Abstract
Muscle fatigability and atrophy are frequent clinical signs in limb girdle muscular dystrophy (LGMD), but their pathogenetic mechanisms are still poorly understood.
We review a series of different factors that may be connected in causing fatigue and atrophy, particularly considering the role of neuronal nitric oxide synthase (nNOS) and additional factors such as gender in different forms of LGMD (both recessive and dominant) underlying different pathogenetic mechanisms.
In sarcoglycanopathies, the sarcolemmal nNOS reactivity varied from absent to reduced, depending on the residual level of sarcoglycan complex: in cases with complete sarcoglycan complex deficiency (mostly in beta-sarcoglycanopathy), the sarcolemmal nNOS reaction was absent and it was always associated with early severe clinical phenotype and cardiomyopathy.
Calpainopathy, dysferlinopathy, and caveolinopathy present gradual onset of fatigability and had normal sarcolemmal nNOS reactivity. Notably, as compared with caveolinopathy and sarcoglycanopathies, calpainopathy and dysferlinopathy showed a higher degree of muscle fiber atrophy.
Males with calpainopathy and dysferlinopathy showed significantly higher fiber atrophy than control males, whereas female patients have similar values than female controls, suggesting a gender difference in muscle fiber atrophy with a relative protection in females. In female patients, the smaller initial muscle fiber size associated to endocrine factors and less physical effort might attenuate gender-specific muscle loss and atrophy.
Key words: LGMD, nNOS, sarcoglycan
Introduction
Limb Girdle Muscular Dystrophies (LGMD) are a group of disorders of skeletal muscle showing a wide clinical and genetic heterogeneity (for classification see: www.musclegenetable.fr). The physio-pathological mechanism underlying LGMDs is different for each form, and for most of them it is only poorly understood. According to the disease mechanism, the LGMDs may be grouped as follows (1): defects of dystrophin-glycoprotein complex (DGC) (LGMD2C, 2D, 2E, 2F, 2P, 2T), enzyme defects affecting glycosylation of α-dystroglycan (DG) (LGMD2I, 2K, 2M, 2N, 2O), sarcomeric defects (LGMD1A, 2G, 2J), enzyme defects affecting sarcomere remodelling (LGMD2A, 2H), defects of signal transduction (LGMD1C), defects affecting membrane repair (LGMD2B, 2L), defects of the nuclear membrane (LGMD1B, 1F).
The sarcolemma of skeletal muscle fibers is characterized by the presence of the DGC, which is composed of cytoskeletal proteins (dystrophin, syntrophins), the DG complex, and the sarcoglycan (SG) complex. The DGC provides a mechanical linkage between the extracellular matrix and the intracellular cytoskeleton. The structural and functional integrity of this connection is crucial to stabilize the sarcolemma during contractions. The SG complex is composed of 4 SG glycoproteins whose mutant genes cause a group of LGMD called sarcoglycanopathies (LGMD2C-2F), where the assembly of the SG complex is compromised, and the sarcolemma integrity and stability are lost. A similar pathogenetic mechanism leads to Duchenne muscular dystrophy (DMD), which is caused by mutations in the dystrophin gene. The DGC has also signalling roles, due to its interaction with other proteins, including neuronal Nitric Oxide Synthase (nNOS), which is anchored at the sarcolemma by binding of α1-syntrophin (2-8). The production of Nitric Oxide (NO), which is a messenger molecule that rapidly transduces signalling events in a calcium-dependent manner, is able to regulate muscle development, contractility and blood flow.
Caveolin-3 appears to be not directly associated with the DGC; caveolins act as scaffolding proteins to organize and concentrate specific caveolin-interacting lipids and proteins. Caveolin-3 has been shown to directly bind nNOS and has a possible interaction with dysferlin. Similar to proteins involved in the DGC, it is recognized that sarcomeric proteins (myotilin, titin, telethonin) have not only important structural roles, but also signalling roles, such as those involved in muscle cell proliferation, fusion, maintenance, regeneration and repair. Dysferlin is involved in the repair of plasmalemma lesions, since it mediates vesicle trafficking and membrane fusion in muscle cells, binding its C2 domain to phospholipids in a calcium-dependent manner.
In this review paper we discuss the results reported in earlier studies from our group and the literature, which independently investigated the role of nNOS, muscle fatigue and muscle fibre atrophy in various forms of LGMD, in order to offer a comprehensive view of their individual role and their relationship in this group of disorders.
Muscle fatigue and nNOS in muscular dystrophies
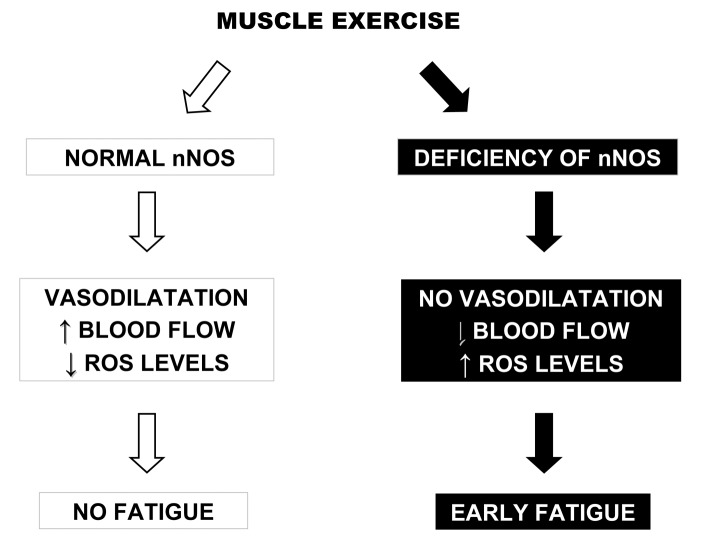
Dystrophic patients have difficulties to support an excessive or long-term physical activity, and frequently complain of fatigue during the exercise of moderate or short-lived intensity (9). The increase of physical exhaustion for the energetic expense for an exercise is the cause of acute fatigue, whereas the inability to maintain a certain level of force is the cause of chronic fatigue. Muscle fatigue can be due to coupling excitement-contraction, to lack of availability of substrates or blood flow and lack of adaptation of vasodilatation by NO (Fig. 1), and to the possible modifications of the intracellular environment and disruption of contractile apparatus (3).
Figure 1.
Cascade of events consequent to muscle exercise when normal (on the left) or defective (on the right) nNOS is present. Modified from Neuromuscular Disorders 2012;22:S214-220.
A secondary deficiency of nNOS has been suggested to contribute to fibre degeneration in muscular dystrophies, because the loss of nNOS would reduce the normal protective action of NO against local ischemia during contraction (vascular hypothesis) and increase the cellular susceptibility to superoxides (oxidative stress hypothesis) (Fig. 1). Indeed, absent nNOS at the sarcolemma was observed not only in muscle from α1-syntrophin knock-out mice, but also from DMD and Becker muscular dystrophy (BMD) patients where dystrophin gene deletions removed a region which is crucial for the interaction between nNOS and α1-syntrophin (10). The loss of nNOS in DMD muscle may result in aberrant regulation of adrenergic vasodilatation, since dystrophin loss was demonstrated to impair the regulation of vasoconstrictor response (11), and dystrophin-deficient mdx mice as well as nNOS null mice are unable to control muscle blood flow during exercise (12). Without proper vascular dilatation and subsequent blood flow, muscles suffer from focal necrosis and are susceptible to fatigue. The potential of nNOS to improve mdx muscle pathology suggested NO-related therapies may be beneficial for treatment of dystrophinopathy.
nNOS in sarcoglycanopathies
Crosbie et al. thoroughly investigated the nNOS expression in the animal models of sarcoglycanopathies (including both the BIO 14.6 hamster with delta-SG deficiency and mice with targeted disruption of alpha- SG, beta-SG, and delta-SG genes) and in patients with sarcoglycanopathies (13), where they clearly demonstrated that nNOS is reduced in sarcoglycan-deficient muscle, and that the deficiency at the sarcolemma was more pronounced in patients with complete SG complex deficiency (beta-sarcoglycanopathy), suggesting a possible direct correlation between the levels of nNOS expression at the sarcolemma and the overall level of SG complex.
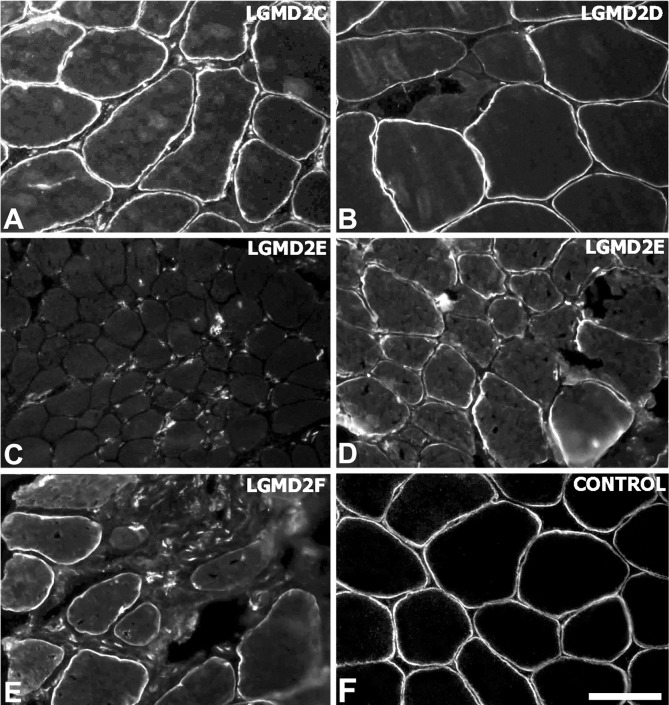
This hypothesis was further validated in another study (14), in which 14 muscles from patients affected with different forms of sarcoglycanopathies (4 alpha-SG, 7 beta-SG, 2 gamma-SG, 1 delta-SG) have been investigated for the expression of both the cytosolic nNOS (by western blotting) and its sarcolemmal localization (by immunohistochemistry). This latter study (14) showed that the sarcolemmal nNOS reaction varied from absent to reduced, depending on the integrity of the SG complex (Fig. 2, Table 1), demonstrating that the integrity of the SG complex is essential for the sarcolemmal localization of nNOS. Indeed, a perturbation in the structural integrity of the DGC may alter syntrophin's PDZ domains, which have been shown to directly interact with nNOS (15).
Figure 2.
Sarcolemmal immunolabelling of nNOS in muscle biopsies from patients with sarcoglycanopathies (A-E) and control (F). The reaction was absent in LGMD2E patients with complete SG complex defect (C) and partial in LGMD2E patients with partial SG complex defect (D), indicating that the reduction of nNOS level depends directly on the residual level of SG complex. Scale bar = 50 μm. Original magnification: 200x.
Table 1.
Clinical and experimental data in different types of LGMD.
| Disease type | N. cases | Mean age at biopsy (years) | Skeletal muscle phenotype | Dilated cardio-myopathy | SG complex deficiency | Cytosolic nNOS (% of control) | Sarcolemmal nNOS |
|---|---|---|---|---|---|---|---|
| Sarcoglycanopathies | |||||||
| LGMD2C | 2 | 20.5 | Childhood-onset LGMD in 2 | None | Partial in 2 | 66.5 | Reduced in 2 |
| LGMD2D | 4 | 16.7 | Childhood-onset LGMD in 3, hyperCKemia in 1 | None | Complete in 1/4, partial in 3/4 | 78.5 | Absent in 1, reduced in 3 |
| LGMD2E | 7 | 14.6 | Childhood-onset LGMD in 7 | Present in 4 | Complete in 5/7, partial in 2/7 | 42.5 | Absent in 5, reduced in 2 |
| LGMD2F | 1 | 34.0 | Childhood-onset LGMD | Present in 1 | Partial in 1 | 52.0 | Reduced |
| Other types of LGMD | |||||||
| LGMD1C | 8 | 26.7 | Childhood-onset LGMD in 2, adultonset LGMD in 2, distal myopathy in 2, rippling in 1, hyperCKemia in 1 | None | None | 98.7 | Reduced in 8 |
| LGMD2A | 8 | 36.5 | Childhood-onset LGMD in 2, adultonset LGMD in 5, hyperCKemia in 1 | None | None | 95.2 | Normal in 3, reduced in 5 |
| LGMD2B | 2 | 46.5 | Distal myopathy in 2 | None | None | 146.0 | Reduced in 2 |
More detailed clinical and laboratory data from the same patients series are reported in Fanin et al. (14).
nNOS and dilated cardiomyopathy in sarcoglycanopathies
In the study by Fanin et al. (14), the sarcolemmal nNOS expression correlated with the clinical severity, as described for some cases also in previous reports (14, 16- 18) and muscle fatigue: absence or severe reduction of sarcolemmal nNOS expression was associated with a severe and childhood-onset form of muscular dystrophy and in most cases also with dilated cardiomyopathy (Table 1).
Mice lacking either γ-SG or δ-SG display progressive focal cardiomyocyte degeneration that ultimately leads to reduced cardiac function and death (19). This model of cardiomyopathy closely parallels what is seen in humans with SG and dystrophin gene mutations (20- 22). Furthermore, null mice for β-SG and δ-SG [but not for α-SG (17)] presented a disruption of the vascular smooth muscle SG complex (23-25). The perturbed vascular function induces ischemic injury in cardiac and skeletal muscle (23), suggesting that this mechanism could contribute to the development of cardiomyopathy and exacerbate skeletal myopathy.
It is well known that vascular spasm is an important contributor to cardiac pathology (19). Elevated levels of intracellular calcium, disturbances of the NOS pathway, and increased activity of protein kinase C, have been implicated in increased contractility and/or spasm of the microvasculature
Therefore, the observation that sarcolemmal nNOS can be absent or mislocalized in sarcoglycanopathy muscle (14, 26, 27) provides a possible link between this pathogenetic mechanism and the development of cardiomyopathy in sarcoglycanopathies, offering further insights for therapeutic interventions.
NO stimulates soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP), and in the absence of dystrophin the NO-sGC-cGMP pathway is disrupted. The nucleotide phosphodiesterases (PDEs) hydrolize the cGMP and regulate their downstream signalling. PDE5 expression in cardiomyocytes is low at baseline and increases in response to ischemia or pressure overload from heart failure. Impaired blood flow in muscle and heart in mdx dystrophin-deficient and NOSdeficient mice was rescued by inhibition of PDE5 (28). Unfortunately, in Duchenne and Becker dystrophy patients a clinical trial with PDE5 inhibitor (Sidenafil) did not improve cardiomyopathy, since 30% of patients progressed to ventricular dilatation (29).
Long term dietary supplementation of L-arginine (a NOS substrate) was not a viable therapy for dystrophinopathy (30), but the use of antioxidants that attenuate the superoxide attack and restore the bioactive NO level, might be useful approaches for the treatment of these disorders.
nNOS in calpainopathy, dysferlinopathy, caveolinopathy
In a study which investigated 18 muscles from calpainopathy, dysferlinopathy and caveolinopathy patients (14), nNOS was found to be present at normal levels in the cytosol (by western blotting) (Table 1) and correctly localized to the sarcolemma by immunohistochemistry, although with variable intensity. In all these disorders, this variability might be in part due to the presence of scattered regenerating fibers which show absent sarcolemmal nNOS staining on serial sections (14). These data suggested that the interactions between the corresponding mutant proteins (calpain-3, dysferlin, caveolin- 3) and nNOS are not crucial for the nNOS signaling pathway.
Muscle fibers atrophy in LGMDs and gender differences in LGMDs
Besides muscle fatigue, LGMD patients experience a loss of muscle mass and the associated muscle weakness. Consequently, patients have an increased risk of suffering from co-morbidities related with reduced physical activity (e.g. reduced mobility, joint contractures). Muscle atrophy results from an imbalance between dynamic anabolic and catabolic reactions, where the increased myofibrillar protein breakdown exceeds the protein synthesis. Muscle atrophy is an active process which is controlled by specific signalling pathways and transcriptional programs involving many atrophy-promoting genes or atrogenes (31, 32). Three proteolytic systems are involved in the maintenance of the sarcomeric function: the cytosolic calpain system, the ubiquitin-proteasome system, and the autophagic-lysosomal pathway. Although they are all activated in muscle atrophy, the key player in the degradation of myofibrillar proteins is the ubiquitin-proteasome system.
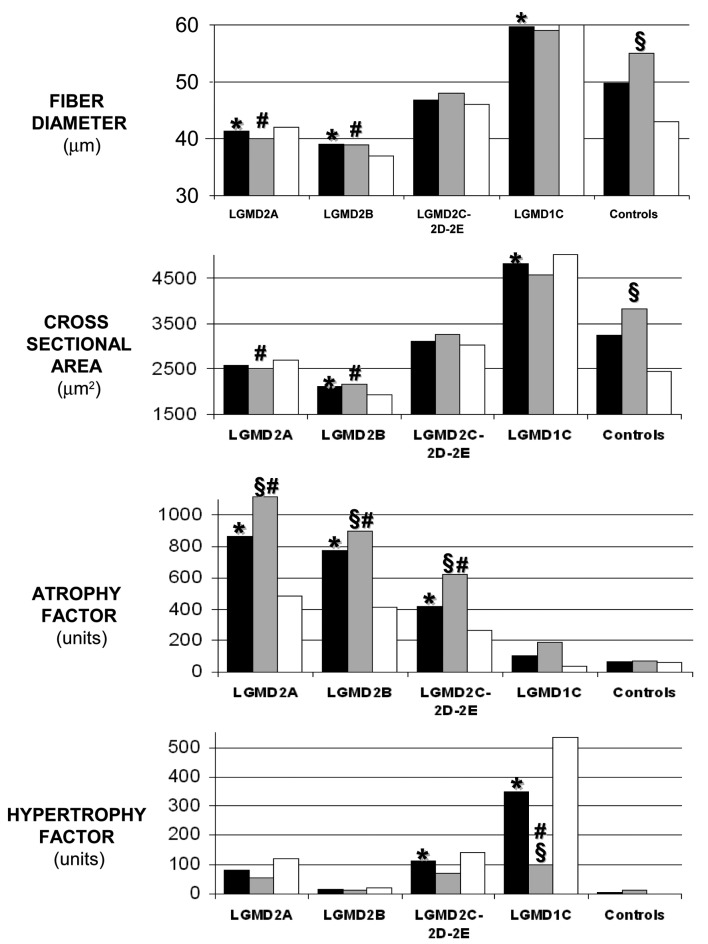
In contrast to muscle strength, which depends on several factors (e.g. exercise, bulk), fiber atrophy seems to be a more precise marker of muscle atrophy because it reflects an abnormal process in the contractile apparatus of muscle. Several studied have investigated muscle fiber size in LGMD using well-known morphometric parameters, such as diameter, cross sectional area, atrophy factor, hypertrophy factor (33). In particular, the degree of muscle fiber atrophy was measured in calpainopathy (34), in dysferlinopathy (35) and in other forms of LGMD including sarcoglycanopathies and caveolinopathy (36).
Calpainopathy and dysferlinopathy muscles were those in which the atrophic process was more relevant, since fiber diameter and fiber cross sectional area were significantly reduced, and atrophy factor was significantly increased as compared to controls (Fig. 3). This observation confirms previous studies that investigated the molecular pathways involved in muscle atrophy program in calpainopathy and dysferlinopathy (34, 35), and has important clinical consequences since possible rehabilitative, pharmacological and nutritional interventions directed at reducing muscle atrophy and wasting could contribute in slowing the disease course.
Figure 3.
Histograms showing the comparison between the mean values of fiber diameter, cross sectional area, atrophy factor and hypertrophy factor in the different groups of LGMD patients and in the control group. Black bars = total cases of both genders, gray bars = male patients, white bars = female patients. Significant difference (p < 0.05) is indicated as: * individual disease group versus control group, § males versus females within the same disease group, # one gender of a disease group versus the same gender of the control group.
Gender differences in muscle fiber atrophy in LGMDs
Previous studies comparing men and women in response to disuse atrophy suggested that there are gender differences in strength loss and atrophy, and one of these studies compared also muscle fiber size in diseased and control muscles of the same gender (36). Male patients affected with calpainopathy and dysferlinopathy have significantly lower values of fiber diameter, cross sectional area and higher values of atrophy factor than male controls, whereas female patients have values not significantly different from female controls (Fig. 3). Earlier studies in LGMD patients suggested that there are gender differences both in strength and atrophy. Among potential factors differentiating women and men, the are gonadal hormone levels (i.e. oestrogen and testosterone) which are known to influence muscle mass (37). Another factor enhancing the degree of atrophy may be the initial muscle mass (38), suggesting that a smaller initial muscle size, rather than endocrine factors attenuate gender-specific muscle loss in women (39).
A study of calpainopathy muscle showed that the degree of muscle fiber atrophy significantly correlated with the clinical-functional severity of the disease (34), and therefore a higher degree of fiber atrophy in males should correspond to a higher degree of clinical muscle impairment. The possibility that male patients with various LGMD may be more severely affected than females has already been explored: among calpainopathy patients, a more rapid progression was observed in males than in females (40-42), but no gender differences were evident in the age at onset or loss of ambulation in other large series of calpainopathy patients (43-45). A more severe phenotype in males than in female was reported in an animal model of dysferlinopathy but not in dysferlinopathy patients (42); similar observations were done also in a large family with gamma-sarcoglycanopathy (46) but not in other series of sarcoglycanopathy patients (42), in LGMD2G (42), and in other myopathies. Furthermore, a male gender predominance is frequently observed in LGMDs, including LGMD2L (47), and one possible explanation is that females might be less severely affected and therefore less likely to be ascertained.
Any intervention aimed to reduce muscle atrophy could improve the course of the disease. Several factors have been described as reducing the otherwise elevated expression of atrogenes and therefore as potentially counteracting muscle atrophy: muscle exercise training (48, 49), treatment with Bortezomib and Fenofibrate (50, 51), branched-chain amino acids (52), L-carnitine (53) and long-chain ω-3 fatty acid (54).
Conclusions
In the recent years, the research on LGMD, besides the successful and continuous work dedicated to the identification of new causative genes, was focused on the investigation of molecular pathways involved in their pathogenetic cascade.
The sarcoglycanopathies share with DMD the disruption of the linkage between the extracellular matrix and the intracellular cytoskeleton, and the consequent loss of sarcolemma integrity and stability, which is also essential for the localization of nNOS. In sarcoglycanopathies the deficiency of nNOS is an adverse modulating factor in the course of muscular dystrophy and dilated cardiomyopathy; indeed, SG complex deficiency in the vascular smooth muscle might lead either to structural changes or to an impairment of metabolic and NOS signalling pathways in tissues involved in the microvascular dysfunction, making cardiomyocytes more susceptible to intermittent ischemia.
While the structure and function of DGC and nNOS does not seem to be affected in other forms of LGMD, in calpainopathy and dysferlinopathy a significant atrophy of muscle fibers is a peculiar characteristic, which originate from the activation of specific intracellular degenerative pathways and slowly progresses leading to chronic pathological changes of muscle.
There is evidence suggesting that male patients with such LGMD are more likely to undergo muscle fibre atrophy than female patients, and may be therefore more likely to suffer from the consequent muscle weakness and clinical disability.
A specific rehabilitative program in LGMD, taking into account muscle fatigue and atrophy, should be planned, avoiding both eccentric exercise and possible local muscle ischemia. It may consist of moderate endurance training and stretching (55).
References
- 1.Nigro V, Savarese M. Genetic basis of limb-girdle muscular dystrophies: the 2014 update. Acta Myol. 2014;33:1–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi YM, Rader EP, Crawford RW, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heydemann A, McNally E. NO more muscle fatigue. J Clin Invest. 2009;119:48–450. doi: 10.1172/JCI38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Percival JM, Anderson KNE, Huang P, et al. Golgi and sarcolemmal nNOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest. 2010;120:816–826. doi: 10.1172/JCI40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sander M, Chavoshan B, Harris SA, et al. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crosbie RH. NO vascular control in Duchenne muscular dystrophy. Nat Med. 2001;7:27–29. doi: 10.1038/83309. [DOI] [PubMed] [Google Scholar]
- 7.Chao DS, Gorospe JR, Brenman JE, et al. Selective loss of sarcolemmal nitric oxide synthase in Becker muscular dystrophy. J Exp Med. 1996;184:609–618. doi: 10.1084/jem.184.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells KE, Torelli S, Lu Q, et al. Relocalization of neuronal nitric oxide synthase (nNOS) as a marker for complete restoration of the dystrophin associated protein complex in skeletal muscle. Neuromusc Disord. 2003;13:21–31. doi: 10.1016/s0960-8966(02)00191-8. [DOI] [PubMed] [Google Scholar]
- 9.Angelini C, Tasca E. Fatigue in muscular dystrophies. Neuromusc Disord. 2012;22:S214–S220. doi: 10.1016/j.nmd.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torelli S, Brown SC, Jimenez-Mallebrera C, et al. Absence of neuronal nitric oxide synthase (nNOS) as a pathological marker for the diagnosis of Becker muscular dystrophy with rod domain deletions. Neuropathol Appl Neurobiol. 2004;30:540–545. doi: 10.1111/j.1365-2990.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 11.Sander M, Chavoshan B, Harris SA, et al. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Nattl Acad Sci USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas GD, Sander M, Lau KS, et al. Impaired metabolic alphaadrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosbie RH, Barresi R, Campbell KP. Loss of sarcolemma nNOS in sarcoglycan deficient muscle. FASEB J. 2002;16:1786–1791. doi: 10.1096/fj.02-0519com. [DOI] [PubMed] [Google Scholar]
- 14.Fanin M, Tasca E, Nascimbeni AC, et al. Sarcolemmal neuronal nitric oxide synthase defect in Limb Girdle Muscular Dystrophy: an adverse modulating factor in the disease course? J Neuropathol Exp Neurol. 2009;68:383–390. doi: 10.1097/NEN.0b013e31819cd612. [DOI] [PubMed] [Google Scholar]
- 15.Brenman JE, Chao DS, Gee SH, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1- syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 16.Angelini C, Fanin M, Freda MP, et al. The clinical spectrum of sarcoglycanopathies. Neurology. 1999;52:176–179. doi: 10.1212/wnl.52.1.176. [DOI] [PubMed] [Google Scholar]
- 17.Melacini P, Fanin M, Duggan DJ, et al. Heart involvement in muscular dystrophies due to sarcoglycan gene mutations. Muscle Nerve. 1999;22:473–479. doi: 10.1002/(sici)1097-4598(199904)22:4<473::aid-mus8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Fanin M, Melacini P, Boito C, et al. LGMD2E patients risk developing dilated cardiomyopathy. Neuromusc Disord. 2003;13:303–330. doi: 10.1016/s0960-8966(02)00280-8. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler MT, Allikian MJ, Heydemann A, et al. Smooth muscle cell-extrinsic vascular spasm arises from cardiomyocyte degeneration in sarcoglycan-deficient cardiomyopathy. J Clin Invest. 2004;113:668–675. doi: 10.1172/JCI20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Politano L, Nigro V, Passamano L, et al. Evaluation of cardiac and respiratory involvement in sarcoglycanopathies. Neuromusc Disord. 2001;11:178–185. doi: 10.1016/s0960-8966(00)00174-7. [DOI] [PubMed] [Google Scholar]
- 21.Barresi R, Blasi C, Negri T, et al. Disruption of heart sarcoglycan complex and severe cardiomyopathy caused by beta sarcoglycan mutations. J Med Genet. 2000;37:102–107. doi: 10.1136/jmg.37.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsubata S, Bowles KR, Vatta M, et al. Mutations in the human delta-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. J Clin Invest. 2000;106:655–662. doi: 10.1172/JCI9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coral-Vazquez R, Cohn RD, Moore SA, et al. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- 24.Durbeej M, Cohn RD, Hrstka RF, et al. Disruption of the betasarcoglycan gene reveals pathogenetic complexity of limb-girdle muscular dystrophy type 2E. Mol Cell. 2000;5:141–151. doi: 10.1016/s1097-2765(00)80410-4. [DOI] [PubMed] [Google Scholar]
- 25.Hack AA, Lam MY, Cordier L, et al. Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. J Cell Sci. 2000;113:2535–2544. doi: 10.1242/jcs.113.14.2535. [DOI] [PubMed] [Google Scholar]
- 26.Crosbie RH, Barresi R, Campbell KP. Loss of sarcolemma nNOS in sarcoglycan-deficient muscle. FASEB J. 2002;16:1786–1791. doi: 10.1096/fj.02-0519com. [DOI] [PubMed] [Google Scholar]
- 27.Heydemann A, Huber JM, Kakkar R, et al. Functional nitric oxide synthase mislocalization in cardiomyopathy. J Mol Cell Cardiol. 2004;36:213–223. doi: 10.1016/j.yjmcc.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Percival JM, Whitehead NP, Adams ME, et al. Sidenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol. 2012;228:77–87. doi: 10.1002/path.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung DG, Herzka DA, Thompson WR, et al. Sidenafil does not improve cardiomyopathy in Duchenne/Becker muscular dystrophy. Ann Neurol. 2014;76:541–549. doi: 10.1002/ana.24214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehling-Henricks M, Jordan MC, Gotoh T, et al. Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One. 2010;5:e10763–e10763. doi: 10.1371/journal.pone.0010763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 32.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubowitz V, Sewry C. Muscle biopsy: a practical approach. 3rd ed. London: Saunders Elsevier; 2007. [Google Scholar]
- 34.Fanin M, Nascimbeni AC, Angelini C. Muscle atrophy in Limb Girdle Muscular Dystrophy 2A: a morphometric and molecular study. Neuropathol Appl Neurobiol. 2013;39:762–771. doi: 10.1111/nan.12034. [DOI] [PubMed] [Google Scholar]
- 35.Fanin M, Nascimbeni AC, Angelini C. Muscle atrophy, ubiquitinproteasome and autophagic pathway in dysferlinopathy. Muscle Nerve. 2014;50:340–347. doi: 10.1002/mus.24167. [DOI] [PubMed] [Google Scholar]
- 36.Fanin M, Nascimbeni AC, Angelini C. Gender difference in limbgirdle muscular dystrophy: a muscle fiber morphometric study in 101 patients. Clin Neuropathol. 2014;33:179–185. doi: 10.5414/NP300728. [DOI] [PubMed] [Google Scholar]
- 37.Tidus PM. Influence of estrogen on skeletal muscle damage, inflammation, and repair. Sport Sci Rev. 2003;31:40–44. doi: 10.1097/00003677-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol. 2000;89:823–839. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- 39.Miles MP, Heil DP, Larson KR, et al. Prior resistance training and sex influence muscle responses to arm suspension. Med Sc Sports Exerc. 2005;37:1983–1989. doi: 10.1249/01.mss.0000176302.99185.be. [DOI] [PubMed] [Google Scholar]
- 40.Piluso G, Politano L, Aurino S, et al. Extensive scanning of the calpain-3 gene broadens the spectrum of LGMD2A phenotypes. J Med Genet. 2005;42:686–693. doi: 10.1136/jmg.2004.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paula F, Vainzof M, Passos-Bueno MR, et al. Clinical variability in calpainopathy: what makes the difference? Eur J Hum Genet. 2002;10:825–832. doi: 10.1038/sj.ejhg.5200888. [DOI] [PubMed] [Google Scholar]
- 42.Zatz M, Paula F, Starling A, et al. The 10 autosomal recessive limbgirdle muscular dystrophies. Neuromusc Disord. 2003;13:532–544. doi: 10.1016/s0960-8966(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 43.Angelini C, Nardetto L, Borsato C, et al. The clinical course of calpainopathy (LGMD2A) and dysferlinopathy (LGMD2B) Neurol Res. 2010;32:41–46. doi: 10.1179/174313209X380847. [DOI] [PubMed] [Google Scholar]
- 44.Fanin M, Fulizio L, Nascimbeni AC, et al. Molecular diagnosis in LGMD2A: mutation analysis or protein testing? Hum Mutat. 2004;24:52–62. doi: 10.1002/humu.20058. [DOI] [PubMed] [Google Scholar]
- 45.Pollitt C, Anderson LV, Pogue R, et al. The phenotype of calpainopathy: diagnosis based on a multidisciplinary approach. Neuromusc Disord. 2001;11:287–296. doi: 10.1016/s0960-8966(00)00197-8. [DOI] [PubMed] [Google Scholar]
- 46.Leal GF, Silva E. Limb-girdle muscular dystrophy with apparently different clinical courses within sexes in a large inbred kindred. J Med Genet. 1999;36:714–718. [PMC free article] [PubMed] [Google Scholar]
- 47.Hicks D, Sarkozy A, Muelas N, et al. A founder mutation in Anoctamin- 5 is a major cause of limb-girdle muscular dystrophy. Brain. 2011;134:171–182. doi: 10.1093/brain/awq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen GQ, Mou CY, Yang YQ, et al. Exercise training has beneficial anti-atrophy effects by inhibiting oxidative stress-induced MuRF-1 up-regulation in rats with diabetes. Life Sci. 2011;89:44–49. doi: 10.1016/j.lfs.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Gielen S, Sandri M, Kozarez I, et al. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age. Circulation. 2012;125:2716–2727. doi: 10.1161/CIRCULATIONAHA.111.047381. [DOI] [PubMed] [Google Scholar]
- 50.Beehler BC, Sleph PG, Benmassaoud L, et al. Reduction of skeletal muscle atrophy by a proteasome inhibitor in a rat model of denervation. Exp Biol Med. 2006;231:335–341. doi: 10.1177/153537020623100315. [DOI] [PubMed] [Google Scholar]
- 51.Castillero E, Nieto-Bona MP, Fernandez-Galaz C, et al. Fenofibrate, a PPAR alpha agonist, decreases atrogenes and myostatin expression and improve arthritis-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab. 2011;300:E790–E799. doi: 10.1152/ajpendo.00590.2010. [DOI] [PubMed] [Google Scholar]
- 52.Herningtyas EH, Okimura Y, Handayaningsih AE, et al. Branchedchain aminoacids and arginine suppress MaFbx/atrogin-1 mRNA expression via mTOR pathway in C2C12 cell lines. Biochim Biophys Acta. 2008;1780:1115–1120. doi: 10.1016/j.bbagen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Keller J, Ringseis R, Priebe S, et al. Dietary L-carnitine alters gene expression in skeletal muscle of piglets. Mol Nutr Food Res. 2011;55:419–429. doi: 10.1002/mnfr.201000293. [DOI] [PubMed] [Google Scholar]
- 54.Huang F, Wei H, Luo H, et al. EPA inhibits the inhibitor of kBa (IkBa)/NF-kB/muscle RING finger 1 pathway in C2C12 myotubes in a PPARγ-dependent manner. Br J Nutr. 2011;105:348–356. doi: 10.1017/S0007114510003703. [DOI] [PubMed] [Google Scholar]
- 55.Voet NB, Kooi EL, Riphagen, et al. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2013;7:CD003907–CD003907. doi: 10.1002/14651858.CD003907.pub4. [DOI] [PubMed] [Google Scholar]