Abstract
The PTPN22 1858T variant was among the first single nucleotide polymorphisms (snp) to be associated with multiple autoimmune diseases. As a coding variant within the tyrosinephosphatase, Lyp, known to participate in antigen receptor signaling, the impact of this variant on the immune response and role in the development of autoimmunity has been a focus of study. These studies have utilized a series of approaches including transfected cell lines, animal models and primary human lymphocytes and have identified multiple alterations in cell signaling and function linked to the PTPN22 variant. Conflicting findings have led to questions of how best to study the role of this variant in human autoimmunity. In this review, we discuss these differences, factors that may account for them, and show how an integrated approach can lead to a more complete understanding of the mechanisms that promote autoimmunity in the context of the PTPN22 1858T risk variant.
Introduction
Genetic variation plays a pivotal role in the development of autoimmune disease. In the past decade the complexity of the genetic factors that influence the development of autoimmunity has become increasingly clear through the explosion of genetic information provided by both genome wide association studies (GWAS) and whole genome/exome sequencing. However, extending our knowledge from the genetic variations associated with disease to the pathogenic mechanisms that trigger, promote or sustain autoimmunity continues to be a challenge. A key goal of such work is to understand how genetic risk variants contribute to development of disease, and, more importantly, to determine whether these variants help to identify a set of common pathways that can be targeted for diagnostic and therapeutic purposes.
Several tools are available that help us address the functional consequences of a genetic variant including: a) use of cell lines into which the genetic variant of interest can be introduced; b) murine models that either lack the gene linked to autoimmunity or contain the variant gene via transgene or knock-in strategies; and c) studies of human cells derived from healthy individuals and from individuals with disease who carry risk variants of interest. Each of these approaches has added to our understanding of the potential impact of risk or protective alleles on the human immune response and these tools are now being increasingly applied to genes associated with autoimmunity. However, it remains crucial to recognize that each approach has unique strengths: cell lines allow for extensive biochemical analysis, murine models allow us to dissect how variants impact the immune system’s development and identify mechanisms that may cause autoimmunity, and human tissue/blood analyses allow us to assess whether a genetic variant in humans may impact intrinsic immune cell function and/or modulates such responses following environment exposures. Each approach also has specific limitations that may lead to conflicting results that must be interpreted within this context. In this review, we will focus on studies of one genetic variant, PTPN22 C1858T, and discuss how the combination of these tools can allow us to develop a better understanding of how genetic variants contribute to autoimmunity.
Genetic association of PTPN22-1858T with multiple human autoimmune diseases
In 2004, a single nucleotide polymorphism (SNP) in the protein tyrosine phosphatase N22 (PTPN22), 1858C>T (rs2476601), was shown to be associated with both type 1 diabetes (T1D) (1) and rheumatoid arthritis (RA)(2). Subsequently, these findings have been confirmed in T1D and RA and the variant has been linked to additional diseases including systemic lupus erythematosus (SLE), Graves’ disease and myasthenia gravis (3–5). This individual SNP is one of the most strongly associated autoimmune risk variants, after HLA class II, with odds ratios ranging from 1.9 to 1.3 (6). PTPN22 encodes the protein commonly referred to as lymphocyte tyrosine phosphatase (Lyp), and the 1858C to T polymorphisms results in a single amino acid change from arginine (R) as position 620 to tryptophan (W). This amino acid change has become the focus of studies to determine how the PTPN22 1858T variant contributes to autoimmunity.
Lyp is part of the PEST group of non-receptor classical class I PTPs and is expressed in all hematopoietic cells and its function has been reviewed extensively elsewhere (7). It is clear that Lyp is a negative regulator of T cell receptor signal transduction via interactions with phosphorylated Lck (Y394), Fyn(Y427), and Zap-70 as well as phospho-sites on TCRζ, CD3ε, Vav and Vcp (8,9). Lyp’s function is thought to be further augmented by its interaction with the C-terminal Src kinase (Csk); mediated by binding of the SH3 domain of Csk to the proline rich region 1 (P1) of Lyp. The interaction between Csk and Lyp results in enhanced inhibitory tuning of Src family kinases and dampens TCR signal transduction (10). However, the negative outcome of this interaction has also been challenged, as additional studies suggest that the Lyp/Csk complex can function to limit Lyp activity via promoting phosphorylation of an inhibitory residue on Lyp (11) and/or by altering its localization within cell membrane lipid rafts (12). More recently, TRAF3 has also been shown to bind the P1 region of Lyp in myeloid cells and impact its turnover (13). Finally, additional binding partners of Lyp have been identified including the adaptor molecule Grb2, the E3 ligase c-Cbl (14) and other signaling effectors ((15) and R. James, XD and DJR, unpublished data)- implying that alterations in Lyp-dependent protein-protein interactions might impact signaling through additional events downstream of TCR, BCR and other immune receptors.
Overview of human studies of the risk variant using cell lines and primary hematopoietic cells
Jurkat T cells lines were used in initial studies of Lyp vs. the Lyp risk variant on TCR signaling. Co-transfection of the variant with Csk resulted in reduced interaction with Csk (compared to wild type Lyp) (1) and an increase in TCR responses (16). In contrast, when Lyp620W was transfected into Jurkat cells, without the addition of Csk, a blunting of the TCR signal was observed (17). These differences with respect to impact on TCR signaling have led to persistent questions as to whether the variant is a gain vs. a loss of function allele. These signaling analyses are clearly impacted by the relative expression levels of Lyp and Csk, which may expose different mechanisms whereby Lyp regulates TCR signaling. In the setting of limiting Csk, the localization of Lyp to the cell membrane may be enhanced resulting in increased inhibitory activity while in cell lines where Lyp and Csk are expressed to a similar level the loss of interaction between the variant and Csk may emphasize its loss of function in inhibition of TCR signaling.
The study of un-manipulated human cells benefits from the ability to determine which functional findings from cell lines are likely most relevant, since the function of cells taken directly from the body reflect the expression and interaction of proteins found in vivo. Such studies, however, are limited by numbers of cells available and the both the genetic and environmental variability within the multiple subjects that must be studied. Despite these limitations, several significant observations have been made related the role of Lyp620W and immune function. Initial studies by Vang et al demonstrated a decrease in IL-2 production after TCR stimulation in T1D subjects carrying the PTPN22 1858T variant, a finding that has been confirmed in a larger cohort (17) (18). Consistent with these findings, studies in healthy carriers of the risk variant identified blunted Ca2+ flux, IL-2 and IL-10 production after stimulation with anti-CD3, a finding that was most pronounced in the memory T cell compartment (19). Analysis of individuals with the variant allele and ANCA vasculitis also showed a decrease in IL-10 production (20). Further evidence of the downstream effect of the PTPN221858T variant on T cell maturation includes the observation that the CD4 memory T cell compartment is expanded (19) and that there is an increase in Th1 cells (21).
Lyp is expressed in B cells and influences BCR signaling and cell survival. An increase in Lyp expression is observed in chronic lymphoid leukemia (CLL) and correlates with blunted BCR signaling via Syk and PLCγ, enhanced Akt activity and pro-survival signals, and an increased ability to escape BCR-mediated cell death (22). Lyp620W expression in B cells has also been shown to alter BCR signaling, B cell maturation and tolerance in studies of normal human B cells. Menard et al have found an increase in the frequency of autoreactive naïve mature B cells in healthy carriers of Lyp620W. Moreover, Habib et al demonstrated an expansion of the transitional B cell compartment and increased numbers of anergic B cells- findings that correlated with a blunting of BCR signaling and resistance to BCR-mediated cell death among immature B cells and anticipated to contribute to a loss of B cell tolerance (23,24). These studies indicate that Lyp620W may directly contribute to the development of autoantibodies and autoimmunity through its impact on B cell development.
Recent work has also implicated Lyp in modulating innate signals in myeloid cells, specifically through its influence on TLR signaling and subsequent production of type 1 interferon. Wang et al described a failure of Lyp variant to promote the ubiquitination of TRAF3 required for downstream IFN production. Primary murine myeloid cells expressing the human Lyp variant (and lacking murine Ptpn22), exhibited a reduction in LPS-triggered in type 1 IFN production while inflammatory cytokine production remained intact (13). These studies potentially implicate Lyp620W in autoimmune pathogenesis by altering innate as well as the adaptive immune responses.
Thus studies of human cells, to this point, demonstrate an impact of the Lyp620W variant on both maturation and function of hematopoietic lineages, each of which may have the potential to contribute to autoimmunity. Notably, despite this information it remains unclear which features either singly or in combination play a crucial role(s) in the events leading to disease. To answer these questions, murine models become increasingly important.
Murine models of Ptpn22 deficiency and risk variant expression
The function of Ptpn22 in murine lymphocytes was first extensively assessed in Ptpn22 knock out (KO) mice generated by the Chan group (25). Ptpn22 deficiency augments TCR signaling in CD4+ CD8+ double positive thymocytes and in peripheral effector/memory T cells, but not in the peripheral naïve T cells, and these events result in enhanced thymic positive selection and expansion of peripheral effector/memory T cells in Ptpn22 KO mice, respectively. Ptpn22 deficiency also leads to increased numbers of thymic and peripheral T regulatory cells (Tregs)(26,27). Although Ptpn22 deficient Tregs exhibited normal suppressive activity in vitro (26), Brownlie et al found they are more suppressive than WT Tregs in vivo, as evidenced by reduced expansion of effector T cells and increased suppression of colitis following adoptive transfer of Ptpn22 KO Tregs with WT effectors into Rag deficient mice (27). Aged Ptpn22 KO mice exhibit increased numbers of spontaneous germinal centers (GCs) and increased serum antibody titers, events presumed to be regulated by B cell extrinsic signals as BCR signaling and B cell development are intact in Ptpn22 KO mice (25). A recent study revealed Ptpn22 deficiency increases GC activity predominately by increasing proliferation, survival and cytokine secretion by T follicular helper cells (28). Ptpn22 KO mice in a non-autoimmune (C57BL/6) background do not develop autoantibodies or overt autoimmunity (25). However, Ptpn22 deficiency in cooperation of the CD45 E613R mutation induces murine lupus-like autoimmune disease in B6 background (16). Ptpn22 KO mice on the KBxN background have increased severity of arthritis (28), while Ptpn22 KO mice on the B6 background are resistant to EAE (26) implying that role of Ptpn22 in autoimmune pathology is dependent on the disease and genetic background of murine model.
The impact of Ptpn22 in T1D pathology has been investigated using the NOD (nonobese diabetic) mouse model. The Kissler group generated transgenic NOD mice in which Ptpn22 is inducibly silenced by RNA interference (29). Notably, Ptpn22 knockdown (KD) mice exhibited reduced diabetic incidence compared with controls indicating Ptpn22 silencing has an overall protective effect against T1D. In related studies, Ptpn22 silencing resulted in hyperactivation and decreased survival in B cells. Conversely, Yeh et al reported that transgenic overexpression of WT Ptpn22 in T cells of NOD mice attenuated autoimmune diabetes in a dose-dependent manner (30). Ptpn22 overexpression lead to a reduction in naïve T cell TCR-mediated signals, inhibited autoreactive T cell proliferation in response to islet antigens and lead to decreased numbers of pathogenic pancreas-infiltrating T cells. One possible explanation for the discrepancy between these studies is a differential impact on the Treg compartment. Ptpn22 silencing leads to an expansion of peripheral Tregs whereas Ptpn22 overexpression has no effect on Treg differentiation or suppressive activity. Alternatively, overexpression of Ptpn22 may also result in decreased activation of diabetogenic effector T cells.
To assess the mechanism by which the risk variant induces autoimmunity three independent groups developed either knock-in (KI) mice expressing Ptpn22-R619W, a mutant analogous to human variant, or transgenic mice overexpressing the human variant (13,15,31,32). The Siminovich group generated Ptpn22-R619W KI mice in a C57/Bl6 (B6) background (31). This strain exhibited phenotypic features similar to Ptpn22 KO mice including lymphocyte and dendritic cell hyperresponsiveness consistent with a loss-of-function effect. Aged Ptpn22-R619W KI mice also developed enlarged spleens and thymuses, expanded effector/memory T cells and spontaneous GCs. Despite these features, however, Ptpn22 KI mice in the B6 background did not develop autoimmunity. Of note, the authors also reported that both the murine and human variant proteins exhibited reduced stability due to enhanced calpain- and proteasome- mediated proteolysis, implying that loss-of-function occurs due to accelerated protein degradation.
Using a similar strategy, Ptpn22-R619W KI mice were also generated and evaluated in a potentially more autoimmune prone, B6 x129 mixed genetic background (15). In contrast to initial findings, the authors showed that the murine and human variant proteins exhibited normal in vitro and in vivo stability, additionally supporting this conclusion using a Rosa26 transgenic model. Importantly, aged KI mice in the mixed genetic background developed autoantibodies and systemic autoimmunity, consistent with the predicted role for the risk variant in promoting disease. Further, using the Rosa26 transgenic model, the authors demonstrated that B lineage-restricted variant expression was sufficient to mediate autoimmunity. Consistent with altered B and T cell homeostasis in human subjects carrying the risk variant (19,24), KI mice developed expanded transitional B cell and effector/memory T cell populations (15). No significant alterations of Treg development or suppressive activity were observed in Ptpn22-R619W KI mice. Ptpn22-R619W enhanced TCR signaling in both naïve and memory T cells and impacted a distinct subset of proximal TCR substrates. These latter data imply that while the variant largely mimics a loss-in-function phenotype, the point mutation also uniquely alters Ptpn22 interactions likely leading to variant-specific changes in function. Intriguingly, in contrast to naïve or newly generated effector T cells, T cells isolated from aged KI mice exhibit attenuated TCR-induced calcium flux indicating the long-term outcome of enhanced signaling ultimately leads to hyporesponsiveness (XD and DJR, unpublished observations). In line with this, as noted above, previous human studies revealed blunted antigen receptor-mediated calcium flux in both memory T and B cells derived from subjects carrying the risk variant (19,24).
The Peterson and Bottini groups have developed and reported two alternative transgenic models that overexpress human Lyp620W under the control of either the proximal Lck promoter (33) or the human endogenous regulatory elements in BAC transgenic mice (13). In the former model, thymocytes overexpressing Lyp620W exhibited reduced TCR signaling- findings that contrast with the augmented TCR signal observed in Ptpn22-R619W KI T cells. Also in contrast to Ptpn22 KO or KI mice, neither thymic positive nor negative selection were altered suggesting that overexpression of the human PTPN22 risk variant was unlikely to alter murine T cell central tolerance. However, as noted with respect to the Jurkat T cell studies described above, potential non-physiological effects due to altered protein:protein interaction following overexpression of the human variant must be considered when interpreting these phenotypes. Interesting, myeloid cells isolated from human PTPN22 risk variant BAC transgenic mice exhibit impaired TLR related type 1 IFN production (13). Similar findings have not been reported using Ptpn22 KI mice and the phenotype of T and B cells in the BAC transgenic model also have not yet been described.
Key similarities and differences between animal and human studies
A number of important observations linking human and mouse studies have emerged from recent work on the PTPN22 risk variant. One striking, albeit underappreciated, feature of human and murine studies has been the impact of the risk variant on B cell biology. While early work was focused on T cells, the near complete association of the risk variant with diseases characterized by high-titer, disease-specific autoantibodies implies a crucial role for the variant in altering B cell function (19). Consistent with this idea, current data demonstrate B cell-intrinsic (as well as B cell-extrinsic) impacts on B lineage function. Indeed, one unifying feature of healthy individuals with the variant and the murine KI model is an alteration in the transitional B cell compartment (15,24). These observations correlate with reduced in vivo apoptosis of early transitional B cells in the murine model and increased resistance to BCR-triggered apoptosis and enhanced CD40-mediated signals in vitro in human transitional B cells (23,24). While it remains unclear as to whether these observations reflect altered negative vs. positive selection of developing B cells, additional studies using the murine KI model are anticipated to address this question. Another emerging theme is that, within an ‘at risk’ genetic setting, individuals with the variant or KI mice each can develop autoantibodies and autoimmunity over time- a concept best illustrated by the requirement for the mixed genetic background to promote disease development in the KI model. A combination of future studies, assessing cell signaling and phenotype in carefully genotyped healthy individuals and using cross-breeding of KI animals with strains bearing alterations in additional candidate genes will be required to more fully understand events that synergize for loss of tolerance. Finally, both individuals with the variant and KI animals exhibit an expansion or skewing of effector memory T cells. As discussed in more detail below, we believe this finding may reflect increased TCR signaling in response to self or environmental antigens in both cases. However, it is also interesting to note that T effector skewing is also observed in murine chimera models wherein only the B cells express the variant protein (XD and DJR, unpublished observations), implying that altered B cell tolerance can be sufficient to promote altered T cell activation.
What is different in human vs. murine studies and why? The most perplexing observations in comparing analysis of primary lymphocytes from human and murine studies have been the disparate response to antigen-receptor triggered signaling. This difference is most evident in experiments using human memory T and B cells where individuals with the variant exhibit significantly blunted signals based upon both intracellular calcium flux and the induction of tyrosine-phosphoryated signaling effectors. These findings contrast with the exaggerated signals in thymocytes, naïve T cells or in vitro expanded effector T cells derived from KI mice. Similarly, transitional and naïve B cells isolated from humans with the variant exhibit blunted signaling that is not evident in primary B cells from young KI mice. While these differences still require definitive explanation, our observations in the KI model suggest that the reduced signaling in human memory and effector cells reflects the outcome of chronic antigen experience (present in both humans and mice) that occurs in the setting of an enhanced TCR signaling program in naïve T cells. Our best evidence for this hypothesis, to date, is the blunted TCR signaling observed in T cells derived from aged KI mice (Fig 1), cells that presumably experienced chronic antigen stimulation. Analogously, in the B cell compartment, we have observed evidence for increased self-antigen driven signaling in transitional B cells in the KI model. This conclusion is based upon evidence for increased positive selection of B cells expressing a phosphorylcholine-specific BCR in the KI model (15), as well as studies assessing the expression level of a BCR signaling-dependent reporter ((16); Genita Mensler, XD and DJR- unpublished data). Reporter expression is elevated in the KI model, implying that KI B cells experience increased self-antigen driven BCR signaling during transitional development. We propose that this increased activation during development and antigen encounter may also help to explain the blunted signals observed in human immature and naïve B cells. Admittedly, these observations remain preliminary and alternative approaches are required to address this ‘signaling dichotomy’. Direct genome editing of PTPN22 in primary human lymphoid cells (leading to cells populations that differ only with respect to this single gene product) would provide a valuable means to address this key question. Alternatively, studies using truly naïve human cells (such as those present in cord blood, in vitro T or B cell culture models, or humanized mice) may need to be used to accurately assess alterations in receptor signaling.
Figure 1.
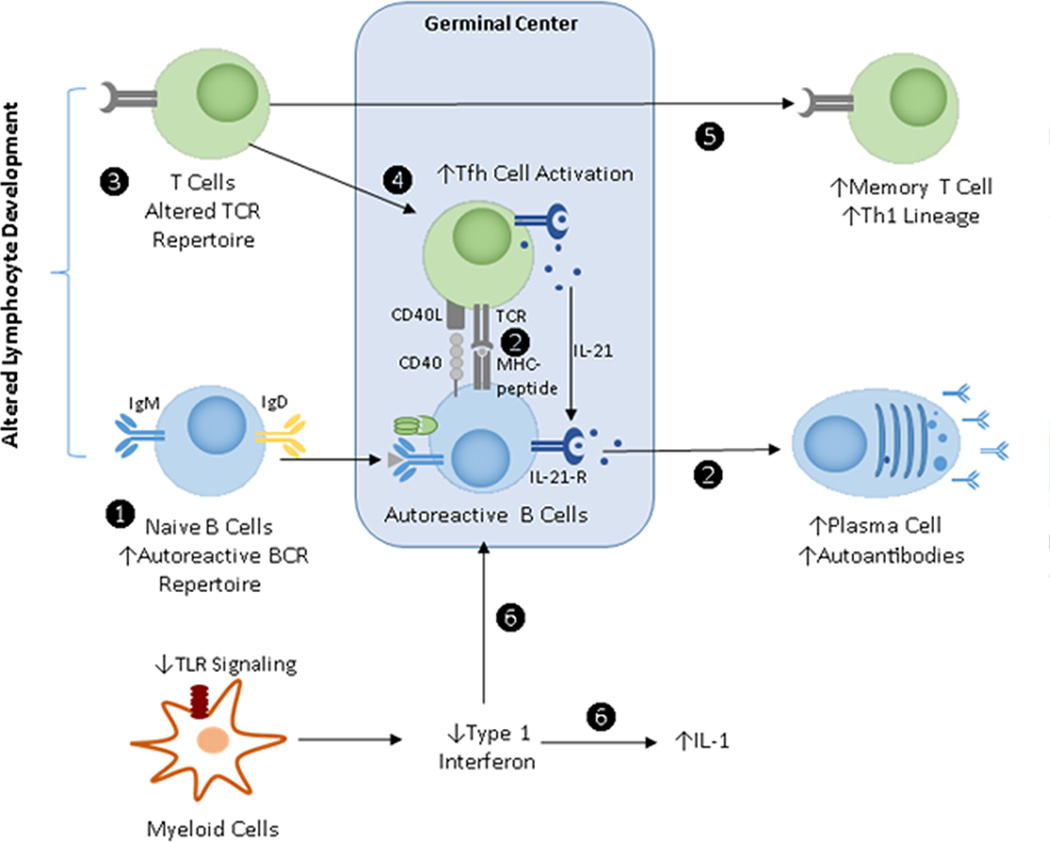
Schematic of Impact of Lyp on the immune response. See text for details.
How do we best integrate functional studies of genetic variants between mouse and man?
The observations reported here highlight the importance of seeking ways to optimally integrate investigation using human cell populations vs. murine models. While work using human cells may identify breaches in tolerance (as demonstrated, for example, by elegant work showing the increased proportion of self-reactive naïve B cells in carriers with the PTPN22 variant; (23)) mechanistic questions regarding how the variant impacts development and repertoire selection remain challenging in humans. Linking KI models with other genetic or transgenic manipulations will be critical for such mechanistic insight and this approach has already begun to bear fruit as evidenced by work showing the impact of the variant on thymic T cell selection and peripheral tolerance events in transitional B cells (15,31). Similarly, human studies have led to important insight via identification of alterations in cell phenotype, function and signaling and such approaches are clearly amenable to an initial survey of combinatorial genetic phenotypes. As noted above, newly developed genome editing tools have significant potential to improve studies in human cells. In addition, expanding technologies including multiparameter flow cytometry or cyTOF, cell type specific RNAseq analysis, and mass spectrometry based assessment of cell-specific signaling proteomes will become increasingly important in these efforts. Murine models and cross-breeding, however, will still be required to further dissect such phenotypes as illustrated by the ‘signaling dichotomy’ described above. Another challenging question with respect to human work is dissection of the specific role for different cell types or developmental subsets in disease pathogenesis. Use of conditional expression of the Ptpn22 variant using the Rosa26 locus, murine cell transfers and bone marrow chimera models have begun to address these questions. Traditional murine knock-in strategies, however, including those used to generate the Ptpn22 KI mice, lead to introduction of the variant allele in all lineages and, therefore, remain limited in their capacity to efficiently assess lineage-specific events in disease. Notably, several alternative genetic KI approaches have emerged including the ‘FlEx’ system using two pairs of heterotypic lox sites to overcome these hurdles (34,35). Thus, combining newer KI models with lineage-specific Cre deletion systems will become increasingly valuable. Finally, an ongoing challenge of murine models will be the capacity to model non-coding variants and/or missense variants wherein the SNP and coding residue may not be highly conserved, and, importantly, to do all of the above in a more timely fashion. While not a complete solution, improved genome editing tools and the ability to multiplex genetic manipulation in both murine and human cells will promote progress.
Another lesson highlighted in studies of the PTPN22 variant is that analyses using human cells must be considered in the context of how the risk variant may impact immune responses that occur following multiple different forms of immune activation and over periods of many years. In vitro studies of human cord blood may yield insight into the functional phenotype seen in naïve cells, while in vivo studies of responses to infection, vaccination or biologic therapies may yield a better understanding of how a genomic variant may impact immune responses in a mature individual. Understanding how a risk variant can alter responses over time is vital as many autoimmune diseases develop in adults. Parallel murine studies will need to be designed to examine such outcomes. Our tools to address these questions using murine systems remain limited and would benefit from consideration of how a specific variant may have emerged within the human population including, in particular, its potential protective role(s) in immune responses to specific pathogens. Using this information may be helpful in both modeling relevant immunologic challenges and predicting lineages most likely to impact immune function in autoimmunity.
Conclusions
In summary, as detailed above and displayed together in Table I, using a combined approach focused on human cells expressing the PTPN22 variant and parallel murine knock-in models, investigators in the field have contributed to an improved understanding of the role for the PTPN22 risk allele in immune tolerance, cell activation and autoimmune pathogenesis.
Table I.
Functional outcomes of PTPN22 variants in mouse and man
| Murine models | Human studies | ||||||
|---|---|---|---|---|---|---|---|
| Cell type and function |
Ptpn22 KO | Ptpn22-R619W KI | WT Ptpn22 Tg under Lck distal promoter |
PTPN22- R619W Tg under proximal Lck or PTPN22 promoter |
Healthy human subjects carrying PTPN22- R620W |
Cell lines | |
| B cells | Homeostasis | Intact (25) | ↑ T1 B cells (15) | N.D. | N.D. | ↓memory B cells, ↑transitional B cells (19,24) | Ramos B cells: ↓ BCR signaling with Lyp620W (24) |
| Signaling | Intact (25) | ↑ BCR signaling (15) | N.D. | N.D. | ↓ BCR signaling (19) | ||
| Selection | ND | ↑self-reactive B cells (15) | N.D. | N.D. | ↑autoreactive transitional and naïve B cells (24) | ||
| T cells | Homeostasis | Expanded memory/effector T cells (25) | Expanded memory/effector T cells (15,31) | Intact (30) | Intact (32) | Expanded memory T cells (19) | Jurkat T cells: ↓ TCR signaling with Lyp620W (17) ↑when over expressed with Csk (16) |
| Signaling | ↑ TCR signaling in effector T cells (25) | ↑ TCR signaling in naïve and effector T cells (15,31) | ↓ TCR signaling (30) | ↓TCR signaling (32) | ↓ TCR signaling in memory T cells (19) | ||
| Selection | ↑thymic positive selection (25) | ↑ thymic positive and negative selection (15,31) | ↓ pathogenic T cells | Intact (32,33) | N.D. | ||
| Tregs | Expanded Tregs, enhanced function in vivo (26,27) | Intact (15) | Intact (30) | N.D. | Intact (unpublished data) | ||
| Tfh | ↑ numbers and activity (28) | Development intact (unpublished data) | N.D. | N.D. | N.D. | ||
| Myeloid cells | Altered macrophage polarization (36,37); ↓type I IFN production production (13) | Enhanced DC maturation and function (31) | N.D. | Impaired type I IFN production (13) | Impaired type I IFN production (13) | THP-1 monocytes: Altered cellular response to IFNγ when knocking down PTPN22 (36,37) | |
| Autoimmunity | Spontaneous GC response, but no autoAbs in B6. (38) Resistant to EAE in B6. (26) Enhanced arthritis in KBxN. (28) | Spontaneous GC response, but no autoAbs in B6. (31) Spontaneous GC response, autoAbs, vasculitic and glomerular lesions in B6/129 background. (15) | Resistant to T1D in NOD (30) | No effect (32); enhanced colitis and arthritis (13) | Increased autoimmunity incidence (1–3) | ||
Based on our current understanding of the impact of Lyp620W on the immune response, several mechanisms can be proposed to help explain its contribution to the development of autoimmunity (Figure 1). As the variant impacts multiple lineages and is linked to multiple distinct immune disorders, we propose that these processes likely function in concert to mediate a break T and B cell tolerance and promote disease.
Disruption of normal tolerance checkpoints during peripheral B cell development leads to enrichment of a naïve B cell repertoire with increased numbers of self- and polyreactive B cells, thereby increasing the potential to facilitate humoral autoimmunity.
Altered BCR signaling promotes self-antigen driven activation of self-reactive B cells and leads to presentation of peptide self-antigens to auto-reactive T cells; this process promotes the development of spontaneous GC reactions resulting in generation of autoantibody secreting plasma cells.
Altered TCR signaling modifies the TCR repertoire (effector and regulatory repertoire).
Possible increases in Tfh cell activity, leading to enhanced IL-21 production, may work in concert with altered B cell activation to promote or sustain autoimmune GC reactions.
Expansion of memory T cells and skewing of T cell differentiation may mediate to direct tissue injury and facilitate dysregulated regulatory processes across a range of effector or memory populations.
Alterations in innate responses, including disrupted TLR-driven type I interferon production, might limit GC responses, yet also promote a pro-inflammatory milieu that facilitates T cell activation and/or other events.
Despite this progress, a number of key unanswered questions remain. It will be important to identify the specific role(s) for the variant in B cell selection and shaping of the naïve B cell repertoire. Further, it will be useful to determine whether specific self-reactive antigen receptor TCR or BCR specificities are sufficient to trigger disease when expressed in primary cells carrying the variant allele. Such experiments would help determine whether the variant’s impact on signaling can contribute to disease in a manner independent of its role in selection. The role for Lyp, in concert with Csk, in modulation of the activation state of src-family kinases is now well established. However, it is also likely that other protein: protein interactions are critical for Lyp function. Thus, it will be valuable to comprehensively identify these interactions and determine whether relevant signaling cascades are impacted by the variant. Finally, while the PTPN22 C1858T variant has been reproducibly associated with multiple autoimmune diseases, it will be informative to perform more detailed sequencing analyses to determine whether rare PTPN22 variants exist within candidate disease cohorts and their impact in assays analogous to those studied to date.
Notably, one might argue that our mechanistic understanding of the variant has benefited from trying to reconcile disparate experimental findings obtained from alternative model systems. For example, discrepant findings regarding antigen receptor signaling in cell lines (that overexpressed the variant protein with vs. without Csk) or in primary human vs. murine lymphocytes has challenged investigators to be open to the new ideas including that altered signals may reflect a critical stoichiometry of protein interaction and that observed changes may reflect a previous history of receptor engagement and selection following immune activation, respectively. This work highlights how a single variant may impact multiple facets and functions of the immune system- and this, in fact, may be part of why it is a risk variant.
Finally, it is important to recognize that most GWAS risk alleles, including PTPN22, exhibit relatively limited individual impact on disease development. Thus, a key argument for performing detailed analyses of a single risk allele (as performed for PTPN22) is that such work will identify shared ‘signatures’ predicted to track with disease. These ‘signatures’ may be driven by other genetic variants that cluster in common pathways. For example, multiple genes involved in BCR and TCR and other co-stimulatory signaling pathways have been implicated in autoimmune diseases that are also associated with the PTPN22 risk allele. Understanding the combined or synergistic impact of such variants represents a critical focus for future human and animal studies. We envision that these ‘signatures’ will facilitate broader phenotypic studies designed to identify individuals at greater risk for disease and also highlight potential candidate therapeutic targets. Based upon this premise, we anticipate that murine modeling of additional common disease-linked variants (as well as of highly penetrant, but rare, dominant autoimmune mutations), paired with carefully designed human genotype:phenotype studies, will continue to be a highly productive avenue for mechanistic and therapeutic insight in human immune diseases.
Acknowledgments
This work is supported by NIAID 5R01AI083455-04 (JHB) and NIDDK 1DP3DK097672 (JHB, XD, DJR)
Footnotes
The authors have declared that no conflict of interest exists.
Reference List
- 1.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D, Mustelin T. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 2.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, Conn MT, Chang M, Chang SY, Saiki RK, Catanese JJ, Leong DU, Garcia VE, McAllister LB, Jeffery DA, Lee AT, Batliwalla F, Remmers E, Criswell LA, Seldin MF, Kastner DL, Amos CI, Sninsky JJ, Gregersen PK. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VE, Chang M, Ramos P, Baechler EC, Batliwalla FM, Novitzke J, Williams AH, Gillett C, Rodine P, Graham RR, Ardlie KG, Gaffney PM, Moser KL, Petri M, Begovich AB, Gregersen PK, Behrens TW. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am. J. Hum. Genet. 2004;75:504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, Ball SG, James RA, Quinton R, Perros P, Pearce SH. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves' disease. J. Clin. Endocrinol. Metab. 2004;89:5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 6.Stanford SM, Bottini N. PTPN22: the archetypal non-HLA autoimmunity gene. Nat. Rev. Rheumatol. 2014;10(10):602–11. doi: 10.1038/nrrheum.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu. Rev. Immunol. 2014;32:83–119. doi: 10.1146/annurev-immunol-032713-120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J. Exp. Med. 1999;189:111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gjorloff-Wingren A, Saxena M, Williams S, Hammi D, Mustelin T. Characterization of TCR-induced receptor-proximal signaling events negatively regulated by the protein tyrosine phosphatase PEP. Eur. J. Immunol. 1999;29:3845–3854. doi: 10.1002/(SICI)1521-4141(199912)29:12<3845::AID-IMMU3845>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Cloutier JF, Veillette A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996;15:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- 11.de la Puerta ML, Trinidad AG, Rodriguez MC, de Pereda JM, Sanchez CM, Bayon Y, Alonso A. The autoimmunity risk variant LYP-W620 cooperates with CSK in the regulation of TCR signaling. PLoS. ONE. 2013;8(1):e54569. doi: 10.1371/journal.pone.0054569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vang T, Liu WH, Delacroix L, Wu S, Vasile S, Dahl R, Yang L, Musumeci L, Francis D, Landskron J, Tasken K, Tremblay ML, Lie BA, Page R, Mustelin T, Rahmouni S, Rickert RC, Tautz L. LYP inhibits T-cell activation when dissociated from CSK. Nat. Chem. Biol. 2012;8:437–446. doi: 10.1038/nchembio.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z, Shaheen ZR, Cheng G, Sawatzke K, Campbell AM, Auger JL, Bilgic H, Shoyama FM, Schmeling DO, Balfour HH, Jr, Hasegawa K, Chan AC, Corbett JA, Binstadt BA, Mescher MF, Ley K, Bottini N, Peterson EJ. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013;39:111–122. doi: 10.1016/j.immuni.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen S, Dadi H, Shaoul E, Sharfe N, Roifman CM. Cloning and characterization of a lymphoid-specific, inducible human protein tyrosine phosphatase, Lyp. Blood. 1999;93:2013–2024. [PubMed] [Google Scholar]
- 15.Dai X, James RG, Habib T, Singh S, Jackson S, Khim S, Moon RT, Liggitt D, Wolf-Yadlin A, Buckner JH, Rawlings DJ. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J. Clin. Invest. 2013;123:2024–2036. doi: 10.1172/JCI66963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J. Immunol. 2009;182:4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P, Nika K, Tautz L, Tasken K, Cucca F, Mustelin T, Bottini N. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 18.Aarnisalo J, Treszl A, Svec P, Marttila J, Oling V, Simell O, Knip M, Korner A, Madacsy L, Vasarhelyi B, Ilonen J, Hermann R. Reduced CD4+T cell activation in children with type 1 diabetes carrying the PTPN22/Lyp 620Trp variant. J. Autoimmun. 2008;31:13–21. doi: 10.1016/j.jaut.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic Variation in PTPN22 Corresponds to Altered Function of T and B Lymphocytes. J. Immunol. 2007;179:4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y, Yang J, Colby K, Hogan SL, Hu Y, Jennette CE, Berg EA, Zhang Y, Jennette JC, Falk RJ, Preston GA. High basal activity of the PTPN22 gain-of-function variant blunts leukocyte responsiveness negatively affecting IL-10 production in ANCA vasculitis. PLoS. ONE. 2012;7:e42783. doi: 10.1371/journal.pone.0042783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vang T, Landskron J, Viken MK, Oberprieler N, Torgersen KM, Mustelin T, Tasken K, Tautz L, Rickert RC, Lie BA. The autoimmune-predisposing variant of lymphoid tyrosine phosphatase favors T helper 1 responses. Hum. Immunol. 2013;74(5):574–585. doi: 10.1016/j.humimm.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negro R, Gobessi S, Longo PG, He Y, Zhang ZY, Laurenti L, Efremov DG. Overexpression of the autoimmunity-associated phosphatase PTPN22 promotes survival of antigen-stimulated CLL cells by selectively activating AKT. Blood. 2012;119:6278–6287. doi: 10.1182/blood-2012-01-403162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, Price C, Abraham C, Motaghedi R, Buckner JH, Gregersen PK, Meffre E. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. The Journal of Clinical Investigation. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habib T, Funk A, Rieck M, Brahmandam A, Dai X, Panigrahi AK, Luning Prak ET, Meyer-Bahlburg A, Sanda S, Greenbaum C, Rawlings DJ, Buckner JH. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J. Immunol. 2012;188:487–496. doi: 10.4049/jimmunol.1102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303:685–689. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- 26.Maine CJ, Hamilton-Williams EE, Cheung J, Stanford SM, Bottini N, Wicker LS, Sherman LA. PTPN22 alters the development of regulatory T cells in the thymus. J. Immunol. 2012;188:5267–5275. doi: 10.4049/jimmunol.1200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brownlie RJ, Miosge LA, Vassilakos D, Svensson LM, Cope A, Zamoyska R. Lack of the Phosphatase PTPN22 Increases Adhesion of Murine Regulatory T Cells to Improve Their Immunosuppressive Function. Sci. Signal. 2012;5:ra87. doi: 10.1126/scisignal.2003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maine CJ, Marquardt K, Cheung J, Sherman LA. PTPN22 controls the germinal center by influencing the numbers and activity of T follicular helper cells. J. Immunol. 2014;192:1415–1424. doi: 10.4049/jimmunol.1302418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng P, Kissler S. PTPN22 Silencing in the NOD Model Indicates the Type 1 Diabetes-Associated Allele Is Not a Loss-of-Function Variant. Diabetes. 2012;62(3):896–904. doi: 10.2337/db12-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh LT, Miaw SC, Lin MH, Chou FC, Shieh SJ, Chuang YP, Lin SH, Chang DM, Sytwu HK. Different modulation of Ptpn22 in effector and regulatory T cells leads to attenuation of autoimmune diabetes in transgenic nonobese diabetic mice. J. Immunol. 2013;191:594–607. doi: 10.4049/jimmunol.1203380. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Zahir N, Jiang Q, Miliotis H, Heyraud S, Meng X, Dong B, Xie G, Qiu F, Hao Z, McCulloch CA, Keystone EC, Peterson AC, Siminovitch KA. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat. Genet. 2011;43:902–907. doi: 10.1038/ng.904. [DOI] [PubMed] [Google Scholar]
- 32.Wu DJ, Zhou W, Enouz S, Orru V, Stanford SM, Maine CJ, Rapini N, Sawatzke K, Engel I, Fiorillo E, Sherman LA, Kronenberg M, Zehn D, Peterson E, Bottini N. Autoimmunity-associated LYP-W620 does not impair thymic negative selection of autoreactive T cells. PLoS. ONE. 2014;9:e86677. doi: 10.1371/journal.pone.0086677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, Jeffery D, Mortara K, Sampang J, Williams SR, Buggy J, Clark JM. Identification of substrates of human protein-tyrosine phosphatase PTPN22. J. Biol. Chem. 2006;281:11002–11010. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 34.Schnutgen F, Doerflinger N, Calleja C, Wendling O, Chambon P, Ghyselinck NB. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat. Biotechnol. 2003;21:562–565. doi: 10.1038/nbt811. [DOI] [PubMed] [Google Scholar]
- 35.Schnutgen F, Ghyselinck NB. Adopting the good reFLEXes when generating conditional alterations in the mouse genome. Transgenic Res. 2007;16:405–413. doi: 10.1007/s11248-007-9089-8. [DOI] [PubMed] [Google Scholar]
- 36.Chang HH, Miaw SC, Tseng W, Sun YW, Liu CC, Tsao HW, Ho IC. PTPN22 modulates macrophage polarization and susceptibility to dextran sulfate sodium-induced colitis. J. Immunol. 2013;191:2134–2143. doi: 10.4049/jimmunol.1203363. [DOI] [PubMed] [Google Scholar]
- 37.Spalinger MR, Lang S, Weber A, Frei P, Fried M, Rogler G, Scharl M. Loss of protein tyrosine phosphatase nonreceptor type 22 regulates interferon-gamma-induced signaling in human monocytes. Gastroenterology. 2013;144:978–988. doi: 10.1053/j.gastro.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa K, Yajima H, Katagiri T, Ogimoto M, Arimura Y, Mitomo K, Mashima K, Mizuno K, Yakura H. Requirement of PEST domain tyrosine phosphatase PEP in B cell antigen receptor-induced growth arrest and apoptosis. Eur. J. Immunol. 1999;29:887–896. doi: 10.1002/(SICI)1521-4141(199903)29:03<887::AID-IMMU887>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]


