Abstract
An ultrasensitive stable isotope dilution liquid chromatography-tandem mass spectrometry method (LC-MS/MS) was developed and validated for multiplexed quantitative analysis of six unconjugated and conjugated estrogens in human serum. The quantification utilized a new derivatization procedure, which formed analytes as pre-ionized N-methyl pyridinium-3-sulfonyl (NMPS) derivatives. This method required only 0.1 mL of human serum, yet was capable of simultaneously quantifying six estrogens within 20 min. The lower limit of quantitation (LLOQ) for estradiol (E2), 16α-hydroxy (OH)-E2, 4-methoxy (MeO)-E2 and 2-MeO-E2 was 1 fg on column, and was 10 fg on column for 4-OH-E2 and 2-OH-E2. All analytes demonstrated a linear response from 0.5 to 200 pg/mL (5–2000 pg/mL for 4-OH-E2 and 2-OH-E2). Using this validated method, the estrogen levels in human serum samples from 20 female patients and 20 male patients were analyzed and compared. The levels found for unconjugated serum E2 from postmenopausal women (mean 2.7 pg/mL) were very similar to those obtained by highly sensitive gas chromatography-mass spectrometry (GC-MS) methodology. However, the level obtained in serum from older men (mean 9.5 pg/mL) was lower than has been reported previously by both GC-MS and LC-MS procedures. The total (unconjugated + conjugated) 4-MeO-E2 levels were significantly higher in female samples compared with males (p<0.05). The enhanced sensitivity offered by the present method will allow for a more specific analysis of estrogens and their metabolites. Our observations might suggest that the level of total 4-MeO-E2 could be a potential biomarker for breast cancer cases.
Keywords: Estrogen, 17β-estradiol, breast cancer, endometrial cancer, prostate cancer, stable isotope dilution
1. Introduction
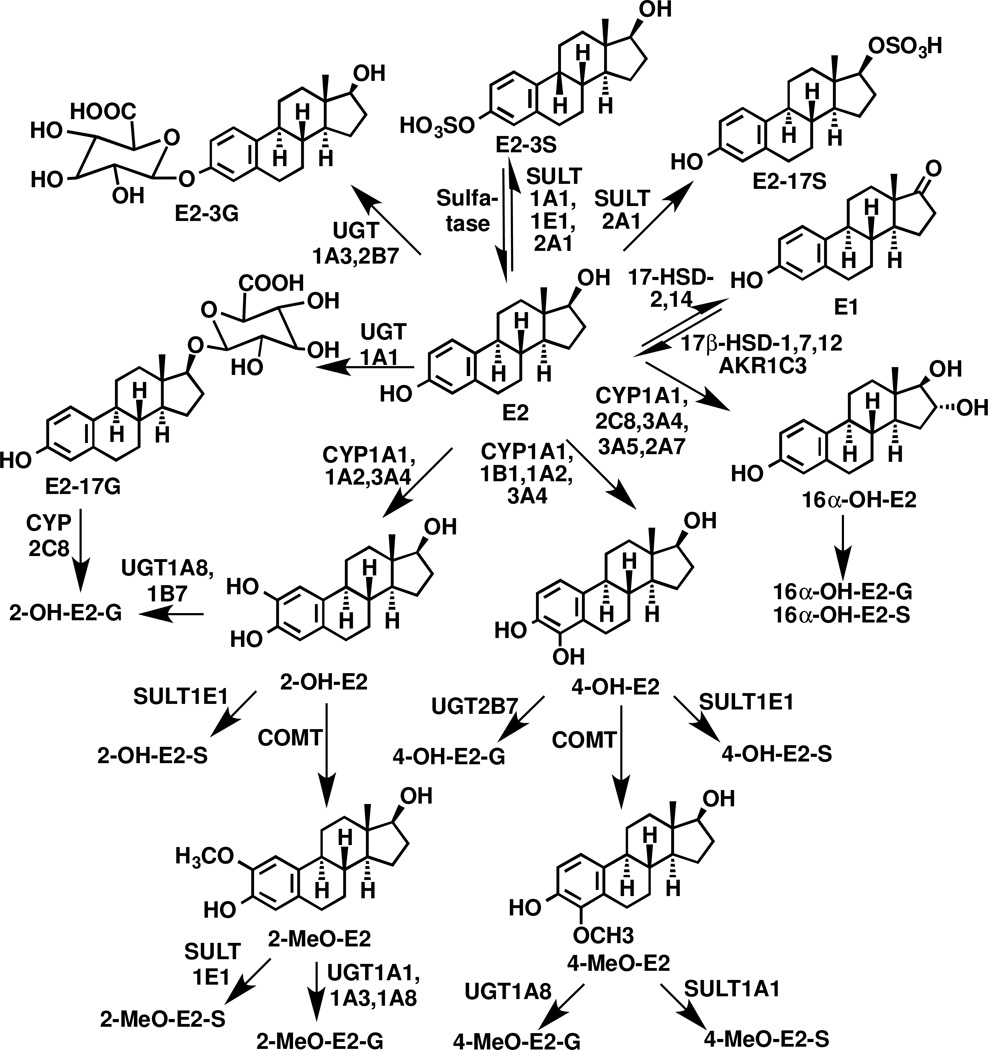
Over the last seven years, gas chromatography (GC)-and liquid chromatography (LC)-mass spectrometry (MS)-based methodology has become increasingly reliable for the quantification of extremely low concentrations of unconjugated estrogens present in the serum and plasma of postmenopausal women (Table 1) [1–3]. Estrone (E1), 17β-estradiol (E2) and 16α-hydroxy (OH)-E2 are the major unconjugated estrogens that are present in serum (Fig. 1). They are largely bound to protein in the circulation, and their free concentrations are estimated from the steroid hormone binding globulin levels [4–6]. Reliable assays for unconjugated circulating estrogens are important because even though their concentrations are extremely low, increased levels are thought to be an important risk factor for breast [7–10] and endometrial cancer [11–13]. Increased circulating estrogens are also a potential risk factor for prostate cancer in men [14–16], although no systematic studies have been conducted to date to test this possibility. Unfortunately, serum concentrations for many of the unconjugated estrogens in postmenopausal women are close to the reported lower limits of quantification (LLOQs), raising concerns that the values could represent an over-estimation of the true values (Table 1). This means that the corresponding sulfate and β-glucuronide conjugates (Fig. 1), which are present in higher concentrations (Table 2), might have some predictive value in determining cancer risk [17–19]. Furthermore, the sulfate conjugates (Fig. 1) could be a potential source of their corresponding unconjugated forms through action of tissue sulfatases [19].
Table 1.
Concentrations of unconjugated estrogens in postmenopausal serum determined by GC-MS and LC-MS.
| Method | LC-MS | GC-MS | GC-MS | GC-MS | GC-MS | GC-MS | LC-MS | GC-MS | LC-MS | LC-MS | GC-MS | LC-MS | LC-MS | Overall Mean pg/mL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Derivative | Dansyl | PFBO | PFBO | PFBO | PFBO | PFBO | Dansyl | PFBO | Dansyl | Dansyl | PFBO | Dansyl | NMPS | |
| Reference | 35 | 23 | 24 | 25 | 26 | 27 | 36 | 12 | 37 | 38 | 28 | 39 | Present study |
|
| pg/mL | Mean | Mean | Mean | MeanL | Mean | Mean | Mean | Median | Mean | Median | Mean | Median | Mean | |
| E2 | 15.0 | 3.5 | 7.3 | 3.0 | 6.6 | 4.2 | 15.9 | 3.4 | 3.1 | 4.2 | 3.1 | 2.7 | 2.7 | 5.1 |
| 16α-OH-E2 | 7.9 | x | x | x | x | x | x | x | x | 13.5 | x | 7.7 | BLQ | 10.6 |
| 4-MeO-E2 | BLQ | x | x | x | x | x | x | x | x | BLQ | x | BLQ | BLQ | BLQ |
| 2-MeO-E2 | BLQ | x | x | x | x | x | x | x | x | 1.4 | x | 0.7 | BLQ | 1.0 |
| 4-OH-E2 | x | x | x | x | x | x | x | x | x | x | x | x | BLQ | BLQ |
| 2-OH-E2 | BLQ | x | x | x | x | x | x | x | x | BLQ | x | BLQ | BLQ | BLQ |
X = not determined
BLQ = below LLOQ
PFBO = pentafluorobenzoyl
Figure 1.
Enzymatic pathways involved in estradiol metabolism.
Table 2.
Concentrations of conjugated estrogens in postmenopausal serum determined by LC-MS.
| Technique | G/S | Intact | Intact | G/S | G/S | G/S | G/S | Overall Mean pg/mL |
|---|---|---|---|---|---|---|---|---|
| Derivative | Dansyl | None | None | Dansyl | Dansyl | Dansyl | NMPS | |
| Reference | 35 | 26 | 12 | 38 | 39 | 40 | Present study |
|
| pg/mL | Mean | Mean | Median | Median | Median | Median | Mean | |
| E2-T | 51.5 | x | x | 9.8 | 6.2 | 10.5 | 20.7 | 19.7 |
| E2-3G | x | 8.4 | 2.5 | x | x | x | x | 5.5 |
| 16α-OH-E2-T | 27.9 | x | x | 126.0 | 78.4 | 70.5 | 32.5 | 67.1 |
| 4-MeO-E2-T | BLQ | x | x | 0.8 | 0.6 | 0.8 | 8.2 | 2.6 |
| 2-MeO-E2-T | BLQ | x | x | 2.3 | 4.7 | 4.0 | 2.5 | 3.4 |
| 2-MeO-E2-3G | x | 6.9 | 2.5 | x | x | x | x | 4.7 |
| 4-OH-E2-T | x | x | x | x | x | x | BLQ | BLQ |
| 2-OH-E2-T | 11.1 | x | x | 10.4 | 6.6 | 3.5 | BLQ | 7.9 |
X = not determined
BLQ = below LLOQ
T = conjugated + unconjugated
G = glucuronide
G/S = β-glucuronidase/arylsulfatase hydrolysis as described in section 2.5.
The very low concentrations of circulating estrogens and their metabolites mean that analysis by MS-based methodology is very challenging. Importantly, LC-MS can overcome potential problems of cross-reactivity that usually occur with more sensitive but less specific immunoassay-based methodology [20–22]. Furthermore, MS-based methods make it possible to quantify multiple estrogens in a single analytical run, which allows for more comprehensive analyses to be conducted. Conventional positive ion GC-MS and LC-MS generally have inadequate sensitivity for routine analyses of endogenous estrogens in the serum of postmenopausal women and older men. Therefore, numerous derivatization methods have been developed to improve the sensitivity of both [1]. High sensitivity GC-MS procedures usually employ electron capture negative chemical ionization using pentafluorobenzoyl (PFBO) derivatives [12,23–28] (Table 1). The corresponding LC-MS method known as electron capture atmospheric pressure chemical ionization, which uses the pentafluorobenzyl (PFB) derivative [29], is widely used for the quantification of lipids [30] but has found only limited utility for the analysis of estrogens and their metabolites [1].
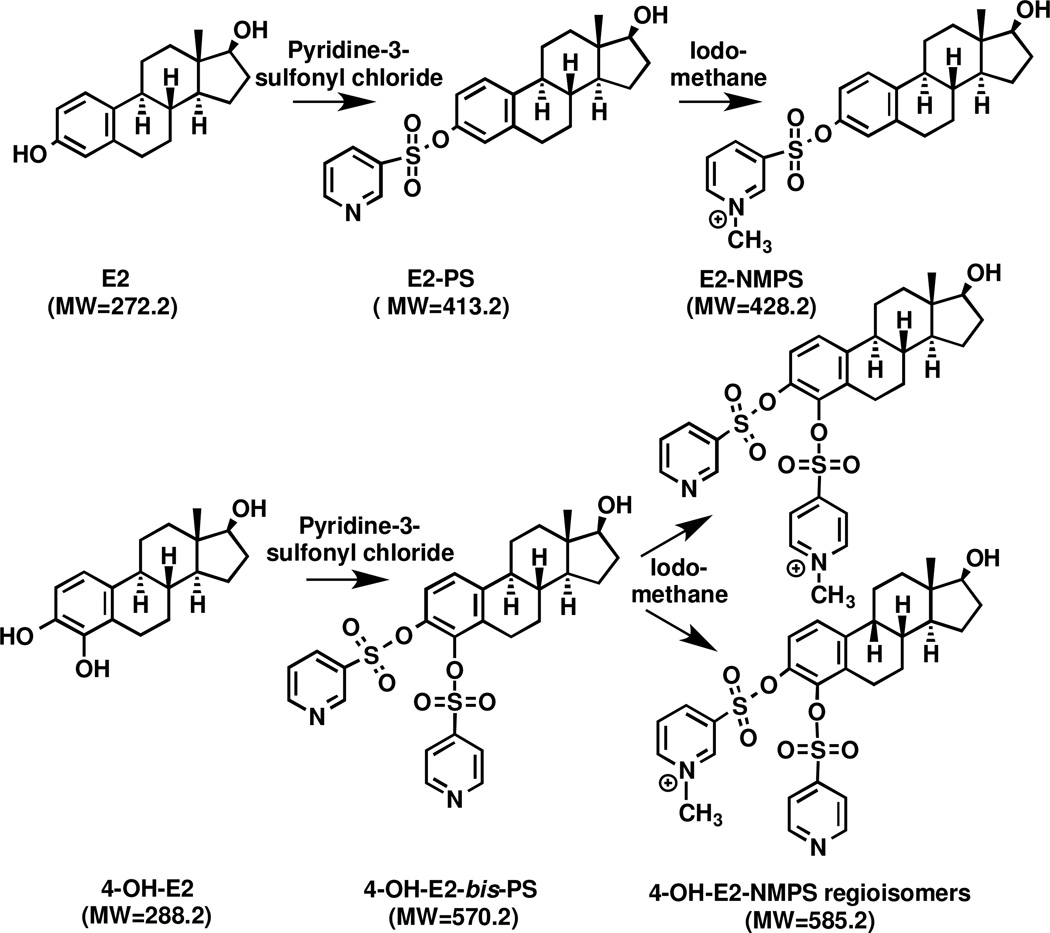
A second LC-MS approach, which has been used much more widely, involves the use of estrogen derivatives that enhance the electrospray ionization (ESI) signal and therefore improves overall sensitivity during LC-ESI/MS analysis. This approach is exemplified by derivatization of the estrogen phenolic moiety to a dansyl ester [31–34]. The dansyl derivative has been used in a number of studies to quantify unconjugated estrogens [35–39] (Table 1) and conjugated estrogens after hydrolysis with β-glucuronidase/arylsulfatase [35,38–40] (Table 2) in serum samples from postmenopausal women. Alternative approaches to improve ESI signal have included the use of picolinoyl [41] and pyridine-3-sulfonyl [42] derivatives. A third LC-MS approach involves the preparation of pre-ionized (quaternized) derivatives, so that protonation of the estrogen derivative is not required. Therefore, suppression of ionization in the ESI source of the mass spectrometer is minimized. This approach has been reported in studies that utilized the N-methyl-2-pyridyl [43], N-methyl-nicotinyl, and 1-(2,4-dinitro-5-fluorphenyl)-4,4,-dimethylpiperaziny [44] derivatives attached to the estrogen 3-phenolic moiety. Our group has also used pre-ionized derivatives to improve sensitivity by adding a Girard P (GP) derivative to the 17-oxo moiety of estrone and its metabolites [45] as well as by adding a Girard T (GT) derivative to the 17-oxo-moiety of androgens [46]. However, this approach cannot be employed for the analysis of E2 and it metabolites that lack a 17-oxo-moiety. We now report development of the pre-ionized N-methyl pyridinium-3-sufonate (NMPS) derivative (Fig. 2), which provides extremely high sensitivity for LC-ESI/MS/MS analysis of E2 and its metabolites. We demonstrate the utility of this new derivatization for the quantification of estrogens in the serum of postmenopausal women and older men.
Figure 2.
Two step reaction of pre-ionized N-methyl pyridine-3-sulfonyl (NMPS) derivatives and their structures.
2. Experimental
2.1. Reagents and Materials
The six estrogens analyzed in this study, E2, 16α-OH-E2, 4-methoxy (MeO)-E2, 2-MeO-E2, 4-hydroxy (OH)-E2 and 2-OH-E2 were purchased from Steraloids Inc. (Newport, RI). [13,14,15,16,17,18-13C6]-E2 ([13C6]-E2), [2,3,4-13C3]-16α-OH-E2 ([13C3]-16α-OH-E2), [13,14,15,16,17,18-13C6]-4-MeO-E2 ([13C6]-4-MeO-E2), [13,14,15,16,17,18-13C6]-2-MeO-E2 ([13C6]-2-MeO-E2), and [13,14,15,16,17,18-13C6]-2-OH-E2 ([13C6]-2-OH-E2) with an isotopic purity of 99% were purchased from Cambridge Isotope Laboratories (Cambridge, MA). [1,4,16,16,17-2H5]-4-OH-E2 ([2H5]-4-OH-E2) with an isotopic purity of 98% was obtained from C/D/N Isotopes, Inc. (Pointe-Claire, Quebec, Canada). β-glucuronidase/arylsulfatase (Helix pomatia) was obtained from Roche (Indianapolis, IN). E2-3-(β-D-glucuronide)-17-sulfate (E2-3G-17S) was obtained from Sigma-Aldrich (St. Louis, MO). Pyridine-3-sulfonyl chloride (97%) was obtained from Matrix Scientific (Columbia, SC). Dry acetonitrile was purchased from Acros Organic (New Jersey, USA). Methyl-tert-butyl-ether (MTBE), iodomethane, methanol, acetone, L-ascorbic acid, formic acid, hydrochloric acid (HCl), sodium chloride, sodium acetate and sodium bicarbonate were obtained from Sigma-Aldrich (Milwaukee, WI). Double charcoal-stripped human serum was obtained from Golden West Biologicals, Inc (Temecula, CA). All solvents used in this study were HPLC Optima grade unless otherwise noted and were purchased from Fisher Scientific (Pittsburgh, PA).
2.2. Clinical study
Twenty postmenopausal women ages 56 to 65 (mean ± SD, 60.0 ± 2.66) and twenty men ages 51 to 69 (mean ± SD, 61.6 ± 5.41) were recruited for the study. Menstrual and menopausal status of the women was based on self-report. All participants were healthy and the women were not taking exogenous hormones. The blood collection protocol was approved by the University of Pennsylvania Review Board (Protocol # 800924). After the blood was collected, it was allowed to clot for 1 h at room temperature, serum was separated and aliquots were stored at −80 °C . Serum samples were allowed to thaw at room temperature and aliquots of 0.1 mL were used for the estrogen analyses.
2.3. Preparation of stock solution and working standard solutions
Estrogen standard and internal standard stock solutions were individually prepared in methanol containing 0.1% (w/v) L-ascorbic acid at a concentration of 1 mg/mL then stored at −20 °C . A mixed stock solution of six estrogens or the corresponding internal standards at 1 µg/mL was prepared by adding 10 µL of each estrogen standard stock solution to a 10 mL volumetric flask with methanol containing 0.1% L-ascorbic acid. Working standard solutions of mixed estrogens and working standard solutions of internal standards (1 ng/mL) were prepared by dilutions of the stock solutions with methanol containing 0.1% (w/v) L-ascorbic acid. Stock solution and working solution of catechols were prepared at concentrations 10 times higher than the other estrogens.
2.4. Preparation of calibration standards and quality controls
Charcoal-stripped human serum was used for preparation of calibration standards and quality controls (QCs). Calibration standards were prepared by spiking appropriate amounts of the working standard solution to charcoal-stripped human serum to make the concentrations of 0.5, 1, 2, 5, 10, 20, 50, 100, 200 pg/mL (10 times higher concentration for 4-OH-E2 and 2-OH-E2). The preparation procedures for QC samples at concentrations of 1.5, 75, and 175 pg/mL were the same as that of the calibration standards.10 µL of working standard solution of internal standards) was added to each calibration standard and QC sample.
2.5. Sample preparation procedure
The sample preparation procedure was designed to determine unconjugated and total estrogens in serum, including hydrolysis, extraction, derivatization and re-suspension. All serum samples were thawed at room temperature and centrifuged at 5300g at 4 °C for 10 min (Z216MK High Capacity Refrigerated Microcentrifuge, Hermel Labortechnik). For the determination of total estrogens, 10 µL of internal standards working solution was spiked into a 0.1 mL aliquot of serum using a calibrated syringe, followed by the addition of 0.1 mL water, 0.1 mL 0.5% L-ascorbic acid, 0.2 mL sodium acetate buffer (200 mM, pH 5.0), and 10 µL of β-glucuronidase/arylsulfatase. Samples were incubated at 37 °C for 19 h. After hydrolysis, samples were acidified with 5 µL of 1N HCl followed by addition of 50 µL saturated sodium chloride. Samples underwent liquid-liquid extraction (LLE) with 1.3 mL of MTBE by vortex-mixing for 10 min, followed by centrifugation at 3400g at 4 °C for 15 min (D37520 Osterode Benchtop Centrifuge, Thermo Electron). The top organic layer was transferred to a clean glass tube and evaporated to dryness under nitrogen.
To each dried sample, 100 µL acetone, 100 µL sodium bicarbonate (100 mM, pH 9), and 10 µL of pyridine-3-sulfonyl chloride (10 mg/mL in acetone) were added and vortex mixed for 5 sec. Samples were then incubated in a water bath (Isotemp 210, Fisher Scientific) at 60 °C for 30 min. The derivatives were extracted with 1 mL of MTBE and the organic layer was evaporated to dryness under nitrogen. The N-methyl derivatization reaction was carried out by adding 100 µL iodomethane (20% in acetonitrile) and followed by incubation at 80 °C for 30 min. The sample was evaporated to dryness under nitrogen. The dry residue was then dissolved in 50 µL 20% aqueous methanol and 1 µL was injected for LC-MS/MS analysis. For the analysis of unconjugated estrogens, identical sample preparation procedures were followed with the exclusion of the hydrolysis step. For analysis of PS derivatives the methylation step was not conducted.
2.6. LC-MS/MS
A Vantage TSQ mass spectrometer (Thermo Scientific, San Jose, CA) equipped with a CaptiveSpray™ ion source (Michrom Bioresources, Inc., Auburn, CA) was used for all analysis. The mass spectrometer was interfaced with a Waters nanoAcquity UPLC system (Waters Corporation, Milford, MA) equipped with an autosampler and sample thermo-controller (set at 4°C). Both the UPLC and mass spectrometer were controlled by Xcalibur software (Thermo Scientific). The syringe was washed with water/acetonitrile (95:5, v/v) containing 0.1% formic acid after every injection. Separations were performed using a Waters BEH130 C18 column (150 µm x 100 mm, 1.7µm, 130 Å) (Waters Corporation, Milford, MA) at 50°C using a partial loop injection. Samples were eluted with a linear gradient at a flow rate of 2 µL/min. For NMPS derivatives, solvent A was water/acetonitrile (99.5:0.5, v/v) containing 0.1% formic acid, and solvent B was acetonitrile/water (98:2, v/v) containing 0.1% formic acid. The gradient started with 30% B, held for 3 min, then linearly increased to 80% B over 17 min. After washing with 90% B for 5 min, the column was re-equilibrated with 30% B for 10 min prior to the next injection. For separation of the PS derivatives, solvent A was water containing 0.1% formic acid, and solvent B was methanol/acetonitrile (40:60, v/v). The gradient started with 50% B, held for 3 min, then linearly increased to 60% B over 30 min. After washing with 80% B for 10 min, the column was re-equilibrated with 50% B for 10 min.
The MS operating conditions were as follows: spray voltage, 1800 V; ion transfer capillary temperature, 300 °C ; collision gas, argon at 1.5 mTorr; ion polarity, positive; scan type, selected reaction monitoring (SRM); chrom filter peak width, 15 s; S-lens,135 v; cycle time, 1 sec; Q1 peak width (FWHM), 0.7 u; Q3 peak width, 0.7 u; DCV, 5 V. The SRM transitions for all the analytes and internal standards are showed in Table 3.
Table 3.
LC-SRM/MS conditions for estrogen NMPS derivatives.
| Analyte | Parent (m/z) |
Product (m/z) |
Collision energy(V) |
Start time (min) |
Stop time (min) |
S-lens (V) |
|---|---|---|---|---|---|---|
| E2 | 428.2 | 364.2 | 35 | 9.5 | 11.5 | 135 |
| [13C6]-E2 | 434.2 | 370.2 | 35 | 9.5 | 11.5 | 135 |
| 16α-OH-E2 | 444.2 | 380.2 | 35 | 7.5 | 9.5 | 135 |
| [13C3]-16α-OH-E2 | 447.2 | 383.2 | 35 | 7.5 | 9.5 | 135 |
| MeO-E2 | 458.2 | 158.2 | 35 | 9.5 | 12.5 | 135 |
| [13C6]-MeO-E2 | 464.2 | 158.2 | 35 | 9.5 | 12.5 | 135 |
| OH-E2 | 585.2 | 379.2 | 35 | 9.0 | 13.0 | 135 |
| [2H5]-4-OH-E2 | 590.2 | 384.2 | 35 | 9.0 | 13.0 | 135 |
| [13C6]-2-OH-E2 | 591.2 | 385.2 | 35 | 9.0 | 13.0 | 135 |
2.7. Method validation
2.7.1. Linearity and lower limit of quantification (LLOQ)
To evaluate linearity of standard curves, calibration standards were prepared at concentrations of 0.5, 1, 2, 5, 10, 20, 50, 100 and 200 pg/mL of each estrogen in charcoal stripped human serum, except for 4-OH-E2 and 2-OH-E2, which were prepared at 10 times higher concentration. 10 µL of internal standard working solution was added to each sample. Calibration curves were generated by plotting the area ratios of the analyte to internal standard peak using linear regression with 1/x weighting. The lower limit of quantification (LLOQ) was defined as the lowest calibration level, which could be fitted to the calibration curve with a residual of less than 10% and peak area ratio deviating less than 25%.
2.7.2. Accuracy and precision
Quality control (QC) samples were prepared at the low quality control (LQC; 1.5 pg/mL for E2, 16α-OH-E2, 4-MeO-E2 and 2-MeO-E2, 15 pg/mL for 4-OH-E2 and 2-OH-E2), medium quality control (MQC; 75 pg/mL for E2, 16α-OH-E2, 4-MeO-E2 and 2-MeO-E2, 750 pg/mL for 4-OH-E2 and 2-OH-E2) and high quality control (HQC; 175 pg/mL for E2, 16α-OH-E2, 4-MeO-E2 and 2-MeO-E2, and 1750 pg/mL for 4-OH-E2 and 2-OH-E2) samples. The accuracy and precision were determined on 5 replicate serum samples run on the same day (intra-day) and on 3 different days (inter-day).
2.7.3. Stability
Stability of the NMPS derivatives was assessed by allowing validation samples to sit in the autosampler and re-analyzing after 24 h. Stability of estrogens in the serum was also assessed following three freeze-thaw cycles for the LQC, MQC and HQC.
2.7.4. Recoveries of unconjugated estrogens
The recovery of each unconjugated estrogen was determined at the LQC, MQC and HQC (n=5). One set of QC samples was spiked with internal standard working solution followed by extraction and derivatization procedures described previously. A second batch of QC samples underwent the same preparation protocol, except the internal standard working solution was added after the extraction procedure. Both sets of samples were derivatized and analyzed by consecutive LC-MS analyses. The recoveries of estrogens were calculated by comparing the ratios of analyte/internal standard of both batches.
2.7.5. Recoveries of conjugated E2
The efficiency of enzymatic hydrolysis was determined using βE2-3G-17S standard as the substrate followed by quantification of the liberated E2 by LC-MS/MS. E2-3G-17S (0.037 pmol/mL, equal to 10 pg/mL of unconjugated E2; and 0.37 pg/mL, equal to 100 pg/mL of unconjugated E2) as well as the internal standards were spiked to 0.1 mL of water, then incubated with 0.1 mL of 0.5% (w/v) L-ascorbic acid, 0.2 mL of sodium acetate buffer (200 mM, pH 5.0), and 10 µL of β-glucuronidase/arylsulfatase at 37 °C for 19 h. After hydrolysis, samples were extracted, derivatized and analyzed by LC-MS/MS. The unconjugated estrogen was quantified from the relevant standard.
2.8. Data Analysis
Serum concentrations of E2 and its metabolites were calculated using Xcalibur software (version 2.6) from Thermo Fisher Scientific. Statistical analyses were performed using GraphPad Prism (v 5.01, GraphPad Software Inc., La Jolla, CA).
3. Results
3.1. Reaction standardization of estrogens and their metabolites with the NMPS derivatives
A scheme showing the NMPS derivatization reaction is shown in Fig. 2. Each of the derivatization steps was essentially complete within 30 min. E2, 16α-OH-E2, 4-MeO-E2 and 2-MeO-E2 contain only one phenolic group and reacted with one molar equivalent of the pyridine-3-sulfonyl chloride, generating pyridine-3-sulfonyl (PS) derivatives that were then converted to NMPS derivatives with iodomethane. The catechol estrogens, 4-OH-E2 and 2-OH-E2, which each contains two phenolic hydroxyl groups, reacted with two molar equivalents of the pyridine-3-sulfonyl chloride generating bis-PS derivatives. Interestingly, each catechol PS derivative only formed mono-NMPS derivatives as a mixture of two regioisomers (Fig. 2).
3.2. LC-MS/MS analysis of estrogen NMPS derivatives
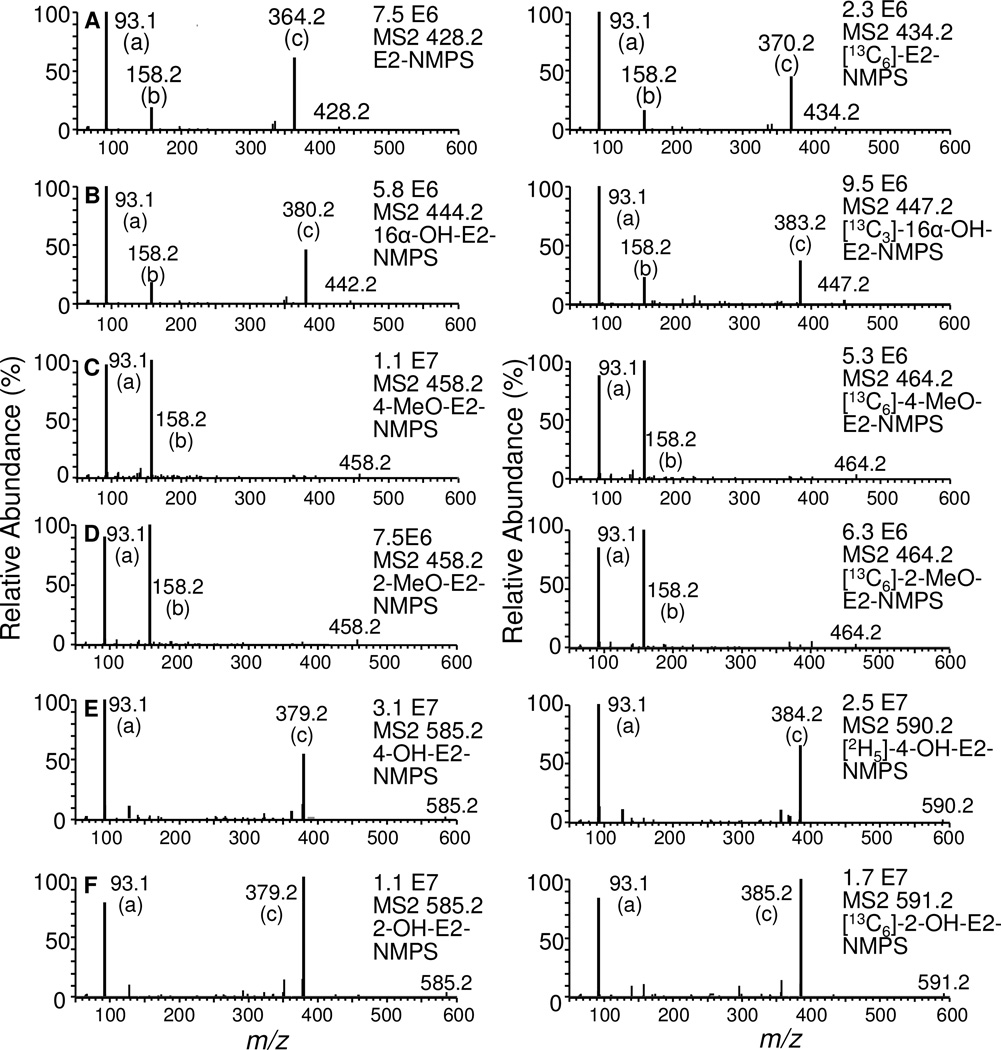
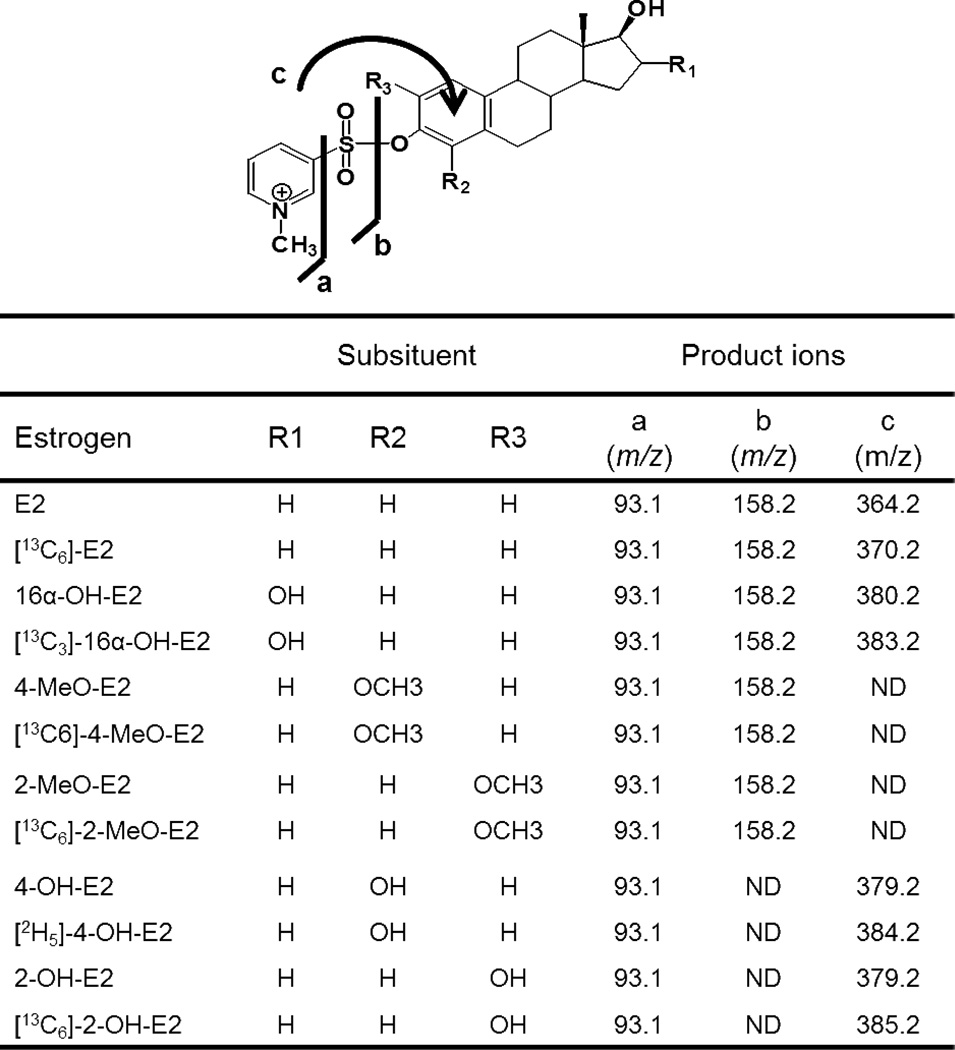
Each of the estrogen derivatives exhibited intense ions corresponding to [M+] ion of the relevant NMPS derivative. Collision induced dissociation (CID) and MS/MS analysis revealed the formation of three major product ions (Fig. 3) from each of the estrogen derivatives. Unique product ions were observed for E2, 16α-OH-E2, isomeric 4-OH-E2 and 2-OH-E2 derivatives at m/z 364.2, 380.2 and 379.2, respectively (Fig. 4). This corresponded to the unusual attachment of the pyridine moiety to the parent molecule after losing SO2 from the derivative (fragment c, Fig. 4). However, this predominant ion was not observed in 4-MeO-E2 and 2-MeO-E2, where the major product ions arose from the loss of the E2 moiety from both of the derivatives (m/z 158.2; fragment b, Fig. 4). The product ion corresponding the pyridinium moiety (m/z 93.1; fragment a, Fig. 4) was observed for all of the estrogen NMPS-derivatives. As expected, the corresponding internal standards gave similar product ions to NMPS derivatives of the endogenous metabolites (Fig. 3).
Figure 3.
Full scan MS/MS analysis of product ions of E2-NMPS, 16α-OH-E2-NMPS, 4-MeO-E2-NMPS, 2-MeO-E2-NMPS, 4-MeO-E2-NMPS and 2-OH-E2-NMPS derivatives. The most intense product ions were selected for the SRM analysis.
Figure 4.
Assignment of product ions from LC-MS/MS analysis of estrogen NMPS derivatives.
3.3. Method validation
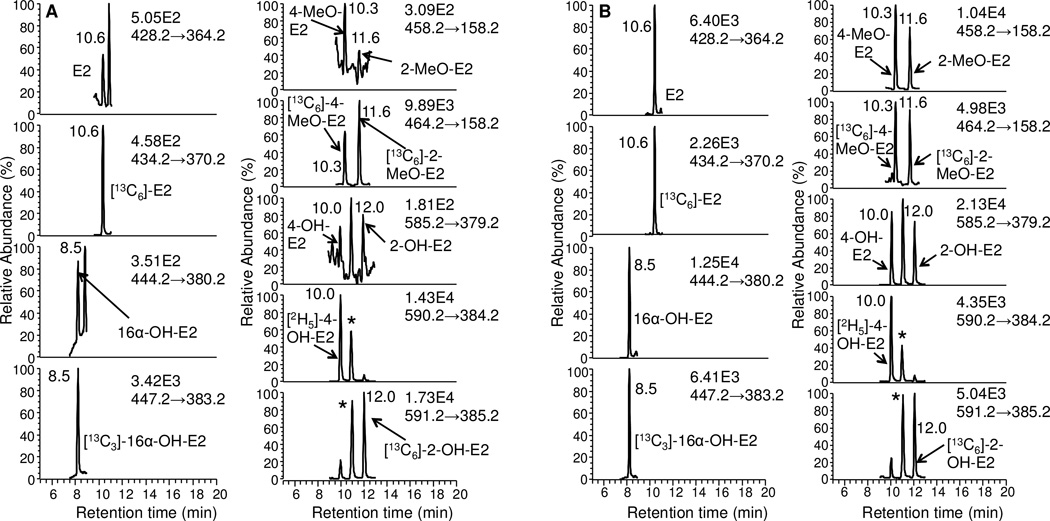
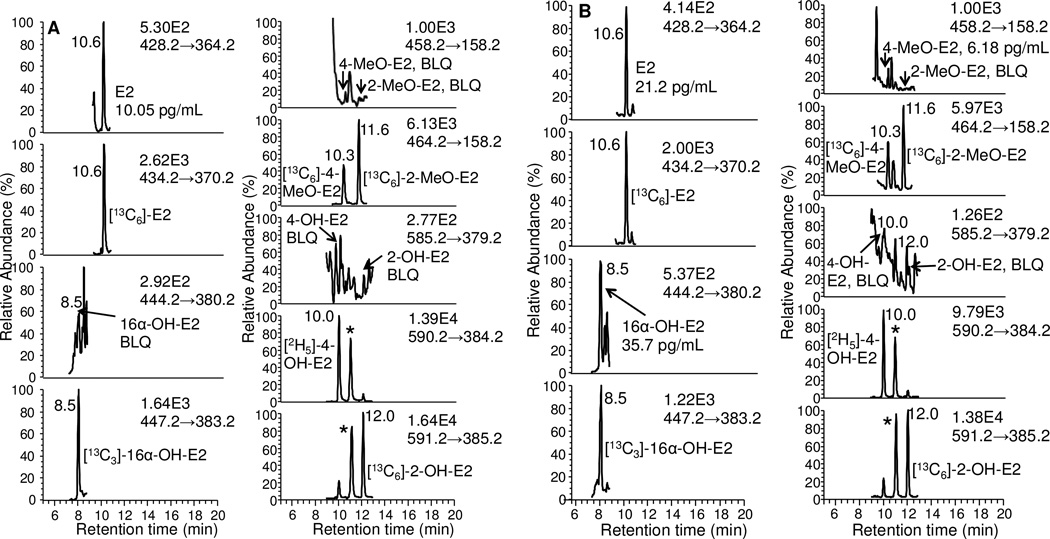
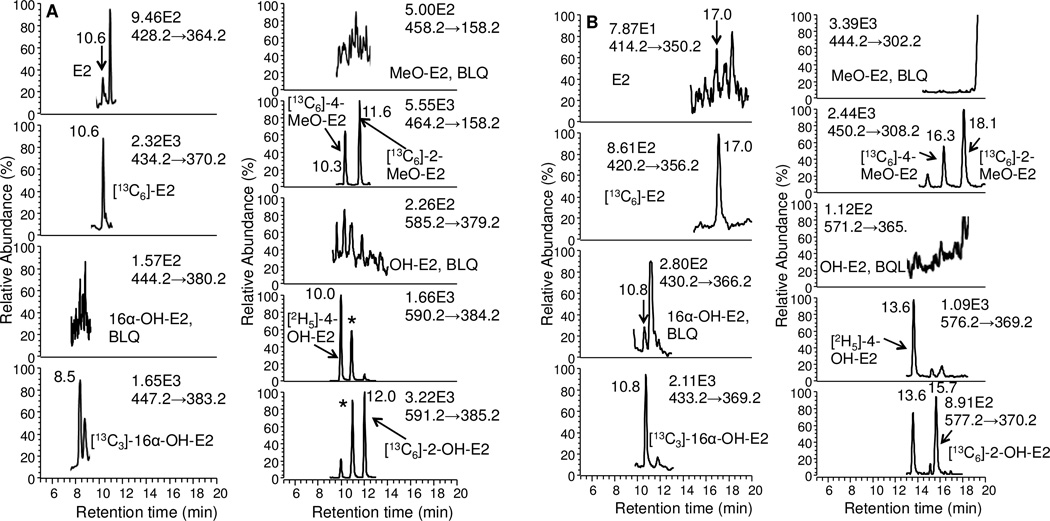
Typical LC/SRM-MS chromatographic profiles of the six estrogen-NMPS derivatives together with their corresponding six internal standards are shown for the LLOQ standard of 0.5 pg/mL for E2, 16 α -OH-E2, 4-MeO-E2 and 2-MeOE2, and 5 pg/mL for 4 and 2-OH-E2 (Fig. 5A). A chromatogram is shown for the HQC standard of 175 pg/mL for E2, 16α-OH-E2, 4-MeO-E2 and 2-MeO-E2, 1750 pg/mL for 4-OH-E2 and 2-OH-E2 (Fig. 5B). The six estrogen derivatives were baseline separated within a 20 min chromatographic run time. The two co-eluting regioisomers from 4-OH-E2 and 2-OH-E2 are shown with an asterisk (Figs. 5 and 6). Comparison of the NMPS and PS derivatives revealed an increase in sensitivity and signal-to-noise for analysis of unconjugated estrogens in the same serum sample (Fig. 7).
Figure 5.
LC-SRM chromatograms for analysis of estrogens and their metabolites extracted from double charcoal-stripped human serum as NMPS derivatives. (A) LLOQ samples (0.5 pg/mL for E2, 16α-OH-E2, 4-MeO-E2 and 2-MeO-E2; 5 pg/mL for 4-OH-E2 and 2-OH-E2). (B) HQC samples (175 pg/mL for E2, 16α-OH-E2, 4-MeO-E2 and 2-MeO-E2; 5 pg/mL for 4-OH-E2 and 2-OH-E2). Asterisks show co-eluting second regioisomers from 4-OH-E2 and 2-OH-E2.
Figure 6.
LC-SRM/MS chromatograms obtained from analysis of estrogens and their metabolites as NMPS derivatives in serum from postmenopausal women. (A) unconjugated estrogens (B) total estrogens. Asterisks show co-eluting second regioisomers from 4-OH-E2 and 2-OH-E2.
Figure 7.
Comparison of derivatives for analysis of unconjugated serum estrogens from the same patient sample. (A) NMPS derivatives. (B) PS derivatives. Asterisks show co-eluting second regioisomers from 4-OH-E2 and 2-OH-E2.
3.3.1. Calibration curve and limit of quantification
Calibration curves for each estrogen were constructed from the ratios of the peak area of the NMPS derivatives to corresponding internal standard with 1/x weighting. Satisfactory linearity was observed over 400-fold concentration with linear regression correlation coefficients all better than 0.99. (Table 4) The lower LLOQ was defined as the lowest concentration on the calibration curve that could be reliably and reproducibly measured with accuracy and precision of less than 20% and a signal-to-noise ratio great than 10.
Table 4.
Typical calibration curves for estrogens as NMPS derivatives.
| Analyte | Equations | Correlation coefficients (R2) |
LLOQ (pg/mL) |
|---|---|---|---|
| E2 | Y=0.015X+0.066 | 0.9997 | 0.5 |
| 16α-OH-E2 | Y=0.011X+0.063 | 0.9992 | 0.5 |
| 4-MeO-E2 | Y=0.012X+0.016 | 0.9998 | 0.5 |
| 2-MeO-E2 | Y=0.010X+0.0031 | 0.9995 | 0.5 |
| 4-OH-E2 | Y=0.025X+0.039 | 0.9987 | 5 |
| 2-OH-E2 | Y=0.0016X+0.0097 | 0.9996 | 5 |
3.3.2. Assay accuracy, precision, recovery and stability of free estrogens
Overall, excellent accuracy and precision were obtained for the analysis of all three QC serum samples (Table 5). For the LQC, MQC and HQC, the intra-day accuracies were 102–118%, 95–108 %, and 98–105%; the intra-day precisions were 4.5–13.9%, 0.9–3.4%, 0.6–4.5%; the inter-day accuracies were 91–124%, 102–111%, and 95–106%, and the inter-day precisions were 4.4–19.9%, 1.1–4.4%, 4.2–5.8%. The recovery of six estrogens after extraction, derivatization, and purification from LQC, MQC, and HQC samples (n=5) was 84–98% for E2, 92–106% for 16α-OH-E2, 99–109% for 4-MeO-E2, 98–112% for 2-MeO-E2, 95–109% for 4-OH-E2, and 90–100% for 2-OH-E2 (Table 6). The precision and accuracy data for analysis of the LQC, MQC, and HQC samples (n=5) after three freeze thaw cycles were similar to that shown in Table 5 for the inter-day validation (data not shown). LQC, MQC, and HQC samples that were re-analyzed after 24 h standing in the autosampler (4 °C) gave essentially identical data to that obtained from the original analyses.
Table 5.
Accuracy and precision for determination of estrogens as NMPS derivatives in serum (n=5).
| Analyte | LQC (1.5 pg/mL) | MQC (75 pg/mL) | HQC (175 pg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | CV | Accuracy | Mean | SD | CV | Accuracy | Mean | SD | CV | Accuracy | |
| Intra-day | ||||||||||||
| E2 | 1.5 | 0.2 | 10% | 102% | 80.7 | 1.7 | 2% | 108% | 183.8 | 3.0 | 2% | 105% |
| 16α-OH-E2 | 1.8 | 0.1 | 5% | 118% | 79.2 | 1.1 | 1% | 106% | 180.2 | 2.7 | 2% | 103% |
| 4-MeO-E2 | 1.6 | 0.2 | 14% | 105% | 77.9 | 1.6 | 2% | 104% | 176.5 | 8.0 | 5% | 101% |
| 2-MeO-E2 | 1.7 | 0.2 | 9% | 112% | 76.0 | 0.7 | 1% | 101% | 179.3 | 2.5 | 1% | 102% |
| 4-OH-E2 | 1.5 | 0.1 | 6% | 102% | 80.2 | 1.3 | 2% | 107% | 180.6 | 1.0 | 1% | 103% |
| 2-OH-E2 | 1.6 | 0.1 | 6% | 107% | 71.5 | 2.4 | 3% | 95% | 171.4 | 5.1 | 3% | 98% |
| Inter-day | ||||||||||||
| E2 | 1.4 | 0.1 | 4% | 91% | 78.5 | 0.9 | 1% | 105% | 173.2 | 9.0 | 5% | 99% |
| 16α-OH-E2 | 1.5 | 0.2 | 11% | 102% | 79.0 | 1.8 | 2% | 105% | 174.1 | 8.1 | 5% | 99% |
| 4-MeO-E2 | 1.6 | 0.2 | 11% | 105% | 83.2 | 3.7 | 4% | 111% | 185.7 | 10.8 | 6% | 106% |
| 2-MeO-E2 | 1.9 | 0.1 | 5% | 124% | 79.0 | 2.2 | 3% | 105% | 173.7 | 7.6 | 4% | 99% |
| 4-OH-E2 | 1.6 | 0.1 | 8% | 104% | 77.5 | 2.4 | 3% | 103% | 172.4 | 7.3 | 4% | 99% |
| 2-OH-E2 | 1.4 | 0.3 | 20% | 95% | 76.3 | 1.5 | 2% | 102% | 166.0 | 8.1 | 5% | 95% |
SD: standard deviation
CV: coefficient of variation
RE: relative error
RE (%) = (found concentration – actual concentration/actual concentration)×100
Accuracy (%) = RE (%) + 100
Table 6.
Recovery of unconjugated estrogens after extraction, derivatization and analysis (n=5).
| Analytes | LQC (1.5 pg/mL) | MQC (75 pg/mL) | HQC (175 pg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post- spike |
Pre- spike |
Recov. (%) |
SD | Post- spiked |
Pre- spiked |
Recov. (%) |
SD | Post- spiked |
Pre- spiked |
Recov. (%) |
SD | |
| E2 | 1.62 | 1.37 | 84% | 4.0 | 80.16 | 78.46 | 98% | 1.1 | 184.23 | 173.24 | 94% | 4.9 |
| 16α-OH-E2 | 1.45 | 1.53 | 106% | 13.9 | 86.31 | 78.98 | 92% | 2.1 | 173.03 | 174.07 | 101% | 4.7 |
| 4-MeO-E2 | 1.44 | 1.57 | 109% | 11.4 | 78.73 | 81.77 | 104% | 3.0 | 186.73 | 185.67 | 99% | 5.8 |
| 2-MeO-E2 | 1.66 | 1.85 | 112% | 5.4 | 78.87 | 78.96 | 100% | 2.8 | 177.55 | 1 73.65 | 98% | 4.3 |
| 4-OH-E2 | 1.43 | 1.56 | 109% | 8.6 | 77.98 | 77.54 | 99% | 3.1 | 182.29 | 172.42 | 95% | 4.0 |
| 2-OH-E2 | 1.42 | 1.43 | 100% | 18.9 | 78.71 | 76.30 | 97% | 1.9 | 184.42 | 166.04 | 90% | 4.4 |
Post-spike: Internal standard spiked into MTBE extracted human serum, followed by derivatization.
Pre- spike: Internal standard spiked into human serum, followed by MTBE extraction and derivatization.
Recov.(%)= [(pre-spike-post-spike)/post-spike]×100
3.3.3. Recovery of estrogens after hydrolysis and extraction procedure
Quantification of total (unconjugated + conjugated) estrogens was performed by way of enzymatic hydrolysis of E2-3G-17S in water, following by extraction, derivatization and analysis by LC-SRM/MS. The hydrolysis efficiency was > 90% and was complete after 19 h. Complete hydrolysis under this condition was confirmed by analyzing triplicate control samples with 0.037 pmol/mL of E2-3G-17S (equivalent of 10 pg/mL of free E2) and 0.37 pmol/mL of E2-3G-17S (equivalent of 100 pg/mL of free E2) (Table 7).
Table 7.
Recovery of E2 from E2-3G-17S after hydrolysis, extraction, derivatization, and analysis (n=5).
| E2-3G-17S (pmol/mL) | β- glucuronidase/ arylsulfatase |
Theoretical E2 from hydrolysis (pg/mL) |
Calculated E2 from hydrolysis (pg/mL) |
Yield (%) |
|---|---|---|---|---|
| 0.037 (10 pg/mL E2 equivalent) |
Without | 0.0 | 0.8 | 8.0 |
| With | 10.0 | 10.5 | 105.0 | |
| 0.370 (100 pg/mL E2 equivalent) |
Without | 0.0 | 0.6 | 6.0 |
| With | 100.0 | 96.6 | 96.6 |
3.4. Analysis of estrogens in patient serum samples
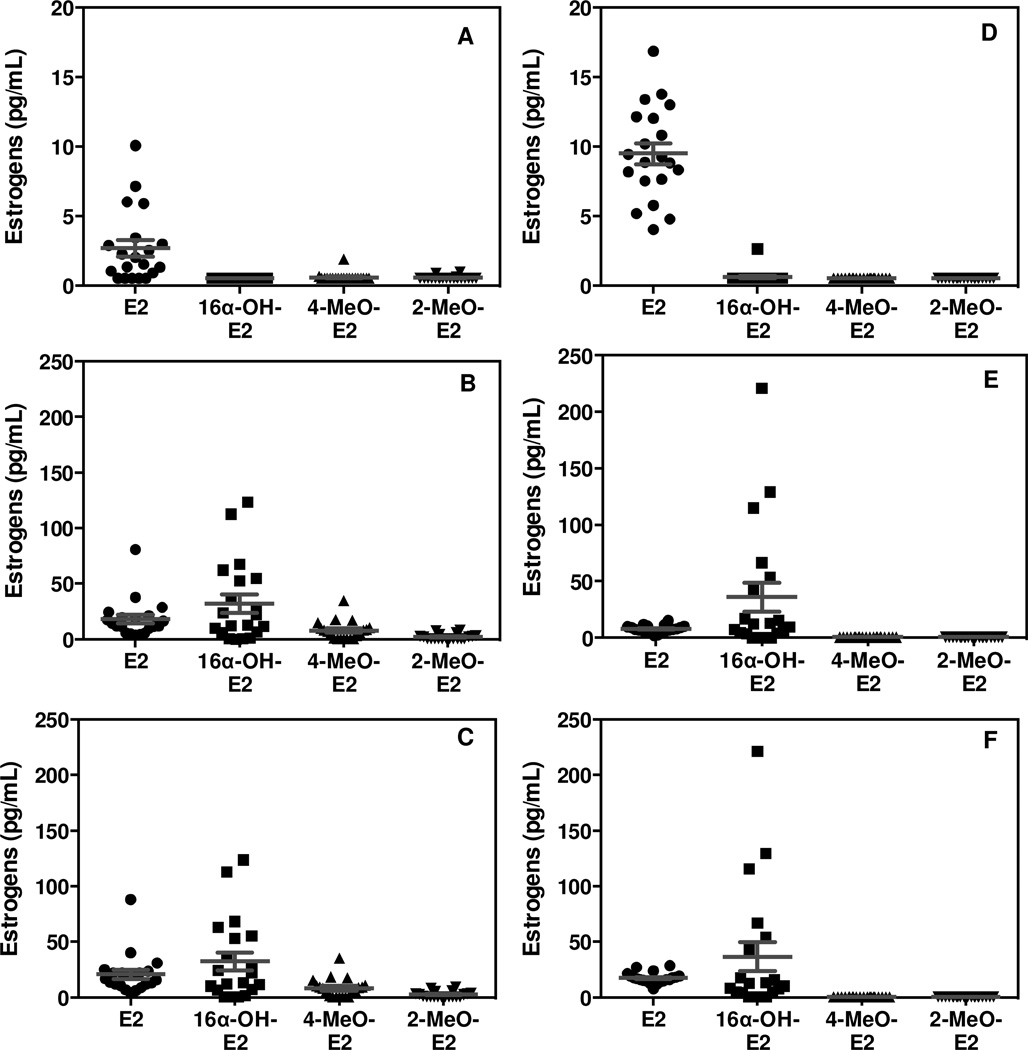
A total of 40 serum samples (20 female and 20 male) were processed using the LC-SRM/MS/MS assay. All estrogen concentrations were within the range of standard curves or below the LLOQs. Representative chromatograms of serum estrogens for a typical female subject are shown in Fig. 6. Unconjugated E2 was quantified in female (Fig. 6A) and male samples (chromatograms not shown). However, 16α-OH-E2, 4-MeO-E2, 2-MeO-E2, 4-OH-E2 and 2-OH-E2 were below the LLOQ in female (Fig. 6A) and male samples (chromatograms not shown). Total E2 and 16α-OH-E2 could be quantified in the female samples (Fig. 6B), whereas only E2 and 16α-OH-E2 could be quantified in the male samples (chromatogram not shown). The mean concentrations (± SEM) of unconjugated E2 in female and male serum were 2.7 pg/mL (± 0.6 pg/mL) (Fig. 8A) and 9.5 pg/mL (± 0.8 pg/mL) (Fig. 8D), respectively. All other unconjugated estrogens were below the LLOQ (0.5 pg/mL for E2, 16α-OH-E2, 4-MeO-E2 and 2-MeO-E2, 5 pg/mL for 4-OH-E2 and 2-OH-E2). The mean concentration (± SEM) of total E2, 16α-OH-E2, and 4-MeO-E2 in the female samples were 20.7 pg/mL (± 4.1 pg/mL), 32.5 pg/mL (± 8.1 pg/mL), and 8.2 pg/mL (± 1.9 pg/mL), respectively (Fig. 8C). The mean concentration (± SEM) of total E2 and 16α-OH-E2, in the male samples were 17.4 pg/mL (± 1.1 pg/mL) and 36.5 pg/mL (± 12.9 pg/mL), respectively (Fig. 8F). There were significant differences in unconjugated E2 and total 4-MeO-E2 concentrations between the female and male groups (p< 0.05).
Figure 8.
Mean values for estrogen concentrations (pg/mL) in serum samples from 20 postmenopausal women (mean age 60.0) and 20 older men (mean age 61.6). (A) Unconjugated serum estrogens in postmenopausal women. (B) Conjugated serum estrogens in postmenopausal women. (C) Total serum estrogens in postmenopausal women. (D) Unconjugated serum estrogens in older men. (E) Conjugated serum estrogens in older men. (F) Total serum estrogens in older men. Individual values ± SEM are shown.
4. Discussion
Estrogens play an important role in the etiology of breast cancer and elevated concentrations of circulating E2 are thought to be associated with an increased risk for breast cancer in postmenopausal women [10,38,47–55]. E2 could increase breast cancer risk through mechanisms in which E2 acts either as a hormone to stimulate aberrant cell proliferation or as the precursor to the formation of genotoxic catechol metabolites [52,55,56]. E2 is metabolized through three hydroxylation pathways - 4-hydroxylation, 2-hydroxylation, and 16α-hydroxylation (Fig. 1) [13,57]. Catechols derived from both the 4- and 2-hydroxylation pathways can be readily oxidized to form electrophilic quinone derivatives. These reactive electrophiles can form stable or depurinating adducts with DNA, which if not repaired could lead to mutations in the DNA that induce tumorigenesis [58]. Methylation of the 4-OH-E2 and 2-OH-E2 (Fig. 1) would prevent their conversion to reactive quinones. In addition, the methylated metabolites 4-MeO-E2 and 2-MeO-E2 could potentially serve as biomarkers of catechol formation.
Over the last decade, MS-based methods for the quantification of unconjugated serum E2 in postmenopausal women have become more specific and accurate so that more recently GC-MS and LC-MS methods have generally given values that are < 10 pg/mL (Table 1). Multiplexed assays for multiple estrogens are easier to conduct by LC-MS and so most of the reported values have employed this technique. Unfortunately, the reported values of all of the unconjugated serum estrogens apart from E2 are close to the limits of sensitivity of the assays and so it is not clear that these concentrations have any diagnostic value. This impedes statistical efficiency for epidemiologic studies, preventing accurate stratification and correlation analysis. Moreover, there is still considerable variability between laboratories in the analysis of individual samples [59]. These issues, coupled with general concerns over the reliability of E2 measurements [60], and the need for improved sensitivity to monitor aromatase inhibition [61], suggested the need for a more specific, sensitive, and reliable method for the analysis of E2 and its major metabolites to complement the high sensitivity method we developed for estrone and its metabolites [62].
Since the levels of estrogens and their metabolites are in the low pg/mL range, it was very important to optimize each sample preparation step to obtain as high recovery as possible. The extraction solvents, the effects of reaction heating time and temperature, the concentration of pyridine-3-sulfonyl chloride and iodomethane, and reaction pH were optimized. Solvents were tested for their ability to efficiently extract the estrogens and their metabolites from human serum, including ethyl acetate, ethyl acetate/hexane (60:40), dichloromethane, MTBE, and MTBE/hexane (9/1, 5/5). MTBE was the most efficient solvent to extract all estrogens tested; furthermore, it removed many of the co-eluting interfering peaks. Acidification of serum and addition of saturated brine also improved recovery. There were almost quantitative recoveries of estrogens and PS-estrogens derivatives from both LLE steps. The methylation reaction is a critical step for NMPS derivatization. High quality acetonitrile is recommended as the solvent for the iodomethane reaction. Additionally, the PS derivatives should be evaporated to complete dryness before methylation to ensure reaction efficiency. We compared aliquots of patient samples using both NMPS and PS derivatization. The NMPS derivatives gave a two-fold increase in response when compared with the PS derivatives. Furthermore, the additional derivatization step lowered the noise in the analyte channel and thus increased the signal/noise ratio (Fig. 7). The highest yield of NMPS derivatives was obtained with sodium bicarbonate at pH 9. When other conditions were held the same, heating sample at 60 °C for 30 min for pyridinium-3-sulfonate derivatization followed by 30 min at 80 °C for N-methyl derivatization, gave the best yield of NMPS derivatives for all estrogens and their metabolites. The major catechol derivatives formed under the conditions that were employed were mono-N-methyl derivatives (Figure 3). However, any minor amounts of bis-N-methyl derivatives that might be formed would not have affected the ratio of analyte to stable isotope-labeled internal standard.
Micro-LC and capillary LC have been widely used as a routine technique in various bioanalytical laboratories. Theoretically, nanospray LC should increase the sensitivity of analysis and so should allow for further improvements in detection limits [63]. Consequently, we compared the sensitivity of a Waters 2695 system (0.2 mL/min) with a Waters nanoAcquity UPLC system (2 µL/min) in the early stages of method development. The signal-to-noise increased by 2.5-fold at the lower flow rate and so method validation and subsequent analyses were conducted with a flow rate of 2 µL/min. In order to obtain both reproducible separation and high sensitivity for estrogens and the internal standards within a reasonable separation time, the nanospray UPLC conditions were carefully optimized. Tests showed that a mobile phase consisting of water and acetonitrile, both containing 0.1% formic acid, was the best mobile phase system.
The NMPS derivatives allowed E2 and five of its metabolites to be analyzed with extremely high sensitivity but less sample consumption. The limit of detection for E2 was 1 fg on column and the LLOQ for serum E2 was 500 fg/mL with only 100 µL of serum. Different to dansyl derivatives [31–34], NMPS derivatives also provided high specificity due to their analyte-dependent product ions, which arise from main structure of estrogen and its metabolites. This new derivative in combination with nano-LC-MS provided a mean value of 2.7 pg/mL (n=20) for unconjugated postmenopausal serum E2 (Table 1, Fig. 8A), which was close to the mean value of 4.4 pg/mL reported for GC-MS assays and lower the mean value of 7.3 pg/mL reported for LC-MS assays that used the dansyl derivative (Table 1). Furthermore, it agrees well with the mean value of 3.3 pg/mL that was reported recently from a novel method, which analyzed underivatized E2 using fluoride-enhanced negative ESI coupled with LC-MS [64]. This confirms that the NMPS derivative confers high specificity as well as high sensitivity for E2 analysis. The mean value of unconjugated serum E2 found for 20 older men of 9.6 pg/mL (Fig. 8C) was significantly higher than that found in postmenopausal women (Fig. 8A). It is noteworthy that higher levels of unconjugated E2 found in men compared with postmenopausal women is similar to many other studies, although the absolute level was somewhat lower. For example, a mean value of 20.6 pg/mL was reported for unconjugated serum E2 in six studies of older men, which used the PFBO derivative coupled with GC-MS-based methodology [27,65–69]. The mean unconjugated serum E2 level we found in men was also lower than studies that employed LC-negative ESI/MS (mean 17.2 pg/mL) [70], LC-negative atmospheric pressure photoionization/MS (mean 20 pg/mL) [71], and regular LC-ESI/MS of the E2 dansyl derivative (mean 22.9 pg/mL) [72–74]. This provides further evidence that the NMPS derivative in combination with LC-MS confers high sensitivity and specificity for E2 analysis. The five other unconjugated serum estrogens were all below the LLOQ for both postmenopausal women (Fig. 8A; Table 1) and older men (Fig. 8D). This together with previous reports of values that were close to the reported assay LLOQs suggests that such measurements have little diagnostic value.
The mean level obtained for total serum E2 of 20.7 pg/mL in postmenopausal women (Table 2, Fig. 8C) was similar to the mean value of 19.7 pg/mL that was obtained using LC-MS of the dansyl derivative (Table 2). A relatively low value for serum E2-3G of 5.5 pg/mL was found using an excellent stable isotope dilution assay of the intact β-glucuronide [26]. In our study the β-glucuronide and sulfate conjugates (total E2 - unconjugated E2) corresponded to 18 pg/mL (Fig. 8B) suggesting that the sulfate was the major conjugate of E2 (Fig. 1) that was present in the serum. This would potentially be a source for E2 through the action of sulfatases that are present in breast tissue [75,75] and so analysis of total serum E2 seems to be a worthwhile endeavor. Perhaps more specific methodology for intact E2-3S similar to that developed for E2-3G [26] and E1-S [76–78] would be even more meaningful.
The concentrations of total serum E2 in older men of 17.4 pg/mL(Fig. 8F) were, surprisingly, quite similar to the levels found in postmenopausal women (Fig. 8C). This means that the β-glucuronide and sulfate conjugates (7.9 pg/mL, Fig. 8E) were lower than the corresponding unconjugated serum E2 (Fig. 8D) concentration in the male subjects. A previous LC-MS study using the dansyl derivative reported a mean serum total E2 of 62.3 pg/mL in older men [79], which was significantly higher than that found in the present study. However, it is noteworthy that this study also reported total serum estrogen concentrations in postmenopausal women that were much higher than has been reported previously (Table 2). This suggests that there was some interference in the method that was used, and that the correct total serum E2 concentrations are closer to value of 17.4 pg/mL determined in the present study.
There is no consensus in the literature on the correct levels of total serum 16α-OH-E2 – reported values span a wide range from 27.9 pg/mL to 126.0 pg/mL (Table 2). The value obtained in the present study of 32.5 pg/mL (Table 2, Fig. 8C) seems to be more realistic in view of the undetectable levels of the unconjugated form in serum. In fact our data would suggest that 16α-OH-E2 is only present as β-glucuronide and/or sulfate conjugates (Fig. 8B). It is conceivable that the differences between the various studies could be due to differences in efficiency of hydrolysis by glucuronidase/sulfatase enzyme and so it will be important in the future to validate our finding through analysis of the intact β-glucuronide and sulfate conjugates. The mean concentration of total serum 16α-OH-E2 of 36.5 pg/mL obtained for older men (Fig. 8F) was very similar to the value observed in postmenopausal women (Table 1, Fig. 8C).
Previous studies have suggested that levels of total 4-MeO-E2 in serum from postmenopausal women are below 1 pg/mL and so analysis of this molecule cannot really be justified. The present study has revealed that the mean level is in fact slightly higher at 8.2 pg/mL (Table 2, Fig. 8C), suggesting that total serum 4-MeO-E2 might be a useful biomarker of catechol formation. Conversely, levels of total serum 4-MeO-E2 in the older men were below the limit of detection of 0.5 pg/mL (Fig. 8F). The levels of total serum 2-MeO-E2 of 2.5 pg/mL (Table 2, Fig. 8C) in postmenopausal women were similar to those reported previously and will have little diagnostic value unless the levels are significantly increased in subjects with high breast cancer risk. The levels of total serum 2-MeO-E2 in older men were below the limit of detection of 0.5 pg/mL (Fig. 8F). There are no reports of total serum 4-OH-E2 levels in postmenopausal women (Table 2) or older men. The present study has confirmed that levels are below our LLOQ of 5 pg/mL in both groups. There are several reports that total serum 2-OH-E2 can reach a measurable level of 7.9 pg/mL in postmenopausal women (Table 2). However, the total serum 2-OH-E2 was below our LLOQ of 5 pg/mL in both menopausal women (Table 2) and older men (data not shown). It is conceivable that the catechol conjugates are poor substrates for typical β-glucuronidase/sulfates enzymes that are used and so the true levels could be higher. Unfortunately, both catechols are labile under acidic conditions so acid catalyzed hydrolysis cannot be used instead. Therefore, it will require the development of assays for the intact β-glucuronide and sulfate conjugates in order to fully confirm that these metabolites are absent from the systemic circulation.
In summary, the new pre-ionized NMPS derivative confers extremely high sensitivity for the analysis of estradiol and its metabolites by LC-ESI/MS. NMPS derivatization requires the initial formation of a pyridinium sulfonate derivative that is then quaternized by methylation with methyl iodide (Figure 2). We are not aware of a derivatization technique that provides a pre-ionized derivative of estradiol in a single step. The NMPS derivatization method can also be applied for the quantification of estrones and other estrogens with a free phenol moiety. Estrone 17-keto groups do not cause any problems during the derivatization or subsequent quantification steps (data not shown). This new NMPS estrogen derivative has made it possible to show that the levels for unconjugated serum E2 were lower than that found in older men and that conjugated E2 was present in somewhat higher levels in both postmenopausal women and older men. In addition, the levels of conjugated serum 16α-OH-E2 were much higher than conjugated E2 in both postmenopausal women and older men. Finally, conjugated serum 4-MeO-E2 was present at low levels in the serum of postmenopausal women but was below the LLOQ in older men.
Highlights.
Estrogen NMPS derivatives factiitate high sensitivity LC-ESI/MS analysis.
Stable isotope dilution LC-MS method developed for six serum estrogens.
Mean unconjugated serum E2 was 2.9 pg/mL in postmenopausal women
Mean unconjugated serum E2 was 9.1 pg/mL in older men
Conjugated serum 4-MeO-E2 is potential cancer biomarker.
Acknowledgements
We acknowledge the support of NIH grants R01CA158328, P30ES013508, and T32GM008076. We thank Dr. Richard Santen from the University of Virginia for useful discussions.
Abbreviations
- E1
estrone
- E2
17β-estradiol
- G
β-glucuronide
- GC
gas chromatography
- LC
liquid chromatography
- HQC
high quality control
- LLE
liquid-liquid extraction
- LLOQ
lower limit of quantification
- LQC
low quality control
- MQC
medium quality control
- MS
mass spectrometry
- m/z
mass to charge ratio
- NMPS
N-methyl pyridinium-3-sulfonyl
- OH
hydroxy
- OMe
methoxy
- PS
pyridine-3-sulfonyl
- S
sulfate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Penning TM, Lee SH, Jin Y, Gutierrez A, Blair IA. Liquid-chromatography mass spectrometry (LC-MS) of steroid hormone metabolites and its applications. J Steroid Biochem Mol Biol. 2010;121:546–555. doi: 10.1016/j.jsbmb.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair IA. Analysis of estrogens in serum and plasma from postmenopausal women: past, present, and future. Steroids. 2010;75:297–306. doi: 10.1016/j.steroids.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Mesaros C, Bottalico L, Blair IA. Analysis of estrogens and androgens in postmenopausal serum and plasma by liquid chromatography-mass spectrometry. Steroids. 2014 Aug 20; doi: 10.1016/j.steroids.2014.08.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidem Biomar. 2002;11:1065–1071. [PubMed] [Google Scholar]
- 5.Endogenous Hormones and Breast Cancer Collaborative Group. Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values. Cancer Epidemiol Biomarkers Prevent. 2003;12:1457–1461. [PubMed] [Google Scholar]
- 6.Longcope C, Hui SL, Johnston CC., Jr Free estradiol, free testosterone, and sex hormone-binding globulin in perimenopausal women. J Clin Endocrinol Metab. 1987;64:513–518. doi: 10.1210/jcem-64-3-513. [DOI] [PubMed] [Google Scholar]
- 7.Santen RJ, Lee JS, Wang S, Demers LM, Mauras N, Wang H, et al. Potential role of ultrasensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids. 2008;73:1318–1321. doi: 10.1016/j.steroids.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 9.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Santen RJ. Assessing individual risk for breast cancer: role of oestrogens and androgens. Breast Cancer Res. 2008;10:S10. doi: 10.1186/bcr2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson SH, Bandera EV, Orlow I. Variants in estrogen biosynthesis genes, sex steroid hormone levels, and endometrial cancer: a HuGE review. Am J Epidemiol. 2007;165:235–245. doi: 10.1093/aje/kwk015. [DOI] [PubMed] [Google Scholar]
- 12.Audet-Walsh E, Lepine J, Gregoire J, Plante M, Caron P, Tetu B, et al. Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: association with risk and relationship to clinical characteristics. J Clin Endocrinol Metab. 2011;96:E330–E339. doi: 10.1210/jc.2010-2050. [DOI] [PubMed] [Google Scholar]
- 13.Rizner TL. Estrogen biosynthesis, phase I and phase II metabolism, and action in endometrial cancer. Mol Cell Endocrinol. 2013;381:124–139. doi: 10.1016/j.mce.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Steiner MS, Raghow S. Antiestrogens and selective estrogen receptor modulators reduce prostate cancer risk. World J Urol. 2003;21:31–36. doi: 10.1007/s00345-002-0316-x. [DOI] [PubMed] [Google Scholar]
- 15.Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biol. 2007;102:899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- 16.Hartman J, Strom A, Gustafsson JA. Current concepts and significance of estrogen receptor beta in prostate cancer. Steroids. 2012;77:1262–1266. doi: 10.1016/j.steroids.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Lonning PE, Ekse D. A sensitive assay for measurement of plasma estrone sulphate in atients on treatment with aromatase inhibitors. J Steroid Biochem Mol Biol. 1995;55:409–412. doi: 10.1016/0960-0760(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 18.Kakugawa Y, Minami Y, Tateno H, Inoue H, Fujiya T. Relation of serum levels of estrogen and dehydroepiandrosterone sulfate to hormone receptor status among postmenopausal women with breast cancer. Breast Cancer. 2007;14:269–276. doi: 10.2325/jbcs.14.269. [DOI] [PubMed] [Google Scholar]
- 19.Purohit A, Foster PA. Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers. J Endocrinol. 2012;212:99–110. doi: 10.1530/JOE-11-0266. [DOI] [PubMed] [Google Scholar]
- 20.Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ. Limitations of direct estradiol and testosterone immunoassay kits. Steroids. 2003;68:1173–1178. doi: 10.1016/j.steroids.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Toniolo P, Lukanova A. The challenge of measuring circulating estradiol at low concentrations. Breast Cancer Res. 2005;7:45–47. doi: 10.1186/bcr987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang DT, Owen WE, Ramsay CS, Xie H, Roberts WL. Performance characteristics of eight estradiol immunoassays. Am J Clin Pathol. 2004;122:332–337. doi: 10.1309/5N2R-4HT4-GM0A-GPBY. [DOI] [PubMed] [Google Scholar]
- 23.Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, et al. Metabolism of DHEA in postmenopausal women following percutaneous administration. J Steroid Biochem Mol Biol. 2007;103:178–188. doi: 10.1016/j.jsbmb.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Labrie F, Cusan L, Gomez JL, Cote I, Berube R, Belanger P, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol. 2008;111:178–194. doi: 10.1016/j.jsbmb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Caron P, Audet-Walsh E, Lepine J, Belanger A, Guillemette C. Profiling endogenous serum estrogen and estrogen-glucuronides by liquid chromatography-tandem mass spectrometry. Anal Chem. 2009;81:10143–10148. doi: 10.1021/ac9019126. [DOI] [PubMed] [Google Scholar]
- 27.Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P, et al. Comparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancer. J Steroid Biochem Mol Biol. 2009;113:52–56. doi: 10.1016/j.jsbmb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Labrie F, Martel C, Berube R, Cote I, Labrie C, Cusan L, et al. Intravaginal prasterone (DHEA) provides local action without clinically significant changes in serum concentrations of estrogens or androgens. J Steroid Biochem Mol Biol. 2013;138:359–367. doi: 10.1016/j.jsbmb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Singh G, Gutierrez A, Xu K, Blair IA. Liquid chromatography/electron capture atmospheric pressure chemical ionization/mass spectrometry: analysis of pentafluorobenzyl erivatives of biomolecules and drugs in the attomole range. Analytical Chemistry. 2000;72:3007–3013. doi: 10.1021/ac000374a. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Williams MV, Dubois RN, Blair IA. Targeted lipidomics using electron capture atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:2168–2176. doi: 10.1002/rcm.1170. [DOI] [PubMed] [Google Scholar]
- 31.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 32.Tai SS, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of estradiol-17beta in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77:6359–6363. doi: 10.1021/ac050837i. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 34.Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, et al. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008;129:530–539. doi: 10.1309/LC03BHQ5XJPJYEKG. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 36.Gao W, Zeng C, Cai D, Liu B, Li Y, Wen X, et al. Serum concentrations of selected endogenous estrogen and estrogen metabolites in pre- and post-menopausal Chinese women with osteoarthritis. J Endocrinol Invest. 2010;33:644–649. doi: 10.1007/BF03346664. [DOI] [PubMed] [Google Scholar]
- 37.Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, et al. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids. 2011;76:177–182. doi: 10.1016/j.steroids.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, et al. Estrogen Metabolism and Risk of Breast Cancer in Postmenopausal Women. JNCI. 2012;104:326–339. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, et al. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15:R34. doi: 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dallal CM, Tice JA, Buist DS, Bauer DC, Lacey JV, Jr, Cauley JA, et al. Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B~FIT. Carcinogenesis. 2014;35:346–355. doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M. Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids. 2007;72:819–827. doi: 10.1016/j.steroids.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Spink DC. Analysis of steroidal estrogens as pyridine-3-sulfonyl derivatives by liquid chromatography electrospray tandem mass spectrometry. Anal Biochem. 2008;375:105–114. doi: 10.1016/j.ab.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin YH, Chen CY, Wang GS. Analysis of steroid estrogens in water using liquid chromatography/tandem mass spectrometry with chemical derivatizations. Rapid Commun Mass Spectrom. 2007;21:1973–1983. doi: 10.1002/rcm.3050. [DOI] [PubMed] [Google Scholar]
- 44.Nishio T, Higashi T, Funaishi A, Tanaka J, Shimada K. Development and application of electrospray-active derivatization reagents for hydroxysteroids. J Pharm Biomed Anal. 2007;44:786–795. doi: 10.1016/j.jpba.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Rangiah K, Shah SJ, Vachani A, Ciccimaro E, Blair IA. Liquid chromatography-mass spectrometry of pre-ionized Girard P derivatives for quantifying estrone and its metabolites in serum from postmenopausal women. Rapid Communications in Mass Spectrometry. 2011;25:1297–1307. doi: 10.1002/rcm.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamae D, Byrns MC, Brettt M, Mostaghel E, Nelson PS, Lange P, et al. Validation and application of a atable isotope dilution Liquid Chromatography electrospray ionization/selected reaction monitoring/mass spectrometry (SID-LC/ESI/SRM/MS) method for quantification of keto-androgens in human serum. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.06.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 48.Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M, et al. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007;14:169–187. doi: 10.1677/ERC-06-0045. [DOI] [PubMed] [Google Scholar]
- 49.Lamar CA, Dorgan JF, Longcope C, Stanczyk FZ, Falk RT, Stephenson HE., Jr Serum sex hormones and breast cancer risk factors in postmenopausal women. Cancer Epidem Biomar. 2003;12:380–383. [PubMed] [Google Scholar]
- 50.ESHRE Capri Workshop Group. Hormones and breast cancer. Hum Reprod Update. 2004;10:281–293. doi: 10.1093/humupd/dmh025. [DOI] [PubMed] [Google Scholar]
- 51.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 52.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 53.Parl FF, Dawling S, Roodi N, Crooke PS. Estrogen metabolism and breast cancer: a risk model. Ann N Y Acad Sci. 2009;1155:68–75. doi: 10.1111/j.1749-6632.2008.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manavathi B, Dey O, Gajulapalli VN, Bhatia RS, Bugide S, Kumar R. Derailed estrogen signaling and breast cancer: an authentic couple. Endocr Rev. 2013;34:1–32. doi: 10.1210/er.2011-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santen RJ, Yue W, Wang JP. Estrogen metabolites and breast cancer. Steroids. 2014 Aug 20; doi: 10.1016/j.steroids.2014.08.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Sepkovic DW, Bradlow HL. Estrogen hydroxylation--the good and the bad. Ann N Y Acad Sci. 2009;1155:57–67. doi: 10.1111/j.1749-6632.2008.03675.x. [DOI] [PubMed] [Google Scholar]
- 57.Yue W, Santen RJ, Wang JP, Li Y, Verderame MF, Bocchinfuso WP, et al. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J Steroid Biochem Mol Biol. 2003;86:477–486. doi: 10.1016/s0960-0760(03)00377-7. [DOI] [PubMed] [Google Scholar]
- 58.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, et al. Catechol estrogen quinones as initiators of breast and other human cancers: Implications for biomarkers of susceptibility and cancer prevention. Biochimica et Biophysica Acta (BBA) -Reviews on Cancer. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Vesper HW, Botelho JC, Vidal ML, Rahmani Y, Thienpont LM, Caudill SP. High variability in serum estradiol measurements in men and women. Steroids. 2014;82:7–13. doi: 10.1016/j.steroids.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab. 2013;98:1376–1387. doi: 10.1210/jc.2012-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pauwels S, Lintermans A, Neven P, Verhaeghe J, Jans I, Billen J, et al. Need for estradiol assays with a lower functional sensitivity in clinical studies examining postmenopausal women treated with aromatase inhibitors. J Clin Oncol. 2013;31:509. doi: 10.1200/JCO.2012.46.2622. [DOI] [PubMed] [Google Scholar]
- 62.Rangiah K, Shah SJ, Vachani A, Ciccimaro E, Blair IA. Liquid chromatography/mass spectrometry of pre-ionized Girard P derivatives for quantifying estrone and its metabolites in serum from postmenopausal women. Rapid Commun Mass Spectrom. 2011;25:1297–1307. doi: 10.1002/rcm.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chervet JP, Ursem M, Salzmann JP. Instrumental requirements for nanoscale liquid chromatography. Anal Chem. 1996;68:1507–1512. doi: 10.1021/ac9508964. [DOI] [PubMed] [Google Scholar]
- 64.Pauwels S, Antonio L, Jans I, Lintermans A, Neven P, Claessens F, et al. Sensitive routine liquid chromatography-tandem mass spectrometry method for serum estradiol and estrone without derivatization. Anal Bioanal Chem. 2013;405:8569–8577. doi: 10.1007/s00216-013-7259-5. [DOI] [PubMed] [Google Scholar]
- 65.Daniels NA, Nielson CM, Hoffman AR, Bauer DC. Sex hormones and the risk of incident prostate cancer. Urology. 2010;76:1034–1040. doi: 10.1016/j.urology.2010.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohlsson C, Labrie F, Barrett-Connor E, Karlsson MK, Ljunggren O, Vandenput L, et al. Low serum levels of dehydroepiandrosterone sulfate predict all-cause and cardiovascular mortality in elderly Swedish men. J Clin Endocrinol Metab. 2010;95:4406–4414. doi: 10.1210/jc.2010-0760. [DOI] [PubMed] [Google Scholar]
- 67.Orwoll ES, Nielson CM, Labrie F, Barrett-Connor E, Cauley JA, Cummings SR, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010;95:E151–E160. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Travison TG, Nguyen AH, Naganathan V, Stanaway FF, Blyth FM, Cumming RG, et al. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: the concord health and ageing in men project. J Clin Endocrinol Metab. 2011;96:2464–2474. doi: 10.1210/jc.2011-0143. [DOI] [PubMed] [Google Scholar]
- 69.Vandenput L, Lorentzon M, Sundh D, Nilsson ME, Karlsson MK, Mellstrom D, et al. Serum estradiol levels are inversely associated with cortical porosity in older men. J Clin Endocrinol Metab. 2014;99:E1322–E1326. doi: 10.1210/jc.2014-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeap BB, Knuiman MW, Divitini ML, Handelsman DJ, Beilby JP, Beilin J, et al. Differential associations of testosterone, dihydrotestosterone and oestradiol with physical, metabolic and health-related factors in community-dwelling men aged 17–97 years from the Busselton Health Survey. Clin Endocrinol (Oxf) 2014;81:100–108. doi: 10.1111/cen.12407. [DOI] [PubMed] [Google Scholar]
- 71.Hsu B, Cumming RG, Blyth FM, Naganathan V, Le Couteur DG, Seibel MJ, et al. Longitudinal and cross-sectional relationships of circulating reproductive hormone levels to self-rated health and health-related quality of life in community-dwelling older men. J Clin Endocrinol Metab. 2014;99:1638–1647. doi: 10.1210/jc.2013-3984. [DOI] [PubMed] [Google Scholar]
- 72.Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008;168:47–54. doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 73.Auyeung TW, Lee JS, Kwok T, Leung J, Ohlsson C, Vandenput L, et al. Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross-sectional study in 1489 older men. Eur J Endocrinol. 2011;164:811–817. doi: 10.1530/EJE-10-0952. [DOI] [PubMed] [Google Scholar]
- 74.Spitzer M, Bhasin S, Travison TG, Davda MN, Stroh H, Basaria S. Sildenafil increases serum testosterone levels by a direct action on the testes. Andrology. 2013;1:913–918. doi: 10.1111/j.2047-2927.2013.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakata T, Takashima S, Shiotsu Y, Murakata C, Ishida H, Akinaga S, et al. Role of steroid sulfatase in local formation of estrogen in post-menopausal breast cancer patients. J Steroid Biochem Mol Biol. 2003;86:455–460. doi: 10.1016/s0960-0760(03)00357-1. [DOI] [PubMed] [Google Scholar]
- 76.Stanway SJ, Purohit A, Reed MJ. Measurement of estrone sulfate in postmenopausal women: comparison of direct RIA and GC-MS/MS methods for monitoring response to endocrine therapy in women with breast cancer. Anticancer Res. 2007;27:2765–2767. [PubMed] [Google Scholar]
- 77.Hosogi J, Tanaka H, Fujita K, Kuwabara T, Ikegawa S, Kobayashi N, et al. LC-MS/MS coupled with immunoaffinity extraction for determination of estrone, 17beta-estradiol and estrone 3-sulfate in human plasma. J Chromatogr B. 2009 doi: 10.1016/j.jchromb.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Giton F, Caron P, Berube R, Belanger A, Barbier O, Fiet J. Plasma estrone sulfate assay in men: Comparison of radioimmunoassay, mass spectrometry coupled to gas chromatography (GC-MS), and liquid chromatography-tandem mass spectrometry (LC-MS/MS) Clin Chim Acta. 2010;411:1208–1213. doi: 10.1016/j.cca.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 79.Masi CM, Hawkley LC, Xu X, Veenstra TD, Cacioppo JT. Serum estrogen metabolites and systolic blood pressure among middle-aged and older women and men. Am J Hypertens. 2009;22:1148–1153. doi: 10.1038/ajh.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]