Abstract
Objective
To investigate the feasibility and reliability of passive muscle stiffness measurements in children through use of shear wave ultrasound elastography.
Methods
This is a prospective cross -sectional study quantifying the passive stiffness of bilateral lateral gastrocnemii muscles during passive stretch in twenty typically developing children (age range, 2.0–12.6 years). Data collected included passive stiffness of the lateral gastrocnemius muscle (shear modulus in kilopascal [kPa]) at four positions of progressive passive foot dorsiflexion; demographic characteristics of the child participants; and comparison of demographic characteristics with the shear modulus.
Results
Passive stiffness increased with increasing stretch (mean [SD] range of stretch, 7.1 [2.0]–36.2 [22.0] kPa). For all four foot positions, no significant difference was found between right and left legs (range P=0.42 to P=0.98) or between the sexes (range P=0.28 to P> 0.99). No correlation of passive muscle stiffness to age, body mass index, or ankle range of motion was found. Reliability of measurements was good to excellent (mean [95% CI] range of reliability 0.67 [0.44–0.83] to 0.80 [0.63–0.90]).
Conclusions
Measurements of passive stiffness of the lateral gastrocnemius muscle are feasible and reliable in children as young as 2 years. Because the present study found no significant difference between sex and the side tested in this age-group, future studies involving children of this age range may not need to be stratified on the basis of these parameters. Defining normal passive muscle stiffness in children is critical for identifying and understanding the implications of abnormal passive muscle stiffness in children with neuromuscular disorders.
Keywords: elastography, feasibility, gastrocnemius muscle, pediatrics, skeletal muscle, sonoelastography
Introduction
Strength, joint range of motion (ROM), and passive muscle stiffness are important factors in predicting physical function. All contribute to total muscle force 1. In children, normal values exist for strength 2 and joint ROM 3 but not for passive muscle stiffness. This latter characteristic is defined as “the ability of skeletal muscles to lengthen without muscle activation.” 1 Too much (hypertonia) or too little (hypotonia) passive muscle stiffness leads to differences in physical function and development in children. Joint ROM and strength measurements can be collected in the clinic during the physical examination, but passive muscle stiffness can only be inferred with reference to muscle tone. Passive muscle stiffness can only be inferred because physical examination maneuvers are unable to isolate the muscle without the influence of tendon or joint capsular stiffness. In addition, clinical muscle tone measurement scales are not sufficient for determining passive muscle stiffness because their reporting contains subjectivity.4
In the past few years, an advanced imaging technique, magnetic resonance elastography, has shown the ability to measure passive muscle stiffness in adults and children, age 8–12 years.5 However, use of this technique for routine clinic care is not practical because of the cost and accessibility of magnetic resonance imaging.
More recently, advances in real-time ultrasound elastography have emerged with the capability to measure passive muscle stiffness. One such technique is supersonic shear imaging (SSI).6 In SSI, high-intensity push pulses from an ultrasound transducer are used to produce shear waves in tissue, and the propagation of the shear waves is detected with the same transducer in imaging mode. As the tissue hardness or stiffness increases, shear wave speed also increases. The shear wave speeds are measured and used to calculate shear modulus—a biomechanical measurement of hardness reported in kilopascal (kPa). Although this technique is still early in the study of muscle 7–10, it has been shown in adults to have capability to detect differences in passive muscle stiffness at varying joint positions 11 and to have good to excellent reliability. 9,12,13 Given the inherent ease of use, real -time imaging, rapid image acquisition, and absence of radiation, ultrasound elastography may be well suited for quantifying passive muscle stiffness in children.
Accurate quantification of passive muscle stiffness in children is the first step in objectively defining normal and abnormal passive muscle stiffness. In combination with measurements of strength and joint ROM, muscle stiffness measurements may improve predictions of physical function, such as walking, in children with abnormal muscle tone. In addition, objective measurement of muscle stiffness may improve assessment of response to interventions for abnormal muscle stiffness, such as techniques for spasticity reduction in children. However, in order to identify abnormal passive muscle stiffness in children, normal passive muscle stiffness must be defined. Therefore, we propose to quantify passive muscle stiffness in children without neuromuscular disorders using SSI in a clinical setting. We will also evaluate the reliability of these measurements.
Materials and Methods
Participants
Twenty typically developing children aged 2 to 12 years were recruited from the community. Children were excluded from the study if 1) they had any medical condition or medication affecting muscle, rheumatologic condition, fracture, or orthopedic intervention to the lower limb in the past 6 months or 2) their parent or guardian did not provide informed consent. Mayo Clinic institutional review board approval was obtained before initiating the study. Written informed consent was obtained from a parent or guardian of the child, with written assent obtained from the child if older than 7 years.
Study Methods
For each child, demographic information was collected, including date of birth, height and weight, sex, medical and surgical history, and medications. Foot dominance was determined by asking the child, parent, or guardian or by having the child demonstrate which foot he or she uses to kick a ball. Physical examination measurements were collected also. Joint ROM of the ankle for foot dorsiflexion and plantar flexion (PF) was performed using a goniometer with the child in supine position with hip at 0° extension and knee at full extension (0° flexion). Maximal ROM was achieved when either the child verbalized or showed body or facial indication of discomfort or a solid end point of dorsiflexion was reached. Manual muscle testing was performed with the child in a seated position.
Measurements of bilateral lateral gastrocnemius hardness were performed with SSI. An Aixplorer ultrasonic scanner (version 4.0; SuperSonic Imagine) with a linear array transducer (SL15-4; SuperSonic Imagine) was used in SSI mode with musculoskeletal preset. Details regarding the physics and mathematical approach for these measurements are further described elsewhere.6,13,14 Briefly, the SSI ultrasound probe generates shear waves in the muscle through brief, repeated ultrasound push beams that rapidly attenuate.14 These shear waves travel along the muscle fiber, causing displacement. This displacement is tracked with the same ultrasound probe and captured by the ultrafast imaging technique. The pixel displacement is used to calculate shear wave speed, which is then incorporated into a mathematical algorithm that results in calculation of shear modulus.6,13,15 The mathematic conversion from shear wave speed to Young’s and shear modulus assumes the material is pure elastic, locally homogeneous, and isotropic. These assumptions do not apply to muscle. However, strong correlations between the muscle shear modulus measurement from shear wave elastography and the Young’s modulus measurements from traditional material testing have been shown.12 This indicates that the shear modulus measurements from shear wave elastography may be used to represent the true Young’s modulus of the muscle, which is otherwise very challenging to measure under in vivo settings.
For the SSI measurements, each child was positioned prone with feet dangling over the edge of the examination table. With a tape measure, circumferential measurements of each calf were obtained, and the area of greatest muscle bulk was marked on the skin. The ultrasound transducer was positioned on the lateral gastrocnemius muscle over the area of greatest muscle bulk and was aligned with muscle fiber direction. The distance from the fibular head to the proximal end of the ultrasound probe was measured and recorded to maintain positioning of the ultrasound transducer in the repeated measures. B-mode imaging and consensus by examiners present at the study visit were used to confirm positioning and alignment of the transducer. Appropriate transducer alignment was achieved when several fascicles of the lateral gastrocnemius could be traced.10 The transducer was held in place with minimal pressure on skin by one of the examiners (JEB, SFE, PS, and HZ). Examiners were trained in use of the SSI machine, identification of the muscle fascicles, and in maintenance of minimal probe pressure. Previous studies have reported good to excellent inter-rater reliability of measurements between multiple examiners for the same subject.9,13 Surface electromyography (U-Control; Thought Technology Ltd) at the lowest setting to detect muscle activation was used to ensure muscle relaxation. Electrodes were placed over the posterior calf near the area of SSI measurement. If electromyographic evidence of muscle activation was present, the image recorded was excluded from analysis and another image was obtained. Foot positioning was determined by goniometer measurement. When correct foot position and muscle relaxation were present, SSI measurement of that position was performed. This measurement process was done for each foot position (ie, 20° PF, 10° PF, and 0° PF and 10° dorsiflexion) and then repeated twice for each leg. A total of 12 measurements were obtained for each leg.
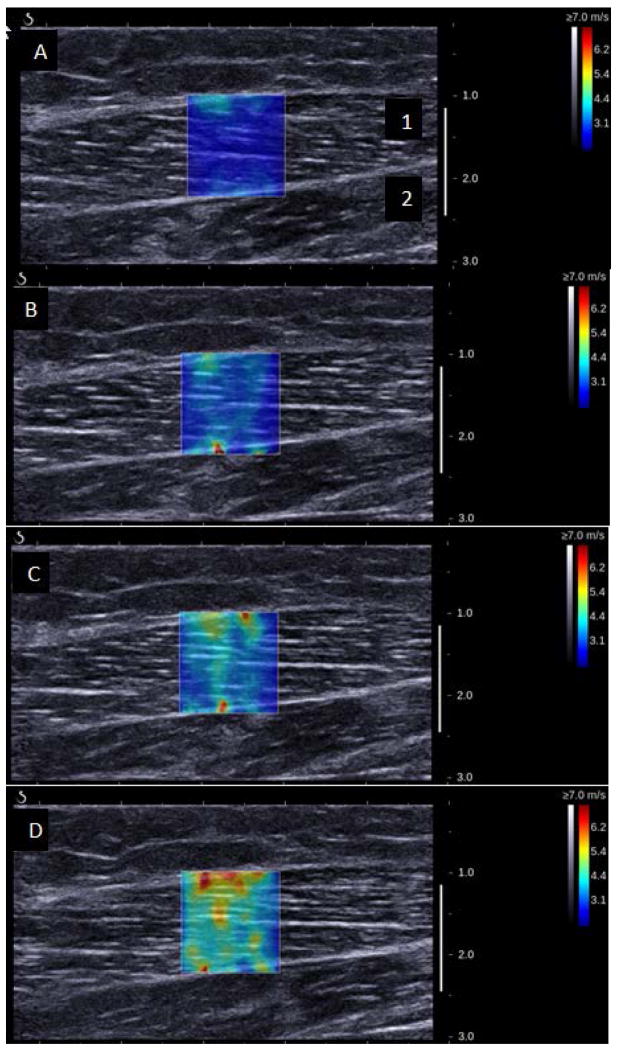
The output for each SSI measurement is a 2-dimensional elastogram (Figure 1). On this elastogram, a region of interest (ROI) over the lateral gastrocnemius muscle was selected at testing. The ROI was a 4.6 to 6.2 mm region within the elastogram. The size of the ROI was maintained across all measurements for each child, but varied slightly between children to avoid fascial planes. Variations in ROI size up to 4 mm have no significant effect on averaged values.16 Young’s modulus (kPa) was averaged over the entire ROI, using an open-source imaging plug-in for the DICOM reader (OsiriX Imaging Software). Young’s modulus was then converted to shear modulus (also kPa) with the formula E=3μ, where E is Young’s modulus and μ is shear modulus. Shear modulus is the accepted measurement for reporting on anisotropic tissues, such as muscle.8,9,13
Figure 1.
Representative 2-Dimensional Elastograms from Right Lateral Gastrocnemius of an 8-Year-Old Girl showing A) 20° plantar flexion, B) 10° plantar flexion, C) 0° plantar flexion, and D) 10° dorsiflexion. The colored box, located over the lateral gastrocnemius muscle (1), represents the area of measurement; the soleus muscle (2) is also shown. The colored bar on the right represents shear-wave speed: purple indicates tissue with low shear-wave speed (softer tissue) and red indicates tissue with high shear-wave speed (more stiff tissue).
Statistical Analysis
Differences between the sexes for demographic and SSI measurements were calculated using Wilcoxon rank sum test. Differences between lateral gastrocnemius SSI measurements for right and left sides and between SSI measurements at each foot position for the same side were calculated using paired t tests. The level for significance for any P value was set at less than .05. Reliability of the 3 repeated measurements at each foot position was calculated using the intraclass correlation coefficient software (version 9; SAS Institute Inc). By convention, intraclass correlation coefficient values are less than 0.4 for slight to fair agreement; 0.4 to 0.6, moderate agreement; greater than 0.6 to 0.75, good agreement; and greater than 0.75 to 1.0, excellent agreement. The level for significance for any P value was set at less than .05. All analyses were conducted using SAS for Unix (version 9; SAS Institute Inc).
Results
A total of 20 children participated in this study. Demographic characteristics are described in Table 1. There were slightly more boys than girls, but no significant difference was present in age or body mass index (BMI) between the sexes. All children had full strength in the muscles tested in the lower extremities. Among the children, 19 (95%) were right-foot dominant. All children were able to achieve complete muscle relaxation. One child was unable to be tested in all 4 positions of 1 leg (left) because of limited dorsiflexion ROM. Two children were able to achieve 10° dorsiflexion during SSI measurement, although their initial measured maximal ankle dorsiflexion was less. Two other children were able to achieve only 5° maximal ankle dorsiflexion. These measurements were included with the 10° dorsiflexion measurements because the degree of dorsiflexion was within one standard deviation for variability with goniometry measurements.17
Table 1.
Demographic Characteristics, Physical Measurements, and Shear Modulus of Children With Respect to Sex and Side of Measurement
| Characteristica | Patients
|
P Value | |
|---|---|---|---|
| Girls (n=12) | Boys (n=8) | ||
| Age, mo | 79.8 (33.1) | 83.3 (46.8) | .94b |
| Age range, mo | 36.0–127.0 | 24.0–151.0 | |
| BMI, kg/m2 | 18.5 (5.1) | 19.6 (3.8) | .28b |
| BMI range, kg/m2 | 14.2–31.7 | 14.8–26.1 | |
| Calf circumference, cm | |||
| Right | 26.7 (5.7) | 28.0 (5.6) | .64b |
| Range | 19.4–38.6 | 21.4–37.7 | |
| Left | 26.9 (5.8) | 27.6 (5.5) | .64b |
| Range | 19.5–39.2 | 21.5–37.5 | |
| Shear modulus, kPa | |||
| 20 PF | |||
| Right | 7.1 (2.0) | 8.6 (3.0) | .22b |
| Left | 7.7 (3.8) | 7.9 (2.7) | .70b |
| 10 PF | |||
| Right | 10.2 (3.0) | 11.3 (4.9) | .64b |
| Left | 10.1 (3.5) | 11.7 (4.3) | .59b |
| 0 PF | |||
| Right | 17.7 (5.9) | 17.5 (7.9) | .88b |
| Left | 15.9 (5.3) | 18.1 (9.0) | .82b |
| 10 DF | |||
| Right | 26.7 (7.5) | 36.2 (22.0) | .40b |
| Left | 30.7 (8.8) | 34.8 (19.7) | >.99b |
| Side of Measurement | |||
| Right (n=20) | Left (n=20) | ||
| Calf circumference, overall, cm | 27.2 (5.6) | 27.2 (5.6) | .88c |
| Range | 19.4–38.6 | 19.5–39.2 | |
| Maximum ankle DF overall | 13.0° (7.2°) | 13.2° (6.3°) | .83c |
| Range | 0°–30° | 1°–31° | |
| Shear modulus overall, kPa | |||
| 20 PF | 7.7 (2.5) | 7.8 (3.3) | .98c |
| 10 PF | 10.6 (3.8) | 10.7 (3.8) | .78c |
| 0 PF | 17.6 (6.6) | 16.8 (6.9) | .42c |
| 10 DF | 30.5 (15.3) | 32.4 (14.1) | .57c |
Abbreviations: BMI, body mass index; DF, dorsiflexion; kPa, kilopascal; PF, plantar flexion.
Values are presented as mean (SD) unless specified otherwise.
From Wilcoxon rank sum test.
From paired t test.
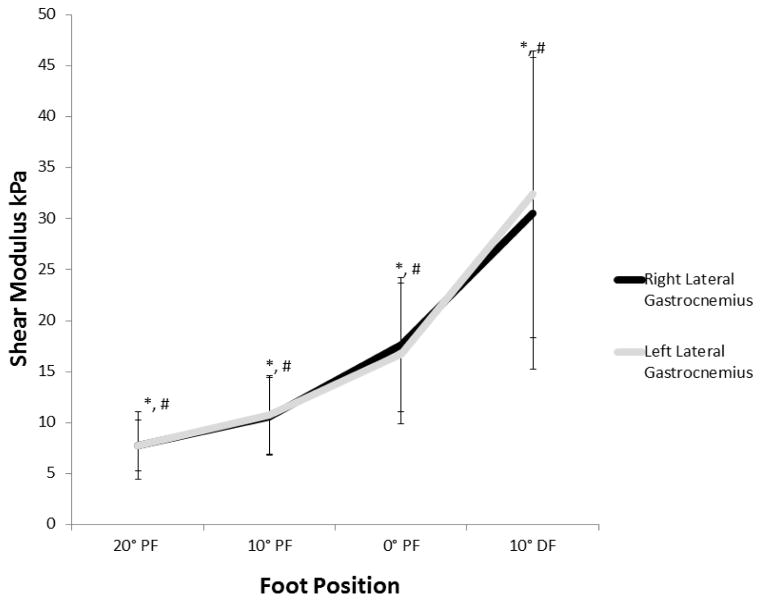
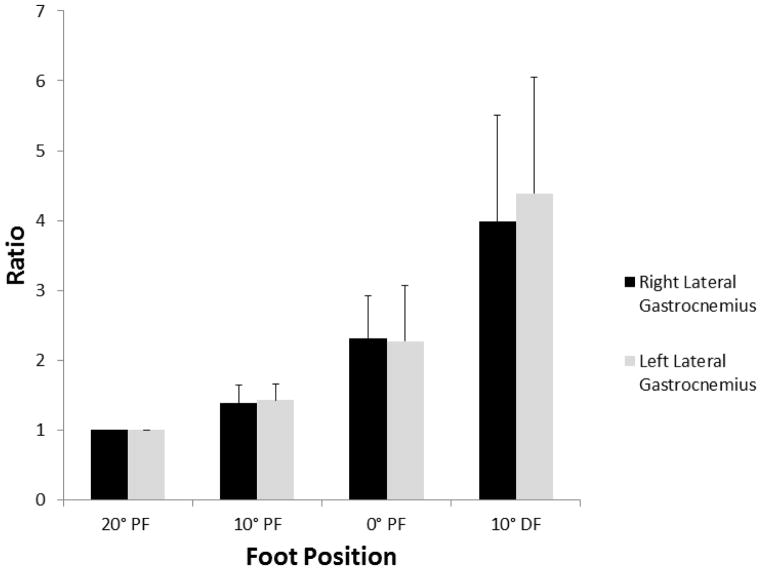
The mean (SD) of shear modulus for each position and each leg are listed in Table 1. When comparing the same position between sides, we found no significant difference between measurements. For each side, a significant difference was found among measurements at differing foot positions, with passive stiffness increasing with increasing dorsiflexion (Figure 2). The ratio of passive stiffness to baseline (20° PF) increased such that at 10° dorsiflexion, the passive lateral gastrocnemius stiffness was approximately 4 times greater than at baseline (Figure 3). Table 2 lists the correlation coefficients of shear modulus for each foot position based on age, BMI, calf circumference, and maximum ankle dorsiflexion. No significant correlations were found between shear modulus and age, BMI, calf circumference, or maximal ankle dorsiflexion. However, though not statistically significant, BMI and maximal ankle dorsiflexion had a negative pattern with shear modulus at all joint positions (ie, as BMI and maximal ankle dorsiflexion increased, shear modulus decreased), and age had a positive pattern with shear modulus at all positions. In comparing the boys and girls, we found no significant difference in shear modulus between the right side and the left side at each foot position (Table 1).
Figure 2.
Shear Modulus (Stiffness) of Right and Left Lateral Gastrocnemius Muscles With Passive Stretch Among Children. There is no significant difference in stiffness between the right and left sides at the same foot position. * indicates that values for each foot position on the right differed significantly from each other (P<.001). # indicates that for each foot position on the left, the values differ significantly (P<.001) from each other. The bars indicate 1 SD from mean value for each foot position. Foot position is in degrees of plantar flexion (PF) and dorsiflexion (DF), with 0° PF being the neutral position. kPa indicates kilopascal.
Figure 3.
Ratio of Shear Modulus (Stiffness) of Right and Left Lateral Gastrocnemius Muscle at Each Foot Position to Shear Modulus at the Baseline of 20° Plantar Flexion (PF). The ratio is 1 at 20° PF and increases to about 4 times greater than the baseline at 10° dorsiflexion (DF). Foot position of 0° PF is neutral position. The bars indicate 1 SD from mean value for each foot position.
Table 2.
Spearman Correlation Coefficient of Age, BMI, Calf Circumference, and Maximal Ankle DF to Shear Modulus at Each Ankle Position
| Characteristic |
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Shear Modulus, Correlation (P valuea)
| ||||||||
| Right Leg
|
Left Leg
|
|||||||
| 20° PF | 10° PF | 0° PF | 10° DF | 20° PF | 10° PF | 0° PF | 10° DF | |
|
|
|
|||||||
| Age | 0.25 (.28) | 0.41 (.07) | 0.27 (.26) | 0.31 (.18) | 0.16 (.51) | 0.25 (.28) | 0.35 (.13) | 0.16 (.51) |
|
|
|
|||||||
| BMI | −0.32 (.16) | −0.21 (.36) | −0.35 (.13) | −0.13 (.58) | −0.38 (.10) | −0.32 (.16) | −0.21 (.38) | −0.45 (.05) |
|
|
|
|||||||
| Right calf circumference | 0.06 (.80) | 0.22 (.35) | 0.08 (.75) | 0.21 (.37) | −0.05 (.84) | 0.02 (.93) | 0.21 (.40) | −0.01 (.94) |
|
|
|
|||||||
| Left calf circumference | 0.05 (.83) | 0.21 (.39) | 0.07 (.77) | 0.19 (.41) | −0.06 (.81) | 0.00 (.99) | 0.17 (.46) | −0.05 (.81) |
|
|
|
|||||||
| Maximum right ankle DF | −0.16 (.51) | −0.24 (.30) | −0.24 (.32) | −0.39 (.09) | −0.18 (.45) | −0.20 (.39) | −0.42 (.06) | −0.45 (.05) |
|
|
|
|||||||
| Maximum left ankle DF | 0.10 (.64) | 0.01 (.96) | −0.02 (.93) | −0.22 (.36) | −0.08 (.75) | −0.00 (.99) | −0.25 (.28) | −0.35 (.13) |
|
|
|
|||||||
Abbreviations: BMI, body mass index; DF, dorsiflexion; PF, plantar flexion.
P <.05 considered significant.
Reliability of the shear modulus value for each foot position for both legs is listed in Table 3. All measurements showed good to excellent agreement.
Table 3.
ICCs for Shear Modulus of the Lateral Gastrocnemius Muscle at Each Tested Foot Position
| Position | Right Side
|
Left Side
|
||
|---|---|---|---|---|
| No. of Patients | ICC (95% CI) | No. of Patients | ICC (95% CI) | |
| 20° PF | 20 | 0.69 (0.49–0.84) | 20 | 0.75 (0.56–0.87) |
| 10° PF | 20 | 0.80 (0.63–0.90) | 20 | 0.71 (0.51–0.85) |
| 0° PF | 20 | 0.67 (0.46–0.83) | 20 | 0.73 (0.54–0.87) |
| 10° DF | 20 | 0.80 (0.63–0.90) | 19 | 0.67 (0.44–0.83) |
Abbreviations: DF, dorsiflexion; ICC, intraclass correlation coefficient; PF, plantar flexion.
Discussion
In typically developing children, lateral gastrocnemius passive muscle stiffness, as calculated by shear modulus, is similar between right and left sides and between sexes. No significant correlations were found between passive muscle stiffness and calf circumference. Both BMI and maximal ankle dorsiflexion had a nonsignificant but negative correlation with passive muscle stiffness (i.e, as BMI and maximal ankle dorsiflexion increased, passive muscle. stiffness decreased). There is also suggestion that passive muscle stiffness may increase with age, though this correlation was also not significant.
The similarity in lateral gastrocnemius passive stiffness between right and left leg is likely related to the similar strength and calf circumference between sides in the group of children we studied. Both strength and volume can affect muscle stiffness.1 The lack of difference in passive muscle stiffness between sides also indicates that foot dominance does not have a significant effect on this measurement in children. In fact, controversy exists about the definition of a dominant foot or leg, citing that the preferred foot or leg depends on the task being performed (eg, kicking a ball vs standing on 1 leg).18 On the basis of the present study’s results, the unilateral measurements of the gastrocnemius muscle in typically developing children appear to be sufficient for future studies.
In our study, calf circumference was used as a surrogate for muscle mass. It has been previously thought there is a direct correlation between skeletal muscle mass and passive muscle stiffness, such that as muscle mass increases, passive stiffness increases.1 Akagi et al 19 measured passive medial gastrocnemius stiffness in men with another ultrasound elastography technique, strain elastography, and found that passive muscle stiffness correlated to muscle thickness only when the muscle was slack.. In children, we also did not find a significant correlation between calf circumference and passive muscle stiffness. The differences in results between these studies may be due to a greater complexity in the association between passive muscle stiffness and mass. Passive stiffness depends not only on muscle mass (ie, resting filament tension), but also on stiffness of the sarcomeric cytoskeleton and connective tissue.1 The balance of these elements may differ between persons, even when calf size is similar, because of a difference in the proportions of these components. This difference in components could lead to differences in passive stiffness measurements despite the measured mass appearing to be the same.
We further explored this apparent discrepancy in the correlation of passive stiffness and mass by looking at the correlation of BMI, a marker of overall body mass. BMI did not significantly correlate with passive muscle stiffness; however, we did find a negative pattern between BMI and passive muscle stiffness. This negative pattern suggests that passive muscle stiffness decreases as BMI increases in children. This negative pattern may be due to changes in the contributors to total mass (relative increase in fat content as opposed to a change in connective tissue or muscle). Further studies are needed to evaluate whether a relation exists between BMI and passive muscle stiffness in children and adults.
Among the children we studied, sex and age did not have a significant correlation with passive muscle stiffness. This finding is similar to a study using SSI to measure passive gastrocnemius stiffness in adults. In that study, passive gastrocnemius stiffness did not differ by sex (P=.40) or age (P=.06).7 In a different study using magnetic resonance elastography to measure passive muscle stiffness of the thigh, Debernard et al 5 found that children aged 8 to 12 years had a slightly lower passive muscle stiffness (P<.05) than young adults (age range, 24–28 years) and middle-aged persons (age range, 52–58 years). Their study did not examine differences in passive muscle stiffness within each group.
Our results show a pattern of increasing passive stiffness with age, but this result was not significant. The lack of a significant difference in passive muscle stiffness with age may be due to sample size and/or the age range of children in our study. Other studies have shown that sex does not appear to affect active muscle stiffness until puberty or at approximately 13 years of age.3,20 This lack of difference between sexes before puberty may also be similar for passive muscle stiffness, though we did not assess pubertal status in the children in our study. In a study measuring passive gastrocnemius stiffness in adults using ultrasound shear wave elastography, sex and age did not correlate to gastrocnemius passive stiffness, though men had a tendency toward greater passive stiffness.7 While our findings suggest the continued grouping of children of this age range, future studies with larger groups of children stratified by age and pubertal status would be helpful to clarify the relationship of age and puberty and passive muscle stiffness.
While not statistically significant, we did find a negative pattern between passive maximal ankle dorsiflexion and passive lateral gastrocnemius muscle stiffness. This finding implies that in children, passive gastrocnemius muscle stiffness is lower in those with greater dorsiflexion ROM. For example, when comparing the passive gastrocnemius stiffness between two children when their ankle positions are the same, the child with greater overall ankle joint ROM (i.e. greater ankle flexibility) may have less passive gastrocnemius muscle stiffness at each position tested as compared to the child with less ankle flexibility at the same position. Importantly, the gastrocnemius is a muscle that crosses both the knee and ankle joints. Therefore, when the knee is in an extended position, the gastrocnemius –as opposed to the soleus -is the muscle that has the most influence on maximal ankle dorsiflexion ROM. Previously, individual muscle contribution to overall joint flexibility had not been directly measured.3,20 Our findings provide preliminary evidence of the contribution of an individual muscle’s passive stiffness to overall joint flexibility. This is a finding that should be investigated further through investigating multiple muscles that influence the joint flexibility.
For all children, as each of their gastrocnemii muscles were stretched, passive muscle stiffness increased with increasing passive stretch (Figure 2). This result has also been found in adult studies of muscle stiffness using SSI and corresponds to the expected pattern seen in the loading portion of a hysteresis curve.10,21 As a muscle is passively stretched, it has an initial region of slack. Once the slack is eliminated, resistance to stretch increases until tissue failure. Although we could not test the muscle to the point of tissue failure (i.e., muscle tear or disruption), we did see an increase in passive muscle stiffness in the ROM we tested.
Finally, the reliabilities of our passive muscle stiffness measurements were good to excellent. Other authors have documented the good to excellent reliability of SSI measurements for passive muscle stiffness in various joint positions in adults.9,10,22 Potential reasons for the variability in our measurements include the participants being studied (children), potential differences between differing muscles, and use of goniometer for positioning.
Limitations
This pilot study evaluated the feasibility of SSI use in children. Small group size limited its power to detect secondary measures, though the suggested associations found in the secondary measures opens areas for further exploration with larger studies. There is also inherent variability in positioning with a goniometer. Positioning with a goniometer has been shown to have a 5°±5° variation with strict measurement protocol in children who have cerebral palsy.17 In typically developing children, we would expect the variation to be less, but this variation may still impact reliability of SSI measurements. However, the goniometer was chosen to maintain clinical applicability of the SSI measurements. Surface electromyography was used, which may not detect very low levels of underlying muscle activation. It is not clear whether a very low level of muscle activity would influence results, though this effect would likely be similar among the children.
Pubertal status may have an effect on muscle measurements.3,20 This status was not evaluated in our study and thus cannot be excluded as a possible source of variability in the measurements. In a different study evaluating the effect of pubertal status on joint hypermobility, the mean (SD) age of puberty was 13.7 (1.0) years for boys and 12.7 (1.1) years for girls.20 The oldest boy and girl in our study were 12.6 and 10.6 years, respectively, placing them at less than 1 standard deviation below the average age of puberty. Thus, pubertal status while a possible source of measurement variability may be less likely to have influenced our results. Effect of pubertal status on muscle properties is an area of interest for further study.
Conclusions
Quantifying passive muscle stiffness in children as young as 2 years is feasible and reliable. In the age range of children in the present study, sex, age, and calf circumference did not significantly correlate with passive muscle stiffness. BMI and maximal ankle dorsiflexion showed patterns of association with passive muscle stiffness. Age, sex, calf circumference, BMI, and effect of joint flexibility on passive muscle stiffness are all areas of interest for further exploration in a larger group of children for creation of normal values for passive muscle stiffness. This study is the first step toward defining normal passive muscle stiffness in children, which is critical for identifying and understanding the implications of abnormal passive muscle stiffness in children with neuromuscular disorders.
Acknowledgments
The authors thank Ann Hoffman PT, DScPT, PCS for assistance with data collection. The authors would also like to thank the ultrasound laboratory of Dr. James F. Greenleaf for encouragement of the project and use of lab resources, without their support this work would not have been possible.
The authors thank the National Institutes of Health (NIH) (grants KL2TR000136-07, K12HD00109, F30 AG044075), and the National Institute of General Medical Sciences (T32 GM 65841) for supporting this research. The opinions expressed are those of the authors and do not necessarily express the opinions of the NIH.
Footnotes
Portions of this work will be presented in poster format at the American Academy of Physical Medicine and Rehabilitation Annual Meeting, November 13 –16, 2014 in San Diego, California.
Relevant commercial interest disclosures include the following. Dr. Chen receives financial support from Sonoscape, Inc. (Shenzhen, China) for general consultation (public domain knowledge) on elastography. Dr. Song has United States and international patents pending for ultrasound elastography. Dr. Chen and Dr. Zhao have United States and international patents for ultrasound elastography. Dr. Chen, Dr. Song, and Dr. Zhao receive royalties from General Electric Medical Systems and Samsung Electronics Company Ltd.
References
- 1.Gajdosik RL. Passive extensibility of skeletal muscle: review of the literature with clinical implications. Clin Biomech. 2001;16:87–101. doi: 10.1016/s0268-0033(00)00061-9. [DOI] [PubMed] [Google Scholar]
- 2.Eek MN, Kroksmark A-K, Beckung E. Isometric muscle torque in children 5 to 15 years of age: normative data. Arch Phys Med Rehabil. 2006;87:1091–1099. doi: 10.1016/j.apmr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Soucie JM, Wang C, Forsyth A, et al. Range of motion measurements: reference values and a database for comparison studies. Haemophilia. 2011;17:500–507. doi: 10.1111/j.1365-2516.2010.02399.x. [DOI] [PubMed] [Google Scholar]
- 4.Yam WKL, Leung MSM. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J Child Neurol. 2006;21:1031–1035. doi: 10.1177/7010.2006.00222. [DOI] [PubMed] [Google Scholar]
- 5.Debernard L, Robert L, Charleux F, Bensamoun SF. Analysis of thigh muscle stiffness from childhood to adulthood using magnetic resonance elastography (MRE) technique. Clin Biomech. 2011;26:836–840. doi: 10.1016/j.clinbiomech.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 7.Arda K, Ciledag N, Aktas E, Aribas BK, Kose K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol. 2011;197:532–536. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- 8.Gennisson J-L, Deffieux T, Mace E, et al. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol. 2010;36:789–801. doi: 10.1016/j.ultrasmedbio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Leong H-T, Ng GY-f, Leung VYF, Fu SN. Quantitative estimation of muscle shear elastic modulus of the upper trapezius with supersonic shear imaging during arm positioning. PLoS ONE. 2013;8:e67199. doi: 10.1371/journal.pone.0067199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maisetti O, Hug F, Bouillard K, Nordez A. Characterization of passive elastic properties of the human medial gastrocnemius muscle belly using supersonic shear imaging. J Biomech. 2012;45:978–984. doi: 10.1016/j.jbiomech.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Shinohara M, Sabra K, Gennisson J-L, Fink M, Tanter M. Real-time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve. 2010;42:438–441. doi: 10.1002/mus.21723. [DOI] [PubMed] [Google Scholar]
- 12.Eby SF, Song P, Chen S, et al. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–2387. doi: 10.1016/j.jbiomech.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacourpaille L, Hug F, Bouillard K, Hogrel J-Y, Nordez A. Supersonic shear imaging provides a reliable measurement of resting muscle shear elastic modulus. Physiol Meas. 2012;33:N19–28. doi: 10.1088/0967-3334/33/3/N19. [DOI] [PubMed] [Google Scholar]
- 14.Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012;85:1435–1445. doi: 10.1259/bjr/93042867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annu Rev Biomed Eng. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 16.Kot BCW, Zhang ZJ, Lee AWC, Leung VYF, Fu SN. Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoSONE. 2012;7:e44348. doi: 10.1371/journal.pone.0044348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allington NJ, Leroy N, Doneux C. Ankle joint range of motion measurements in spastic cerebral palsy children: intraobserver and interobserver reliability and reproducibility of goniometry and visual estimation. J Pediatr Orthop B. 2002;11:236–239. doi: 10.1097/00009957-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Velotta J, Weyer J, Ramirez A, Winstead J, Bahamonde R. Relationship between leg dominance tests and type of task. Portugese Journal of Sport Sciences. 2011;11:1035–1038. [Google Scholar]
- 19.Akagi R, Chino K, Dohi M, Takahashi H. Relationships between muscle size and hardness of the medial gastrocnemius at different ankle joint angles in young men. Acta Radiologica. 2012;53:307–311. doi: 10.1258/ar.2011.110481. [DOI] [PubMed] [Google Scholar]
- 20.Quatman CE, Ford KR, Myer GD, Paterno MV, Hewett TE. The effects of gender and pubertal status on generalized joint laxity in young athletes. J Sci Med Sport. 2008;11:257–263. doi: 10.1016/j.jsams.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chernak LA, Dewall RJ, Lee KS, Thelen DG. Length and activation dependent variations in muscle shear wave speed. Physiol Meas. 2013;34:713–721. doi: 10.1088/0967-3334/34/6/713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordez A, Gennisson JL, Casari P, Catheline S, Cornu C. Characterization of muscle belly elastic properties during passive stretching using transient elastography. J Biomech. 2008;41:2305–2311. doi: 10.1016/j.jbiomech.2008.03.033. [DOI] [PubMed] [Google Scholar]