Abstract
Fibroblast growth factor receptor 1 (FGFR1) is a type 4 receptor tyrosine kinase. The receptor and its ligands play an important role in development and physiology. However, constitutive activation of FGFR1 by gene amplification, translocation or mutation is associated with various malignancies as, for example, breast cancer or myeloproliferative diseases. We have recently reported that FGFR1 amplification occurs in 20% of pulmonary squamous cell carcinomas, and preclinical tests have shown that these alterations are therapeutically tractable. These findings make FGFR1 amplification a potential biomarker for lung cancer treatment.
Squamous cell carcinomas of the lung are characterized by an uneven FGFR1 gene copy number distribution. Therefore, fluorescence in situ hybridization assays need to address focality and heterogeneity of FGFR1 in these tumors. Here, we review our proposal for a reading and evaluation strategy. Furthermore, we highlight the emerging landscape of clinical trials with selective and unselective FGFR inhibitors and provide first response data from early clinical trials.
Key Words: Lung cancer, squamous cell carcinoma, fibroblast growth factor receptor 1 (FGFR1), FISH
Introduction
Fibroblast growth factor receptor 1 (FGFR1) amplification represents now one of the most promising predictive biomarkers in lung cancer. This alteration seems to become the first therapeutically relevant genetic change in pulmonary squamous cell carcinomas, which occurs frequently in these tumors. In contrast to adenocarcinomas of the lung, squamous cell carcinomas do not significantly harbor EGFR mutations or ALK, ROS1 or RET translocations, which are therapeutically tractable. Therefore, FGFR1 amplifications in pulmonary squamous cell carcinomas are currently in the focus of many researchers and various ongoing clinical trials.
Squamous cell carcinoma is a common subgroup of lung cancer, which is strongly associated with smoking. The estimated annual incidence is approximately 123 newly diagnosed cases per 100,000 inhabitants in Europe (1,2). It is suspected that both incidence and prevalence will still increase, especially among female patients. The current therapeutic regimen for locally advanced or metastatic tumors consists of conventional platinum based chemotherapy and radiation (3). Very recently, data from our group indicated, however, that a focal amplification of chromosome band 8p12, representing the second most common genetic alteration, occurs in pulmonary squamous cell carcinomas which was proven to be related to FGFR1 amplification (4). Subsequently, we could confirm this finding in a large cohort of 420 clinical lung cancer samples by fluorescence in situ hybridization (5). Furthermore, data from in vitro studies provided first evidence that FGFR1 amplified squamous cell lines are in fact exploitable by FGFR inhibitors (4).
The FGFR family of receptor tyrosine kinases
FGFR1 is a member of the type 4 family of receptor tyrosine kinases, which consists of the closely related and highly conserved FGFRs 1 to 4. All these proteins are transmembrane receptors which are composed of an extracellular ligand binding domain, a transmembrane domain and an intracellular part which contains the functionally relevant tyrosine kinase domain. Three immunoglobulin-like loops (IgI-III) build the extracellular part, with IgI and II being separated by a so-called acid box of few amino acid residues. IgII and III form the ligand binding site. The binding specificity of the receptors is regulated by alternative splicing of the IgIII portion as exons 8 and 9 build alternatively the C-terminal part of this domain, thus forming the IIIb or IIIc variant of the receptor, respectively (6). Epithelial tissues express mostly the IIIb variant, whereas IIIc predominates in mesenchymal cells (7). This alternative splicing is, however, restricted to FGFR1-3 with FGFR4 being expressed always in the IIIc form.
FGFRs are activated by binding of their specific ligands - the fibroblast growth factors (FGFs) of which 18 different types are currently known. FGFs can function in an autocrine or paracrine manner and may even have hormonal long-distance effects. Furthermore, FGFs can also be liberated from the stroma, for instance during invasive tumor growth. FGFs bind with high specificity to FGFRs and form a complex with dimerized receptor molecules and a heparan sulphate proteoglycan chain. These activated complexes undergo conformation change and activation of the tyrosine kinase domains which finally trans-phosphorylate. After binding and phosphorylation of adapter proteins FGF signaling functions via different downstream effectors, e.g., the signal transducer and activator of transcription (STAT) pathway. Another signaling axis consists of phospholipase Cγ, protein kinase C and ends in the RAS - MAP kinase pathway. An important regulator of FGFR signaling is FGFR substrate 2 (FRS2) which binds to the juxtamembrane domain of activated FGF receptors and which recruits GRB2 and other downstream molecules finally leading again to an activation of the RAS - RAF - MAP kinase pathway as well as the PI3K - AKT pathway. Among others proteins FGFR-like 1 (FGFRL1 or FGFR5) functions as a negative regulator. FGFRL1 has the capability of binding (or “trapping”) FGFs without subsequent tyrosine kinase activity.
FGFR activity and FGF signaling play a major role in development, proliferation, differentiation and survival. Thus, FGFRs are crucial for embryogenesis, e.g., for limb development and organogenesis, and are highly important for many physiological processes including wound healing. In this context, FGFRs can act even as tumor suppressors.
The role of FGFR1 in oncogenesis
Gains of function of the FGF receptors were found to be associated with various malignancies. Constitutive activation of FGFR1 occurs basically by three major mechanisms: gene amplification, translocation or activating mutations (for overview and selected references see Table 1). FGFR1 mutations have been reported in melanomas but this appears to be a rather rare event. FGFR1 amplification, however, belongs to the most frequent genetic changes in breast cancer. Amplification of FGFR1 has additionally been reported in squamous cell carcinomas of the head and neck as well as from the esophagus. Translocations of FGFR1 have originally been described in a myeloproliferative hematological disorder which has now been referred to as “8p11 myeloproliferative syndrome characterized by FGFR1 translocation” by the current WHO classification system. Very recently FGFR1 translocations were additionally found in a subset of glioblastoma multiforme and in a rhabdomyosarcoma.
Table 1. FGFR1 alterations in malignancies.
| FGFR1 alterations | Tumor entities (estimated frequency) | Selected references |
|---|---|---|
| Amplification | Breast cancer (10%) | Reis-Filho et al. [2006] (8) |
| Ovarian cancer (5%) | Gorringe et al. [2007] (9) | |
| Bladder cancer (3%) | Simon et al. [2001] (10) | |
| Rhabdomyosarcoma (3%) | Missiaglia et al. [2009] (11) | |
| Squamous cell carcinoma of the lung (20%) | Schildhaus et al. [2012] (5) | |
| Small cell carcinoma of the lung (5%) | Peifer et al. [2012] (12) | |
| Head and neck squamous cell carcinomas (17%) | Freier et al. [2007] (13) | |
| Esophageal squamous cell carcinoma | Randla et al. [2012] (14) | |
| Translocation | 8p11 myeloproliferative syndrome [ZNF198-FGFR1] (100%, entity defining alteration as part of myeloid and lymphoid neoplasms with abormalities of FGFR1 and various translocation partners of FGFR1) | WHO classification [2008] (15) |
| Chronic myeloid leukemia (rare) | WHO classification [2008] (15) | |
| Rhabdomyosarcoma [FOXO1-FGFR1] (one case) | Liu et al. [2011] (16) | |
| Glioblastoma multiforme [TACC-FGFR1/3] (3%) | Singh et al. [2012] (17) | |
| Activating mutation | Melanoma (rare) | Lin et al. [2008] (18) |
Altered FGF receptor activity contributes to cancer development by regulating different key processes. Meanwhile, there is clear evidence that unscheduled FGFR activation leads to an increase in cell proliferation and prolonged survival but also cell migration and angiogenesis are stimulated.
FGFR1 amplification in lung cancer - epidemiology
Very recently we have reported on the frequency of FGFR1 amplifications in pulmonary carcinomas. In the so far largest series we found 20% FGFR1 amplified tumors among squamous cell carcinomas (5) which was recently confirmed in a second independent study (19). Therefore, FGFR1 amplification represents one of the most frequent driver lesions in lung cancer next to EGFR mutations, and far more often than ALK, ROS1 or RET rearrangements or other therapeutically targetable alterations. The high frequency as well as the large list of potential inhibitors which are currently in early or advanced clinical trials make FGFR1 amplification one of the most promising biomarkers for lung cancer treatment.
It is, however, noteworthy that - at least until now - no convincing case of an adenocarcinoma has been proven to be FGFR1 amplified. In our series of nearly one hundred pulmonary adenocarcinomas all cases were clearly negative for gene amplifications whereas polysomic cases were frequently noticed (5). This finding seems to be restricted to pure adenocarcinomas as we have seen FGFR1 amplification occasionally in adeno-squamous carcinomas. Furthermore, we have found additionally a pulmonary large cell carcinoma FGFR1 amplified (5). This might reflect the fact that emerging data from expression profiles provide evidence that some pulmonary large cell carcinomas represent a dedifferentiation endpoint of squamous carcinomas. Taken together, among non small cell carcinomas, FGFR1 amplification seems to be strongly associated with squamous morphology.
Very recently, we further reported that also small cell carcinomas of the lung can harbor FGFR1 amplifications (12). Preliminary and not yet published data from our screening program provide first evidence that this is a reproducible finding which can be confirmed in clinical routine cohorts. The frequency of FGFR1 amplifications among small cell carcinomas seems to be lower than in squamous cell carcinomas. Based on our current and still ongoing epidemiologic studies we estimate the frequency of FGFR1 amplifications among small cell carcinomas around 5%.
Detection of FGFR1 amplifications by fluorescence in situ hybridization
Therapeutic effects of FGFR1 tyrosine kinase inhibitors seem to be dependent on significantly increased FGFR1 gene copy numbers. It still needs to be clarified whether FGFR1 amplification only serves as a surrogate marker for receptor protein overexpression since the receptor itself should represent obviously the therapeutic target. There are currently no validated antibody assays on the market, which could reliably detect FGFR1 expression levels quantitatively or semiquantitatively by using paraffin embedded tumor samples. Thus, comprehensive studies on the correlation between FGFR1 gene copy numbers and protein expression levels are still missing. Therefore, current clinical trials with FGFR1 inhibitors enroll patients who are found to be “FGFR1 amplified”. Reliable FGFR1 FISH probes are now commercially available. Therefore, fluorescence in situ hybridization assays on formalin fixed and paraffin embedded material are carried out to screen patients for clinical trials. This is an important fact as lung cancer samples per se are often hard to diagnose. Biopsy samples, which are obtained endoscopically or by transthoracic CT-guided biopsy are often very small and contain only little tumor tissue. Tumor cells are frequently damaged by manipulations and show often crushing artifacts. Surgical samples contain often large tumor areas of necrosis or dense fibrosis which regularly influence hybridization quality. Therefore, lung cancer tissue is basically a challenge for FISH. Despite this fact we were able to establish a robust and reliable FISH assay, and we have noticed a drop out rate below 5% in our laboratory by using our protocol (5).
FGFR1 amplification is not yet convincingly defined. Some authors have simply applied the criteria which are commonly used for detection of her2 amplifications in breast cancer. From our experience, however, these criteria are not useful to evaluate FGFR1 FISH assays for squamous cell carcinomas of the lung. FGFR1 in these tumors is characterized by some unique features which make FGFR1 FISH assays challenging. One major issue is heterogeneity and focality of gene amplifications (Figure 1). Thus, adequate screening for amplification hot spots is a prerequisite for reliable FGFR1 evaluation. Based on our reading and evaluation strategy (5), we recommend careful scanning of the entire tumor area by using a 40× or 63× oil objective. FGFR1 and centromer 8 (CEN8) signals should be counted for individual tumor cells (63× or 100× oil objective). We suggest counting of 20 tumor cell from three areas, resulting in a total of 60 nuclei. Counting areas should be selected from prior screening as the hot spot areas containing the highest number of FGFR1 copies. If the signals are found to be evenly distributed random areas should be used. Another important phenomenon is focality of FGFR1 gene copy distribution. Very often isolated tumor cells with a very high number of FGFR1 gene copies occur which are surrounded by tumor cells with normal or only slightly increased gene copy numbers. Therefore, it turned out to be mandatory to count contiguous and cohesive tumor cells from each area. It should be avoided to pick only suspicious tumor cell nuclei with increased FGFR1 and/or CEN8 copy numbers because this approach might lead to an overestimation of gene copy numbers.
Figure 1.
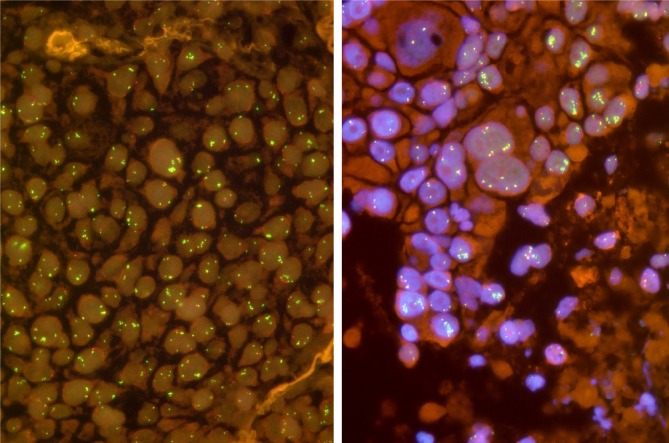
Fluorescence in situ hybridization for detection of FGFR1 amplification in pulmonary squamous cell carcinomas. A. In this tumor, FGFR1 (green) and chromosome 8 (CEN8, orange) copies are more or less evenly distributed. Only occasionally, microclusters are seen (arrow); B. Heterogeneity occurs frequently in these tumors. Beside tumor cells with normal or only slight increase in copy numbers, there are many tumor cell nuclei with tight clusters of amplified FGFR1, some of which are indicated by arrows. Note that the occurrence of FGFR1 clusters is not necessarily related to the size of the nuclei.
Furthermore, the gene copy distribution in pulmonary squamous cell carcinomas is different from e.g., her2 in breast cancer. A significant proportion of tumors show colocalized clusters with numerically balanced increase in both FGFR1 and CEN8 copy numbers. Another more or less specific feature represents so-called microclusters which consist of a tight accumulation of more than three FGFR1 signals. We have proposed to regard these microclusters as 5 signal copies.
Having counted 60 tumor cells a final decision has to be made whether a given sample is “amplified” or not. As already mentioned there are until now no convincing criteria to judge FGFR1 FISH assays in squamous cell carcinomas. From our point of view this FISH assay should finally serve as a predictive biomarker which should be capable to predict response to anti-FGFR treatment. However, criteria for thresholds and cut-off values still have to be determined retrospectively after finishing the currently ongoing clinical trials individually for each compound. Therefore, we have developed evaluation criteria (“Cologne Score”) which are suitable to detect patients with the highest gene copy numbers and to enroll them in clinical trials. In this context, it seems to be important to us not only to use FGFR1/CEN8 ratio as criterion for FISH positivity since we have noticed tumors with an enormous increase in FGFR1 gene copy numbers in a background of co-localized clusters, i.e. an additional increase of centromeric DNA material. These cases would finally result in a ratio of nearly 1.0 and would be considered negative if the decision would rest solely on FGFR1/CEN8 ratio. Therefore, it appears useful also to consider average gene copy number as a criterion for positivity. Furthermore, we have seen isolated tumor cells with high level cluster amplification which were surrounded by lesional cells with normal or only slightly increased gene copy numbers. Thus, we proposed to include additionally the percentage of tumor cell with gene clusters of at least 15 gene copies in the catalogue of FISH criteria.
Considering all these items we have proposed to diagnose FGFR1 amplification (“high level amplification”) if one of the following criteria is fulfilled: (I) FGFR1/CEN8 ratio is ≥2.0, (II) average gene copy per nucleus is ≥6.0, and (III) percentage of tumor cells containing ≥15 gene copies or large cluster is ≥10%.
Beside unamplified tumors with nearly normal gene count and amplified cases as defined above we became aware of a third category of squamous cell carcinomas which is characterized by a moderate increase of FGFR1 gene copies. For these more or less polysomic cases we have defined a “low level amplification” category which we defined by a percentage of ≥50% of tumor cells containing ≥5 FGFR1 gene copies. This criterion was derived and adapted from previous studies on squamous cell carcinomas of the head and neck as well as from reports on breast cancer (8,13). Since polysomy is a common phenomenon in cancer, one might expect that many squamous cell lung cancers might fall into this category. It is, however, important to emphasize the fact that this low level amplification contributes for only one fifth of the amplified pulmonary squamous carcinomas. Low level amplification is found in only 4% of these tumors.
Therapeutic implications resulting from oncogenic FGFR dependence
The identification of FGFR alteration in various types of human cancer led to rapid development of compounds targeting FGFR. As described above, the FGFR family comprises 4 members (FGFR1, 2, 3 and 4). The small molecules act either as selective pan inhibitors of the FGFR family or as non-selective inhibitors, which usually target not only FGFR but also other intracellular proteins. Table 2 summarizes FGFR inhibitors in current clinical development.
Table 2. Selective and non-selective FGFR inhibitors in current clinical development.
| FGFR inhibition | Drugs | Receptor targets | Provider |
|---|---|---|---|
| Selective | |||
| AZD4547 | FGFR 1, 2, 3 | Astra Zeneca | |
| BGJ398 | FGFR 1, 2, 3 | Novartis | |
| JNJ-42756493 | pan FGFR | Janssen Research & Development | |
| Non-selective | |||
| ARQ087 | pan FGFR | ArQuele | |
| Brivanib | FGFR 1 | Bristol-Meyers-Squibb | |
| VEGFR 2 | |||
| Danusertib | FGFR 1 | Nerviano Medical Sciences | |
| Aurora kinase A, B, C | |||
| Abl, Ret, TrkA | |||
| Dovitinib | FGFR 1, 2, 3 | Novartis | |
| VEGFR 1, 2, 3 | |||
| PDGFR β | |||
| E-3810 | FGFR 1 | Ethical Oncology Science (EOS) | |
| VEGFR 1, 2, 3 | |||
| FP-1039 | FGF ligand trap | Five Prime Therapeutics | |
| LY2874455 | FGFR 1, 2, 3, 4 | Eli Lilly | |
| VEGFR 2 | |||
| MK-2461 | FGFR 1, 2, 3 | Chemfun Medical technology | |
| c-Met, Ron | |||
| Flt-1, 3, Mer | |||
| PDGFR β | |||
| Nintedanib | FGFR 1, 2, 3 | BoehringerIngelheim | |
| VEGFR 1, 2, 3 | |||
| PDGFR α/β | |||
| Orantinib | FGFR 1 | TaihoPharmaceutical Co., Ltd. | |
| VEGFR 2 | |||
| PDGFR β | |||
| Ponatinib | FGFR 1, 2, 3, 4 | Ariad Pharmaceuticals | |
| Abl, Src | |||
| FLT-3, c-KIT | |||
| XL228 | FGFR 1 | Exelixis | |
| IGF1R | |||
| Aurora A, B | |||
| FAK, Src | |||
| BCR-Abl | |||
| XL999 | FGFR 1, 3 | Exelixis | |
| VEGFR 2 | |||
| PDGFR α/β | |||
| FLT-3, Src |
Currently ongoing phase Ia/Ib and phase II studies recruit patients either with diagnosed FGFR alterations only or include unselected patient populations [for overview of FGFR trials see ref (20).].
The phase I/II studies with AZD4547, BGJ398, E-3810 and dovitinib include only patients with genetic FGFR alterations. The phase II with ponatinib is recruiting patients with squamous cell carcinoma with retrospective outcome analysis of FGFR altered patients (20).
Nintedanib and XL999 were investigated in advanced non small cell lung cancer (NSCLC) without further selection (1,21). All other trials recruited patients with different solid tumors without any further molecular analysis.
Trials recruiting patients with FGFR alterations
BGJ398 is a selective FGFR inhibitor which blocks FGFR1, FGFR2 and FGFR3. The drug is supposed to be effective in tumors with activated FGFR axis due to activating mutations or gene amplification. The BGJ398 phase Ia (first in man) trial recruited patients with solid tumors in an escalating dose schedule starting from 5 mg daily. After cohort 3, only patients with FGFR1 or FGFR2 amplification or FGFR3 mutation are included. Preliminary analysis was conducted after 26 recruited patients including 10 patients with FGFR1 amplified breast cancer and 3 patients with FGFR1 amplified squamous cell carcinomas of the lung. One patient with FGFR1 amplified squamous cell lung cancer with an FGFR1/CEP8 ratio of 2.6 by FISH analysis treated with 100 mg BGJ398 showed partial response in CT scan at 8 weeks, confirmed at 12 weeks with an substantial SUV decrease on PET scan at week 4 (22). The trial is currently treating patients with FGFR alterations on maximal tolerated dose in the expansion part of the phase I.
The phase I study with the selective FGFR inhibitor AZD4547 (FGFR 1, 2, 3 inhibitor) is currently recruiting patients with FGFR amplified tumors at a maximal tolerated dose.
Dovitinib - as an unselective FGFR inhibitor - blocks also VEGFR 1, 2, 3 and PDGFR β besides FGFR 1, 2, 3. First phase I/II studies were conducted in patients with metastatic melanoma without any pre-selection according to genetic alterations. A moderate clinical benefit of stable disease was reached in 12 from 47 enrolled patients (23). The phase I/II study in patients with advanced or metastatic renal cell cancer showed 2 partial responses from 20 recruited patients (24). A phase II study treating 77 metastatic breast cancer patients showed 13% partial responses in the group of patients who were FGFR1 amplified and hormone positive (25). Phase II studies in patients with metastatic gastric cancer and FGFR2 amplification and in patients with advanced endometrium cancer and FGFR2 mutation (stratified to non-mutated patients) are currently ongoing (20).
The expansion part of the phase I study with E-3810, a combined inhibitor of FGFR 1 and VEGFR 1, 2, 3, is recruiting patients with FGFR1 amplification and patients relapsing after response or long stable disease after anti-angiogenic treatment (26).
Ponatinib is a multikinase inhibitor of FGFR-1, 2, 3, 4, Abl, Src, FLT-3 and c-KIT showing high clinical activity in heavily pretreated patients with chronic myeloid leukemia resistant to other tyrosine kinase inhibitors (27). The phase II study enrolling squamous cell lung cancer patients with retrospective analysis of FGFR alterations is currently ongoing (20).
Trials recruiting NSCLC patients without molecular testing
Nintedanib, a FGFR 1, 2, 3, VEGFR 1, 2, 3 and PDGFR α/β inhibitor showed clinical activity in phase I in advanced solid tumors with one complete and two partial responses occurred in patients with renal (n=2) and colorectal cancer (n=1) among 61 recruited patients (28). The phase II study in unselected patient population with NSCLC showed one partial response and 35 stable diseases in 73 treated patients (21).
XL999 is a multikinase inhibitor of FGFR 1, 3, VEGFR 2, PDGFR α/ß, FLT-3 and Src. The preliminary results of a phase II study in nine NSCLC patients showed one partial response (29).
Trials recruiting other cancer entities without molecular testing
Brivanib (FGFR 1 and VEGFR 2 inhibitor) is one of the few FGFR compounds in late clinical development. The phase I was performed in an unselected patient population. In a dose finding part, the best response was stable disease in 1 patient with NSCLC from 5 patients with different tumor entities (30). Another large phase I study recruited 68 patients with different tumor entities. Two patients achieved partial response, one with renal cell carcinoma and one with a carcinoma of Vaters’s ampulla (31). The phase II study on brivanib in 55 patients with hepatocellular carcinoma (HCC) showed one complete and 3 partial responses. The median progression free survival (PFS) and overall survival (OS) were 2.7 and 10 months, respectively (32).
In the phase II discontinuation trial, patients with various tumors and stable disease after initial treatment with brivanib was stratified according to FGF-2 expression and randomized to receive either brivanib or placebo. Fifty-three patients with FGF-2 expression and soft tissue sarcoma showed a PFS of 2.8 months in a brivanib group comparing to 1.4 months in the placebo group (33). Regarding the phase III trials, the study in patients with HCC after failure on sorafenib did not meet its primary endpoint in improving of OS (34).
Phase I study with JNJ-42756493, a selective pan FGFR inhibitor is currently ongoing in an unselected patient population with solid tumors and lymphomas after standard treatment (20). Similarly, phase I study with ARQ087 recruiting unselected patients with solid tumors is currently ongoing (20).
The phase I study with danusertib, which inhibits besides FGFR 1 also Aurora kinase A, B, C, Abl, Ret and TrkA was perfomed in unselected patient population. Although the compound showed some clinical benefit in small cell lung, colorectal, breast and ovarian cancer, the adverse effect were characterized due to pronounced hematological toxicity with febrile neutropenia (35). The phase II study on danusertib in metastatic castration resistant prostate cancer showed moderate clinical activity with median PFS of 3 months (36).
FP-1039 is a ligand trap, which binds to FGF ligands and prevents them from binding to FGFRs. A phase I study recruiting patients with all solid tumors showed moderate clinical activity with tumor shrinkage of 20% in a patient with a prostate cancer (37). Phase II study enrolling patients with FGFR2 mutated endometrial cancer is currently ongoing (20).
LY2874455 is an inhibitor of all FGF receptors with low VEGFR 2 activity (38). The phase I study recruiting patients with all types of advanced cancer (20).
MK-2461 is a common inhibitor of FGFR 1, 2, 3, c-Met, Ron, Flt-1, 3 and PDGFR β. The phase I in solid tumors showed mild clinical activity (39).
Orantinib, a multikinase inhibitor of FGFR 1, VEGFR 2 and PDGFR ß was investigated mainly in hepatocellular carcinoma (HCC). A phase I/II study in 35 patients with HCC showed one complete and two partial responses (40).
XL228 is a multikinase inhibitor of FGFR1, IGF1R, Aurora A, B, FAK, Src and bcr-abl. The phase I study in advanced solid tumors and lymphoma showed one partial response in a patient with adenocarcinoma of the lung (41).
Conclusions
A great variety of FGFR inhibitors is currently in clinical development. First results show that these drugs basically have therapeutic effects on solid and hematologic tumors. Data from trials enrolling lung cancer patients indicate that genetic prescreening increases antitumoral efficacy since we could provide first evidence for therapeutic response of a pulmonary FGFR1 amplified squamous cell carcinoma patient to treatment with a selective FGFR inhibitor. Therefore, FISH is currently the method of choice to detect squamous cell carcinomas of the lung with FGFR1 amplification.
Acknowledgements
Disclosure: HUS has received honoraria and reimbursements from Pfizer, Abbott Molecular, Zytomed Systems and Roche Pharma, and grants, honoraria and reimbursements from Novartis Oncology. LN has received honoraria from Roche and Novartis, and travel support from Pfizer, Bayer, Novartis, Roche and Lilly. JW has served as an advisory board member of Novartis and Astra Zeneca and has received research support from Novartis and honoraria from Novartis and Astra Zeneca. RB declares no conflict of interest.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Moreno P, Brambilla E, Thomas R, et al. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res 2012;18:2443-51. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [DOI] [PubMed] [Google Scholar]
- 4.Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010;2:62ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schildhaus HU, Heukamp LC, Merkelbach-Bruse S, et al. Definition of a fluorescence in-situ hybridization score identifies high- and low-level FGFR1 amplification types in squamous cell lung cancer. Mod Pathol 2012;25:1473-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner N, Grose R.Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 2010;10:116-29. [DOI] [PubMed] [Google Scholar]
- 7.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 2005;16:139-49. [DOI] [PubMed] [Google Scholar]
- 8.Reis-Filho JS, Simpson PT, Turner NC, et al. FGFR1 emerges as a potential therapeutic target for lobular breast carcinomas. Clin Cancer Res 2006;12:6652-62. [DOI] [PubMed] [Google Scholar]
- 9.Gorringe KL, Jacobs S, Thompson ER, et al. High-resolution single nucleotide polymorphism array analysis of epithelial ovarian cancer reveals numerous microdeletions and amplifications. Clin Cancer Res 2007;13:4731-9. [DOI] [PubMed] [Google Scholar]
- 10.Simon R, Richter J, Wagner U, et al. High-throughput tissue microarray analysis of 3p25 (RAF1) and 8p12 (FGFR1) copy number alterations in urinary bladder cancer. Cancer Res 2001;61:4514-9. [PubMed] [Google Scholar]
- 11.Missiaglia E, Selfe J, Hamdi M, et al. Genomic imbalances in rhabdomyosarcoma cell lines affect expression of genes frequently altered in primary tumors: an approach to identify candidate genes involved in tumor development. Genes Chromosomes Cancer 2009;48:455-67. [DOI] [PubMed] [Google Scholar]
- 12.Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freier K, Schwaenen C, Sticht C, et al. Recurrent FGFR1 amplification and high FGFR1 protein expression in oral squamous cell carcinoma (OSCC). Oral Oncol 2007;43:60-6. [DOI] [PubMed] [Google Scholar]
- 14.Bandla S, Pennathur A, Luketich JD, et al. Comparative genomics of esophageal adenocarcinoma and squamous cell carcinoma. Ann Thorac Surg 2012;93:1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swerdlow SH, Campo E, Harris NL, et al. eds. WHO classification of tumours of haematopietic and lymphoid tissues. Lyon: IARC, 2008. [Google Scholar]
- 16.Liu J, Guzman MA, Pezanowski D, et al. FOXO1-FGFR1 fusion and amplification in a solid variant of alveolar rhabdomyosarcoma. Mod Pathol 2011;24:1327-35. [DOI] [PubMed] [Google Scholar]
- 17.Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012;337:1231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin WM, Baker AC, Beroukhim R, et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res 2008;68:664-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heist RS, Mino-Kenudson M, Sequist LV, et al. FGFR1 amplification in squamous cell carcinoma of the lung. J Thorac Oncol 2012;7:1775-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Available online: www.clinicaltrials.gov
- 21.Reck M, Kaiser R, Eschbach C, et al. A phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancer. Ann Oncol 2011;22:1374-81. [DOI] [PubMed] [Google Scholar]
- 22.Wolf J, Lorusso PM, Camidge RD, et al. A phase I dose escalation study of NVP-BGJ398, a selective pan FGFR inhibitor in genetically preselected advanced solid tumors. Cancer Res 2012;72:abstr LB122.
- 23.Kim KB, Chesney J, Robinson D, et al. Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma. Clin Cancer Res 2011;17:7451-61. [DOI] [PubMed] [Google Scholar]
- 24.Angevin E, Lin C, Pande AU, et al. A phase I/II study of dovitinib (TKI258), a FGFR and VEGFR inhibitor, in patients (pts) with advanced or metastatic renal cell cancer: Phase I results. J Clin Oncol 2010;28:abstr 3057.
- 25.Andre F, Bachelot TD, Campone M, et al. A multicenter, open-label phase II trial of dovitinib, an FGFR1 inhibitor, in FGFR1 amplified and non-amplified metastatic breast cancer J Clin Oncol 2011;29:abstr 289.
- 26.Soria J, De Braud FG, Cereda R, et al. First-in-man study of E-3810, a novel VEGFR and FGFR inhibitor, in patients with advanced solid tumors. J Clin Oncol 2011;29:abstr TPS149.
- 27.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med 2012;367:2075-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mross K, Stefanic M, Gmehling D, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res 2010;16:311-9. [DOI] [PubMed] [Google Scholar]
- 29.March RJ, Mirtsching B, Modi S, et al. A phase II study of XL999 in patients (pts) with NSCLC. J Clin Oncol 2007;25:abstr 18112.
- 30.Mekhail T, Masson E, Fischer BS, et al. Metabolism, excretion, and pharmacokinetics of oral brivanib in patients with advanced or metastatic solid tumors. Drug Metab Dispos 2010;38:1962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonker DJ, Rosen LS, Sawyer MB, et al. A phase I study to determine the safety, pharmacokinetics and pharmacodynamics of a dual VEGFR and FGFR inhibitor, brivanib, in patients with advanced or metastatic solid tumors. Ann Oncol 2011;22:1413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JW, Finn RS, Kim JS, et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2011;17:1973-83. [DOI] [PubMed] [Google Scholar]
- 33.Chou T, Finn RS. Brivanib: a review of development. Future Oncol 2012;8:1083-90. [DOI] [PubMed] [Google Scholar]
- 34.Llovet JM, Decaens T, Raoul JL, et al. Brivanib versus placebo in patients with advanced hepatocellular carcinoma (HCC) who failed or were intolerant to sorafenib. Results from the phase 3 BRISK-PS study. J Hepatol 2012; 56:S549. [DOI] [PubMed] [Google Scholar]
- 35.Cohen RB, Jones SF, Aggarwal C, et al. A phase I dose-escalation study of danusertib (PHA-739358) administered as a 24-hour infusion with and without granulocyte colony-stimulating factor in a 14-day cycle in patients with advanced solid tumors. Clin Cancer Res 2009;15:6694-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meulenbeld HJ, Bleuse JP, Vinci EM, et al. Randomized phase II study of danusertib in patients with metastatic castration-resistant prostate cancer after docetaxel failure. BJU Int 2013;111:44-52. [DOI] [PubMed] [Google Scholar]
- 37.Tolcher A, Papadopoulos K, Patniak A, et al. Preliminary results of a dose escalation study of the fibroblast growth factor (FGF)“trap” FP-1039 (FGFR1: Fc) in patients with advanced malignancies. Eur J Cancer 2010;8:abstr 381.
- 38.Zhao G, Li WY, Chen D, et al. A novel, selective inhibitor of fibroblast growth factor receptors that shows a potent broad spectrum of antitumor activity in several tumor xenograft models. Mol Cancer Ther 2011;10:2200-10. [DOI] [PubMed] [Google Scholar]
- 39.Camacho LH, Moulder SL, LoRusso PM, et al. First in human phase I study of MK-2461, a small molecule inhibitor of c-Met, for patients with advanced solid tumors. J Clin Oncol 2008;26;abstr 14657.
- 40.Kanai F, Yoshida H, Tateishi R, et al. A phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol 2011;67:315-24. [DOI] [PubMed] [Google Scholar]
- 41.Smith DC, Britten C, Clary DO, et al. A phase I study of XL228, a potent IGF1R/AURORA/SRC inhibitor, in patients with solid tumors or hematologic malignancies. J Clin Oncol 2009;27;abstr 3512.


