). Participants also recorded when they went to bed for the final time and got out of bed in the morning. These records were used with the SenseWear data to define the nighttime sleep bout. From the sleep and lying down estimates within the nighttime sleep bout, WASO (minutes spent awake following sleep onset), and TST (average time spent sleeping) were calculated. Average WASO and TST for young and older groups are displayed in Table 1.
Analytic Techniques
Moderation analysis
We conducted hierarchical regression analyses on each of the 5 cognitive domains with each sleep measure and age group as predictors. The regression included 2 hierarchical models. Sex and education were included in model 1 as covariates because they were significantly related to at least one cognitive factor. Model 2 included age group and centered sleep variables. Model 3 included an interaction term between age and sleep to test the moderating effect of age group on the relationship between cognition and sleep. To test these relationships within the two age groups, moderation analyses were followed-up with separate regression analyses for each age group with sex, education, and age as a continuous covariate in Model 1 and sleep as a continuous predictor variable in Model 2. Separate regressions were performed for WASO and TST.
Results
Age differences in sleep and cognition
Older adults had significantly greater WASO, t(110) = 2.18, p = 0.032, and significantly shorter TST, t(110) = 2.21, p = 0.029. Means and standard deviations are displayed in Table 1. We have reported elsewhere on age effects for these sleep variables from a subset of the participants who also performed a task-switching paradigm in the present study (Wilckens, et al., 2014). These effects of age group on sleep are consistent with prior studies (Ohayon, et al., 2004). Although WASO and TST were highly correlated, r = -0.44 p < 0.001), they differed in age group effects and have been shown to relate to cognition differentially (Blackwell, et al., 2006; Wilckens, et al., 2014). Thus we examined the relationship between both of these sleep measures and performance. Table 3 displays means and age differences in performance for each of the 5 cognitive domains separated by young and older age groups.
Table 3.
Means and standard deviations in performance for each cognitive domain in young and older adults and statistical differences between groups
| Young | Older | Difference | |||||
|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | t | df | p | |
| Working Memory | 0.19 | 0.45 | −0.13 | 0.57 | 2.60 | 110 | 0.01 |
| Inhibition | 0.12 | 0.49 | −0.12 | 0.47 | 2.65 | 109 | 0.01 |
| Verbal Fluency | 0.20 | 0.63 | −0.22 | 0.77 | 3.13 | 110 | 0.002 |
| Recall | 0.36 | 0.86 | −0.40 | 1.00 | 4.26 | 110 | < 0.001 |
| Processing Speed | 0.50 | 0.55 | −0.56 | 0.74 | 8.71 | 110 | <0.001 |
Table 4 displays the degree of dependence between the different cognitive domains for the young and older groups. Working memory, verbal fluency, and processing speed were all significantly associated with one another in the younger group. Recall and processing speed were also related in the younger group. There were no significant correlations between cognitive domains in the older group.
Table 4.
Correlation matrix r and (p values) among cognitive domains for young and older adults; significant effects are highlighted in bold
| Young | Inhibition | Verbal Fluency | Recall | Processing Speed |
|---|---|---|---|---|
| Working Memory | −0.02 (0.87) | 0.48 (<0.001) | 0.05 (0.71) | 0.28 (0.034) |
| Inhibition | −0.06 (0.68) | −0.19 (0.16) | −0.02 (0.87) | |
| Verbal Fluency | 0.25 (0.06) | 0.42 (0.001) | ||
| Recall | 0.33 (<0.01) |
| Older | Inhibition | Verbal Fluency | Recall | Processing Speed |
|---|---|---|---|---|
| Working Memory | 0.29 (0.13) | 0.06 (0.69) | 0.10 (0.49) | 0.004 (0.98) |
| Inhibition | .22 (0.12) | 0.06 (0.69) | 0.008 (0.96) | |
| Verbal Fluency | 0.19 (0.16) | 0.23 (0.10) | ||
| Recall | 0.001 (.995) |
Wake after Sleep Onset (WASO)
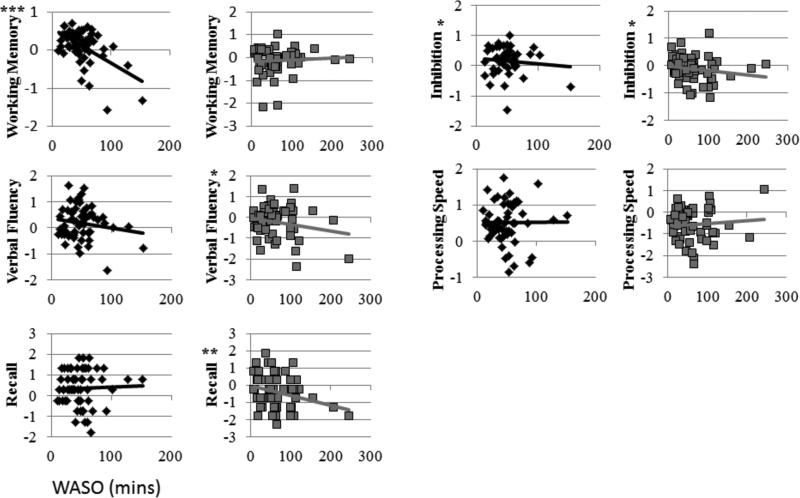
Regression analyses revealed significant main effects of WASO on inhibition, verbal fluency, and delayed recall, such that lower WASO was associated with better performance. The moderating effect of age on the relationship between WASO and working memory was significant; such that WASO was significantly related to working memory in the younger group, but not the older group. Analyses to follow up on main effects of WASO revealed significant relationships between WASO and inhibition for both young and older groups. Relationships with WASO were significant only in the older group for verbal fluency and recall. There were no main effects or interactions involving WASO and processing speed for either age group. Regression coefficients for the linear relationship between WASO and performance across age groups and the interaction term are displayed in Table 5. Analyses stratified by age group are displayed in Table 6. Scatterplots of the bivariate relationships with WASO are displayed in Figure 1.
Table 5.
Regression coefficients for each of the cognitive domains with WASO as a predictor; significant effects are highlighted in bold
| WASO | Age × WASO | |||
|---|---|---|---|---|
| Beta | p | Beta | p | |
| Working memory | −0.13 | 0.19 | 1.39 | 0.001 |
| Inhibition | −0.208 | 0.03 | −0.25 | 0.52 |
| Verbal Fluency | −0.24 | 0.009 | −0.14 | 0.71 |
| Recall | −0.198 | 0.027 | −0.49 | 0.18 |
| Processing Speed | −0.02 | 0.79 | −0.03 | 0.93 |
Table 6.
Regression coefficients stratified by age group for each of the cognitive domains with WASO as a predictor; significant effects are highlighted in bold
| WASO | Young | Older | ||
|---|---|---|---|---|
| Beta | P | Beta | P | |
| Working memory | −0.45 | < 0.001 | 0.12 | 0.42 |
| Inhibition | −0.28 | 0.046 | −0.29 | 0.049 |
| Verbal Fluency | −0.07 | 0.62 | −0.30 | 0.045 |
| Recall | 0.12 | 0.41 | −0.40 | 0.003 |
| Processing Speed | 0.11 | 0.45 | −0.02 | 0.92 |
Figure 1.
Scatter plots of the linear bivariate relationships between WASO (x axis) and each cognitive domain (y axis) for young (black) and older participants (gray). Trend lines reflect linear relationships. Significant linear relationships are denoted, p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***
Total Sleep Time (TST)
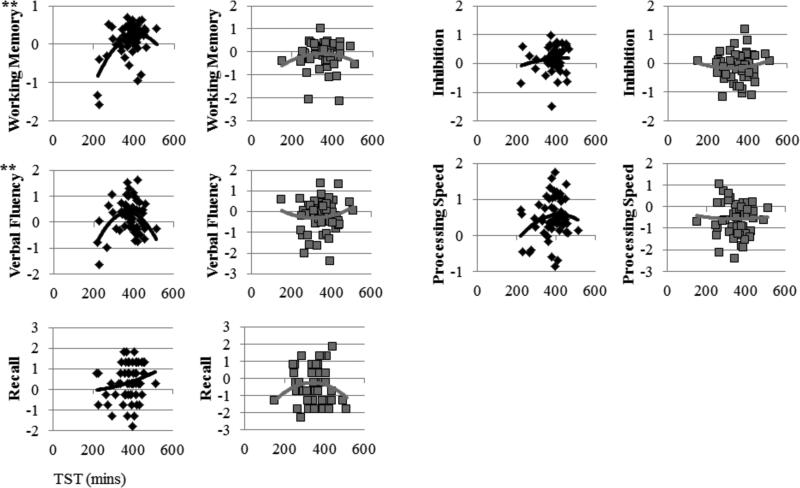
We next assessed whether TST was associated with cognitive performance in young and older adults. TST is often associated with an inverted U-shaped curvilinear relationship with health and daytime function (Goldman, et al., 2007; Grandner & Kripke, 2004; Hall, et al., 2008). Further, longer time in bed or sleep times may decrease sleep drive, leading to lower sleep continuity and sleep depth, which may be detrimental for cognition. Thus, we tested both linear TST terms and curvilinear (TST2) terms. In terms of linear relationships, longer TST was associated with better working memory, and this main effect was moderated by age group, whereby the relationship was significant in the young group, but not the older group. In terms of curvilinear relationships, there was a significant TST2 × age group interaction for verbal fluency and a marginally significant curvilinear relationship for working memory. Both curvilinear effects were driven by very short and very long TST associated with poorer performance in the young group only. Overall, both linear and curvilinear TST terms explained performance in the young group, but no effects of TST were significant in the older group. Regression coefficients for the linear and curvilinear relationships between TST and performance across age groups and the interaction term are displayed in Table 7. Analyses stratified by age group are displayed in Table 8. Scatterplots of relationships between TST and cognition are displayed in Figure 2.
Table 7.
Regression coefficients for each of the cognitive domains with TST and TST2 as a predictor; significant effects are highlighted in bold
| TST | Age × TST | TST2 | Age × TST2 | |||||
|---|---|---|---|---|---|---|---|---|
| Beta | p | Beta | p | Beta | p | Beta | p | |
| Working memory | 0.20 | 0.034 | −0.67 | 0.031 | −0.22 | 0.052 | 0.34 | 0.38 |
| Inhibition | 0.10 | 0.27 | −0.10 | 0.74 | −0.20 | 0.86 | 0.26 | 0.57 |
| Verbal Fluency | 0.08 | 0.37 | −0.25 | 0.40 | −0.10 | 0.39 | 1.08 | 0.006 |
| Recall | −0.019 | 0.854 | −0.146 | 0.144 | −0.02 | 0.84 | −0.35 | 0.35 |
| Processing Speed | 0.09 | 0.25 | −0.18 | 0.45 | −0.03 | 0.70 | 0.23 | 0.49 |
Table 8.
Regression coefficients stratified by age group for each of the cognitive domains with TST and TST2 as a predictor; significant effects are highlighted in bold
| TST | TST2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Young | Older | Young | Older | |||||
| Beta | p | Beta | p | Beta | p | Beta | p | |
| Working memory | 0.38 | 0.003 | −0.02 | 0.89 | −0.35 | 0.007 | −0.11 | 0.58 |
| Inhibition | 0.18 | 0.19 | 0.13 | 0.37 | −0.16 | 0.36 | 0.09 | 0.66 |
| Verbal Fluency | 0.08 | 0.58 | 0.10 | 0.48 | −0.43 | 0.003 | 0.28 | 0.14 |
| Recall | 0.15 | 0.29 | 0.04 | 0.79 | 0.07 | 0.65 | −0.03 | 0.85 |
| Processing Speed | 0.17 | 0.21 | 0.08 | 0.58 | −0.14 | 0.35 | −0.03 | 0.89 |
Figure 2.
Scatter plots of the bivariate relationships between TST (x axis) and each cognitive domain (y axis) for young (black) and older participants (gray). Trend lines reflect quadratic relationships. Significant quadratic relationships are denoted, p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***
Discussion
Sleep continuity assessed with WASO significantly predicted cognitive performance in both young and older adults. WASO was significantly associated with working memory and inhibition in the young group, and inhibition, controlled recall and verbal fluency in the older group.
Although effects of WASO on recall and verbal fluency were not moderated by age group, age group- stratified analyses revealed that the relationship with WASO was only significant in the older group. Further, the relationship between working memory and WASO was only significant in the younger group. This was a significant age difference. This suggests that the extent to which a relationship between sleep and cognition exists depends on the cognitive domain, and possibly task difficulty. Thus, for some cognitive domains, age differences may actually be diminished with higher sleep continuity.
Consistent with our hypothesis, sleep continuity was not significantly associated with processing speed in either age group. It may be that more continuous or consolidated sleep allowing for adequate progression through sleep stages and NREM (non-rapid eye movement)-REM cycling, is particularly important for executive functions.
It is plausible that lower sleep continuity prevents people from progressing normally through sleep stages and limits the amount of time spent in slow-wave sleep. The benefits of slow-wave sleep to cognition and frontal lobe function are supported by a range of findings: The greatest reductions in brain activity during slow-wave sleep occur in the frontal lobes (Muzur, et al., 2002), delta activity which is highest during slow-wave sleep is associated with cognitive performance (Anderson & Horne, 2003; Edinger, Glenn, Bastian, & Marsh, 2000; Mander, et al., 2013; Scullin, 2012) and selective disruption of slow-wave sleep is associated with poor cognitive performance (Ferrara, De Gennaro, & Bertini, 1999). Synchronized neural activity during slow-wave sleep may enhance connectivity between functionally related brain regions (Muzur, et al., 2002). Alternatively, sleep continuity may be associated with other factors such as motivation, stress, depression, and physical activity, all of which may directly influence cognition. Future studies should assess the direct benefits of sleep continuity to cognition through sleep interventions.
In contrast to WASO, we examined whether a linear or quadratic function explained variability in performance in relation to TST. An inverted U-shaped relationship with TST has been demonstrated in prior studies with other forms of daytime function and overall health (Goldman, et al., 2007; Hall, et al., 2008). One recent epidemiological study (Xu et al., 2011) found that very short (3-4 hours) and very long (> 10 hours) self-reported sleep durations were associated with memory impairment. Indeed, we found such a relationship for working memory and verbal fluency. Very short and very long TSTs were associated with poorer working memory and verbal fluency in young, but not older adults. This was a significant age difference for verbal fluency. Thus, there may be a “moderate” TST range ideal for cognitive performance in young adults. Our findings build upon existing studies by demonstrating that the U-shaped relationship applies to objectively measured TST in young adults.
The moderating effect of age group on relationships between TST and performance was significant for both working memory and verbal fluency. This finding reflects that TST was not associated with performance in any domains for the older group. The lack of a relationship between TST and performance in older adults was not due to lower variability in the older group because variability in TST was numerically greater in the older group (Table 1). In contrast, older adults showed relationships between sleep continuity and performance in multiple domains. One explanation for this TST-specific age difference is that sleep continuity plays a greater role in performance in older adults, but both TST and sleep continuity are important for performance in young adults, possibly due to age differences in sleep need. Further, there may be fundamental age differences in reasons for very short sleep times that may influence performance differently. It is conceivable that in the younger group, very short TST more often reflects acute self-imposed sleep restriction, whereas in the older group, shorter TST may result from a chronic reduction in homeostatic sleep drive and a shift in circadian rhythms (Dijk, Duffy, & Czeisler, 2000). Individual differences in TST may also reflect differences in morningness and eveningness which may influence performance (Yaffe, Blackwell, Barnes, Ancoli-Israel, & Stone, 2007). Alternatively, it may be that older adults improve performance through daytime naps (Buysse et al., 1992; Yoon, Kripke, Youngstedt, & Elliott, 2003), a factor that was not captured in the present study. The lack of a relationship between TST and performance in older adults is consistent with prior reports (Blackwell, et al., 2006; Nebes, et al., 2009). In a separate report focused on task-switching (Wilckens, et al., 2014), we found that switch costs were sensitive to individual differences in TST, particularly in the young group. However, within older adults there were no significant relationships between TST and switch costs or task-preparation. Consistent with the cognitive domains reported here, older adults with higher sleep continuity exhibited better switching and preparation abilities.
While the present study is a first step in identifying a role of sleep in age-related decline in executive function, it is possible that other variables such as stress, depression, physical activity, or other health factors drove associations between sleep and performance. Although beyond the scope of this manuscript, we hope to incorporate these and other health factors including sleep into a more comprehensive model of how modifiable health factors influence age-related losses in executive functions. Additionally, although it is generally accepted that sleep benefits the brain (Maquet et al., 1997), brain volume and function also affect sleep. Age-related decreases in PFC structure and function may contribute to decreased sleep, especially slow-wave sleep production (Nicholas, Sullivan, Pfefferbaum, Trinder, & Colrain, 2002). Interventions aimed at ameliorating age-related sleep deficits are necessary to establish a direct benefit of improved sleep to executive function.
The present study has some limitations that should be noted. While participants were cognitively healthy, they were not excluded based on any sleep measures or sleep disorders. It is plausible that effects of sleep on cognition differ among sleep disorders and in comparison with older adults with no sleep disorders. In addition, we used an accelerometer-based sleep detection device, whereas polysomnography (PSG) is considered the “gold standard” for sleep measurement. Nonetheless, the sleep detection device used here avoids some confounds associated with wrist actigraphy, such as knowing when the device is off the body by collecting additional physiological data (Sunseri, et al., 2009). Further, the SenseWear device demonstrates concordance with PSG (Sunseri, et al., 2009), but does not require that participants wear electrodes while sleeping or sleep in a laboratory setting. Future studies will benefit from examining whether PSG-measured sleep predicts cognitive performance across a wide range of cognitive domains in young and older adults. Further, brain activity reflected in PSG may shed light on the role of neural oscillations during sleep in cognitive deficits (Mander, et al., 2013).
Conclusions
Sleep continuity is associated with cognitive performance, especially for executive functions. This relationship exists in young and older adults. Very short and very long TST was associated with poorer performance only in young adults. The present results set the stage for future work investigating whether adults who exhibit fragmented sleep are more likely to exhibit cognitive decline. Experimental sleep interventions should examine benefits of increased sleep continuity to cognitive performance in older adults on a wide range of cognitive tasks to establish a direct benefit of sleep to executive function.
Acknowledgement
We thank Krupa Patel, Marina Lukac, and Leslie Denlinger for assistance with data collection.
This project was supported by the National Institute of General Medical Sciences (T32GM081760 to K.A.W.) and the National Institute of Mental Health (MH086492 to M.E.W.) at the National Institutes of Health.
Contributor Information
Kristine A. Wilckens, University of Pittsburgh School of Medicine, Department of Psychiatry
Sarah G. Woo, University of Pittsburgh, Learning Research and Development Center
Afton R. Kirk, University of Pittsburgh, Department of Psychology in Education
Kirk I. Erickson, University of Pittsburgh, Department of Psychology and the Center for the Neural Basis of Cognition
Mark E. Wheeler, University of Pittsburgh, Department of Psychology, Learning Research and Development Center and the Center for the Neural Basis of Cognition
References
- Anderson C, Horne JA. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40(3):349–357. doi: 10.1111/1469-8986.00038. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Frontal Lobes and Memory. In: Baddeley, Kopelman, editors. Handbook of Memory Disorders. 2nd Edition John Wiley & Co.; London: 2002. [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Stone KL. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2006;61(4):405–410. doi: 10.1093/gerona/61.4.405. doi: 61/4/405 [pii] [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buysse D, Browman K, Monk T, Reynolds C, 3rd, Fasiczka A, Kupfer D. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. Journal of the American Geriatrics Society. 1992;40(8):779. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiology & Behavior. 1999;66(3):485–492. doi: 10.1016/s0031-9384(98)00316-3. doi: 10.1016/S0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiology International. 2000;17(3):285–311. doi: 10.1081/cbi-100101049. doi: 10.1081/CBI-100101049. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. Journal of the American Geriatrics Society. 2009;57(7):1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Glenn DM, Bastian LA, Marsh GR. Slow-wave sleep and waking cognitive performance II: Findings among middle-aged adults with and without insomnia complaints. Physiology & Behavior. 2000;70(1):127–134. doi: 10.1016/s0031-9384(00)00238-9. [DOI] [PubMed] [Google Scholar]
- Ferrara M, De Gennaro L, Bertini M. The effects of slow-wave sleep (SWS) deprivation and time of night on behavioral performance upon awakening. Physiology of Behavior. 1999;68(1-2):55–61. doi: 10.1016/s0031-9384(99)00150-x. doi: 10.1016/S0031-9384(99)00150-X. [DOI] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2009;29(4):320–339. doi: 10.1055/s-0029-1237117. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SE, Stone KL, Ancoli-Israel S, Blackwell T, Ewing SK, Boudreau R, Newman AB. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30(10):1317. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Kripke DF. Self-reported sleep complaints with long and short sleep: a nationally representative sample. Psychosomatic medicine. 2004;66(2):239–241. doi: 10.1097/01.psy.0000107881.53228.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31(5):635. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Medicine Reviews. 2001;5(6):463–475. doi: 10.1053/smrv.2001.0203. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. Journal of Sleep Research. 2006;15(1):7–13. doi: 10.1111/j.1365-2869.2006.00487.x. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nature Neuroscience. 2013;16(3):357–364. doi: 10.1038/nn.3324. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Degueldre C, Delfiore G, Aerts J, Peters JM, Luxen A, Franck G. Functional neuroanatomy of human slow wave sleep. Journal of Neuroscience. 1997;17(8):2807–2812. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Noda A, Iwamoto K, Kawano N, Okuda M, Ozaki N. Poor sleep quality impairs cognitive performance in older adults. Journal of Sleep Research. 2013 doi: 10.1111/jsr.12054. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends in Cognitive Sciences. 2002;6(11):475–481. doi: 10.1016/s1364-6613(02)01992-7. doi: 10.1016/S1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2009;64(2):180–187. doi: 10.1093/geronb/gbn037. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CL, Sullivan EV, Pfefferbaum A, Trinder J, Colrain IM. The effects of alcoholism on auditory evoked potentials during sleep. Journal of Sleep Research. 2002;11(3):247–253. doi: 10.1046/j.1365-2869.2002.00298.x. doi: 10.1046/j.1365-2869.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. Progress in Brain Research. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Philip P, Taillard J, Sagaspe P, Valtat C, Sanchez-Ortuno M, Moore N, Bioulac B. Age, performance and sleep deprivation. Journal of Sleep Research. 2004;13(2):105–110. doi: 10.1111/j.1365-2869.2004.00399.x. doi: 10.1111/j.1365-2869.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- Scullin MK. Sleep, Memory, and Aging: The Link Between Slow-Wave Sleep and Episodic Memory Changes From Younger to Older Adults. Psychology and aging. 2012;28(1):105–114. doi: 10.1037/a0028830. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunseri M, Liden C, Farringdon J, Pelletier R, Safier S, Stivoric J, Vishnubhatla S. The SenseWear armband as a Sleep Detection Device. 2009:1–9. [Google Scholar]
- Szelenberger W, Niemcewicz S. Severity of insomnia correlates with cognitive impairment. Acta Neurobiologiae Experimentalis. 2000;60(3):373–376. doi: 10.55782/ane-2000-1356. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Administration and scoring manual. 3rd ed. The Psychological Corporation; San Antonio: 1997. Wechsler Adult Intelligence Scale. WAIS-III. [Google Scholar]
- Wilckens KA, Erickson KI, Wheeler ME. Age-related decline in controlled retrieval: The role of the PFC and sleep. Neural Plasticity, 2012. 2012 doi: 10.1155/2012/624795. doi: 10.1155/2012/624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Woo SG, Erickson KI, Wheeler ME. Sleep continuity and total sleep time are associated with task-switching and preparation in young and older adults. Journal of Sleep Research. 2014 doi: 10.1111/jsr.12148. doi: 10.1111/jsr.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Jiang CQ, Lam TH, Liu B, Jin YL, Zhu T, Thomas GN. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34(5):575–580. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Barnes DE, Ancoli-Israel S, Stone KL. Preclinical cognitive decline and subsequent sleep disturbance in older women. Neurology. 2007;69(3):237–242. doi: 10.1212/01.wnl.0000265814.69163.da. doi: 10.1212/01.wnl.0000265814.69163.da. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA: The Journal of the American Medical Association. 2011;306(6):613. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon IY, Kripke DF, Youngstedt SD, Elliott JA. Actigraphy suggests age related differences in napping and nocturnal sleep. Journal of Sleep Research. 2003;12(2):87–93. doi: 10.1046/j.1365-2869.2003.00345.x. [DOI] [PubMed] [Google Scholar]