Abstract
Introduction. The burden of tuberculosis is high in eastern Malaysia, and rates of Mycobacterium tuberculosis drug resistance are poorly defined. Our objectives were to determine M. tuberculosis susceptibility and document management after receipt of susceptibility results. Methods. Prospective study of adult outpatients with smear-positive pulmonary tuberculosis (PTB) in Sabah, Malaysia. Additionally, hospital clinicians accessed the reference laboratory for clinical purposes during the study. Results. 176 outpatients were enrolled; 173 provided sputum samples. Mycobacterial culture yielded M. tuberculosis in 159 (91.9%) and nontuberculous Mycobacterium (NTM) in three (1.7%). Among outpatients there were no instances of multidrug resistant M. tuberculosis (MDR-TB). Seven people (4.5%) had isoniazid resistance (INH-R); all were switched to an appropriate second-line regimen for varying durations (4.5–9 months). Median delay to commencement of the second-line regimen was 13 weeks. Among 15 inpatients with suspected TB, 2 had multidrug resistant TB (one extensively drug resistant), 2 had INH-R, and 4 had NTM. Conclusions. Current community rates of MDR-TB in Sabah are low. However, INH-resistance poses challenges, and NTM is an important differential diagnosis in this setting, where smear microscopy is the usual diagnostic modality. To address INH-R management issues in our setting, we propose an algorithm for the treatment of isoniazid-resistant PTB.
1. Introduction
Tuberculosis (TB) is a disease of major significance in eastern Malaysia (Malaysian Borneo), especially in Sabah state. Eastern Malaysia has a disproportionally high burden of the country's TB cases [1], and Sabah's estimated TB incidence rate (131/100,000) [2] far exceeds national rates (80/100,000) [3]. Rates of Mycobacterium tuberculosis drug resistance are not well defined in Sabah. Where testing has occurred, it has often been reserved for treatment failure cases, limiting the generalizability of such findings. Malaysian rates of multidrug resistant- (MDR-) TB have been estimated at 0.3% in 2005 and 1.3% in 2011 [4]. In unpublished data from the Sabah state reference laboratory, 16 MDR-TB cases occurred in 2011, representing 2.1% of isolates submitted that year [5]. Isoniazid resistance (INH-R) is reported in approximately 4% of M. tuberculosis in western Malaysia [6, 7], but rates have not been published from eastern Malaysia.
Isoniazid resistance (INH-R) is now generally acknowledged to be associated with poorer outcomes than INH-susceptible disease [8–11]. Despite INH-R TB being so common (4–10% globally [12–14]), the level of evidence to guide management is mostly level III-IV (Table 1), resulting in practices being nonuniform regarding drug choice, duration, and dosing frequency. The standard short-course regimen (isoniazid (H), rifampicin (R), ethambutol (E), and pyrazinamide (Z)) has traditionally been considered adequate [15, 16]. However 9 months of REZ (9REZ) or variations on this theme [10, 17–19] are increasingly used, and some advocate for the addition of a fluoroquinolone routinely [20], or in extensive INH-R pulmonary disease [18]. A new trial from the Tuberculosis Trials Consortium [21] provides much-needed evidence in this field; although the study was single-arm and nonrandomised, it provides evidence that twice or thrice weekly REZ for 6 months in HIV negative patients is mostly effective (3.5% failed or relapsed) but poorly tolerated [21]. Poor tolerability, especially of pyrazinamide, is a key challenge in the treatment of INH-R pulmonary TB (PTB) and a reason to require third-line options such as 12 months of a 2-drug regimen [22] or substitution with a fluoroquinolone [18].
Table 1.
Treatment of isoniazid-resistant pulmonary tuberculosis: recommendations from selected references and level of evidence.
| Treatment element | Reference | Study type | Level of evidence* |
|---|---|---|---|
| Drugs | |||
| R throughout | [11] | Meta-analysis of trials and cohort studies | III |
| REZ | [21] | Nonrandomised trial | IIa |
| [22] | Observational study | III | |
| [17, 23] | Expert opinion | IV | |
| HREZ | [16] | Retrospective review | III |
| Add a fluoroquinolone in all instances (moxifloxacin 400 mg or levofloxacin 750–100 mg substituted for isoniazid) | [20] | Expert opinion | IV |
| Add a fluoroquinolone if pyrazinamide is not tolerated | [23] | Expert opinion | IV |
| Add a fluoroquinolone if disease is extensive | [18] | Expert opinion | IV |
| High-dose H is not recommended | [23] | Expert opinion | IV |
| Duration | |||
| 6 months (REZ) | [21] | Nonrandomised trial | IIa |
| 6 months (REZ) if disease is not extensive | [23] | Expert opinion | IV |
| 6 to 9 months (REZ) | [17] | Expert opinion | IV |
| 6 to 12 months (variety of regimens) | [22] | Observational study | III |
| 9 months (REZ) if culture-positive at 2 months | [23] | Expert opinion | IV |
| Dosing frequency ** | |||
| Dosing should be daily during the intensive phase | [11] | Meta-analysis of trials and cohort studies | III |
| [24] | Systematic review | ||
| [23] | Expert opinion | IV | |
| Dosing can be given intermittently (thrice weekly) during the continuation phase | [25] | Expert opinion | IV |
| Twice or thrice weekly RZE for 6 months in HIV negative disease | [21] | Nonrandomised trial | IIa |
*Ia: evidence from meta-analysis of randomized controlled trials, Ib: evidence from at least one randomized controlled trial, IIa: evidence from at least one well designed controlled trial which is not randomized, IIb: evidence from at least one well designed experimental trial, III: evidence from case, correlation, and comparative studies, IV: evidence from a panel of experts [26].
**Where dosing is self-administered rather than directly observed (as in Malaysia), there may be no advantage in an intermittent regimen.
The time taken to obtain M. tuberculosis susceptibility results is long if solid culture medium only is used, or where results are batched for dispatch to a reference laboratory; both these factors apply in our setting. If susceptibility results are delayed and the patient has already been switched to HR continuation phase treatment, the question of what to do after a period of effective monotherapy with rifampicin arises. Tailoring of regimens for pulmonary INH-R TB depending on factors, such as patient age, HIV status, and cavitary disease, can be difficult in high TB-burden settings accustomed to standardized regimens.
Knowledge of local susceptibility not only allows tailoring of treatment in individuals but provides local antibiogram data to aid clinicians in constructing regimens to use empirically and in culture-negative suspected tuberculosis (e.g., paediatric and extrapulmonary TB) and latent TB. Our first objective was to determine TB drug susceptibility profiles among patients with new or retreatment pulmonary TB presenting to a large community TB clinic in Kota Kinabalu, Sabah, Malaysia. Our second objective was to document clinical practice on receipt of susceptibility results.
2. Methods
This is a prospective observational study of smear-positive PTB outpatients, enrolled at Luyang Tuberculosis Outpatient Clinic, Kota Kinabalu, Malaysia. This is the main referral TB clinic in Kota Kinabalu, diagnosing and treating almost 200 patients annually. Kota Kinabalu district has a population of approximately 600,000. Participants were enrolled over 2 years, 4 July 2012–3 July 2014. Additionally, during the study period, clinicians at the Kota Kinabalu tertiary adult referral hospital, Queen Elizabeth Hospital (QEH), were able to access the services of the reference laboratory for clinical purposes, for patients in whom mycobacterial infection, particularly drug resistance, was suspected. QEH manages approximately 200 smear-positive PTB inpatients annually.
2.1. Informed Consent
Potentially eligible participants were provided with information about the study using written and pictorial materials and explanations from research staff and were required to provide written, informed consent. Consent was obtained from a parent/guardian for participants aged between 15 and 17, who additionally provided their assent.
2.2. Participants
Outpatient participants were eligible if they had sputum smear-positive pulmonary TB, were aged ≥15 years, and had received <7 days' TB treatment. They were referred for enrolment by the TB clinic doctor after being diagnosed with TB on the basis of clinical and X-ray assessment, and at least one sputum positive for acid fast bacilli (AFB) on Ziehl-Neelsen stain performed at the clinic's smear microscopy laboratory.
2.3. Procedures
A new sputum sample was provided on the day of enrolment. Samples were batched in a domestic refrigerator and transported to an international reference laboratory (Singapore General Hospital Laboratory, Singapore) as described elsewhere [27]. The BACTEC Mycobacterium Growth Indicator Tube (MGIT) 960 tube system was employed for culture. Drug susceptibility testing was performed for M. tuberculosis isolates using the nonradiometric MGIT system for isoniazid, rifampicin, ethambutol, streptomycin, and, in the instance of any first-line resistance, also for ofloxacin, kanamycin, and ethionamide. Nontuberculous mycobacteria (NTM) were identified using a DNA probe (ProbeTec, Becton-Dickinson). Further speciation, if done, was achieved using a second DNA probe (INNO-LiPA MYCOBACTERIA, Innogenetics, Ghent, Belgium) and high-performance liquid chromatography (HPLC) of mycolic acids.
HIV was tested using two rapid tests (Abbott Determine HIV-1/2 and a second locally supplied point-of-care test, as described elsewhere [28]). Positive results were confirmed by enzyme immunoassay, particle agglutination, and line immunoassay.
2.4. Ethical Approval
Ethical approval was obtained from the Medical Research Ethics Committee, Malaysian Ministry of Health, and the Health Research Ethics Committee of Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research, Australia.
2.5. Clinical Specimens from Hospital Patients
During the study period, clinicians at Queen Elizabeth Hospital were also able to utilise the arrangement established with the reference laboratory. Clinicians could send sputum specimens or extrapulmonary samples, with the batched research specimens, to obtain mycobacterial culture and susceptibility testing to guide clinical management. No specific inclusion criteria were applied apart from the suspicion of mycobacterial infection and difficulty obtaining results locally.
2.6. Statistics
Data were analysed using Stata 13.1. (Stata Corp., College Station, Texas, USA). Continuous variables were compared using Student's t-test or Wilcoxon Rank Sum test. Categorical variables were compared using Chi-squared or Fisher's Exact test, as appropriate.
3. Results
3.1. Outpatients
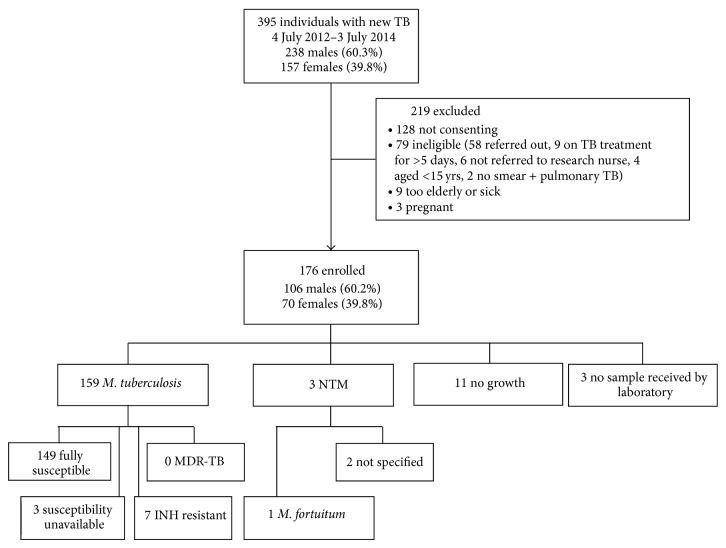
During the 2-year study period, 176 outpatients consented and were enrolled (Figure 1, Table 2). Details relating to patient demographics and HIV status are described elsewhere [28]. A sputum sample was received by the laboratory for 173 individuals. Thirteen participants reported having received TB treatment in the past (median 12.5 years ago according to patients' recollection, range 1–39 years).
Figure 1.
Outpatient study diagram showing enrolment and microbiological results.
Table 2.
Characteristics of outpatients enrolled at Luyang Clinic.
| Baseline characteristic | Number |
|---|---|
| Number | 176 |
| Age in years: median (range) | 30.0 (16–73) |
| Male: number (%) | 104 (59.0%) |
| Nationality | |
| Malaysian: number (%) | 117 (66.5%) |
| Non-Malaysian: number (%) | 59 (33.5%) |
| HIV positive: number (%) | 3 (1.7%) |
| Current smoker | 40 (22.7%) |
| Past TB | 13 (7.4%) |
3.2. Outpatient Culture and Susceptibility Results
Mycobacterial culture was positive in 162 of 173 (93.6%) samples received. One hundred and fifty-nine (91.9%) yielded M. tuberculosis, and three (1.7%) yielded NTM; one was M. fortuitum; the others were not speciated. Of patients in whom NTM was isolated, two had underlying bronchiectasis.
Susceptibility results were unavailable in 3 M. tuberculosis isolates due to overgrowth of the culture by contaminating organisms. In the 156 isolates from outpatients with susceptibility data, none had MDR-TB or rifampicin monoresistance. Seven people (4.5%) had infection with INH-R M. tuberculosis, none of whom recalled having previously received TB treatment. INH-R disease was more common in people of non-Malaysian ethnicity: 6 of the 7 (86%) were Filipino or Indonesian, compared with 50/159 (33.6%) with INH-S disease (P = 0.009).
One M. tuberculosis isolate with isoniazid resistance had additional streptomycin and ethionamide resistance. One instance of streptomycin monoresistance was also identified.
3.3. Management of Drug Resistant Cases
Among people identified as having INH-R, there were substantial delays between commencement of the first-line tuberculosis treatment regimen and change to a second-line regimen. The median delay was 13 weeks (range 8 to 28 weeks); see Table 3. Opportunities for delay included the time taken to dispatch sputum samples to the laboratory, receive the resistance report, inform the treating doctor, and recall the patient and for nursing staff to implement the doctor's new treatment regimen. The usual regimen prescribed after notification of INH-R results was rifampicin, pyrazinamide, and ethambutol (Table 3). Three patients with INH-R disease had follow-up sputum cultures performed at two months; all three had converted their culture to negative.
Table 3.
Isoniazid resistance among outpatients with pulmonary tuberculosis.
| Previous TB | Change in therapy after INH-R result | Time elapsed between treatment start & change of regimen | Second line regimen | Recommended duration of second line regimen | Outcome | |
|---|---|---|---|---|---|---|
| Patient 1 | No | Yes | 28 weeks | RZE | 6 | Cured |
| Patient 2 | No | Yes | 15 weeks | RZE | 9 | Defaulted but already smear negative and received 6 months' treatment in total |
| Patient 3 | No | Yes | 8 weeks | RZE | 4.5 | Transferred |
| Patient 4 | No | Yes | 12 weeks | RZE | 9 | Cured |
| Patient 5 | No | Yes | 11 weeks | RZE | 6 | Cured |
| Patient 6 | No | Yes | 16 weeks | RZE | 6 | Cured |
| Patient 7 | No | Yes | 8 weeks | RZE | 6 | Cured |
| Median | 12 Weeks |
3.4. Hospital Patients
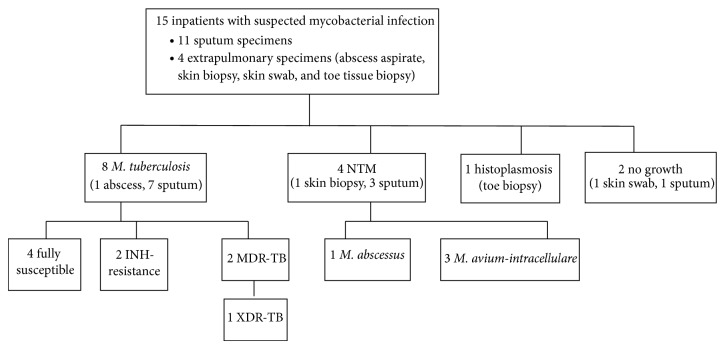
Fifteen additional samples were submitted for culture from hospital inpatients with suspected pulmonary TB (10 patients), or suspected extrapulmonary TB (five patients). Of those with suspected pulmonary TB, three had prior TB treatment failure, one had defaulted, three had unknown past treatment history, and three had underlying lung disease.
Findings from hospital inpatients are shown in Figure 2. Two cases of pulmonary MDR-TB, including one case of XDR-TB, were identified, both in heavily pretreated people. The XDR-TB case is the subject of a separate case report [29]. Two instances of INH-R were also identified, one monoresistant (in a pretreated patient) and one combined with streptomycin resistance (in a TB treatment-naïve health care worker). Regarding NTM, these were isolated in specimens obtained from four of the hospitalised individuals and identified by DNA probe and HPLC as M. abscessus or M. avium-intracellulare complex (from sputum) and M. avium-intracellulare complex (from a skin biopsy).
Figure 2.
Hospital inpatients with suspected mycobacterial infection.
4. Discussion
We have found low rates of drug resistance among outpatient cases of tuberculosis in Sabah, Borneo, but occasional cases of MDR-TB in pretreated patients in the inpatient setting. The rate of isoniazid resistance (4.5%) is, reassuringly, at the lower end of what is reported globally [12–14]. Thus, while the TB burden is high in eastern Malaysia, our study illustrates that this appears to be predominantly drug-susceptible disease. While the uncommon finding of MDR-TB reflects well on the local TB control program, high ongoing rates of drug-susceptible disease in relatively young people (median age 30 years) suggest that community transmission of TB is an important area requiring targeted intervention.
The MDR-TB rates we have found are low compared with Malaysia's immediate neighbours, where rates have been estimated as follows in new and retreatment cases, respectively: Singapore 0.1% new and 2.9% retreatment, Thailand 1.7% and 34.5%, Indonesia 2% and 14.7%, and Philippines 4.0% and 20.9% [30]. There were few retreatment cases in our series (13 outpatients and 3 hospitalised patients) to provide an accurate assessment among these patients, but the two cases of MDR-TB both occurred in pretreated individuals. These low rates also compare favourably with other member states of the WHO Western Pacific Region; MDR-TB rates in Papua New Guinea, for instance, have been estimated to be at least 26% of cases [31].
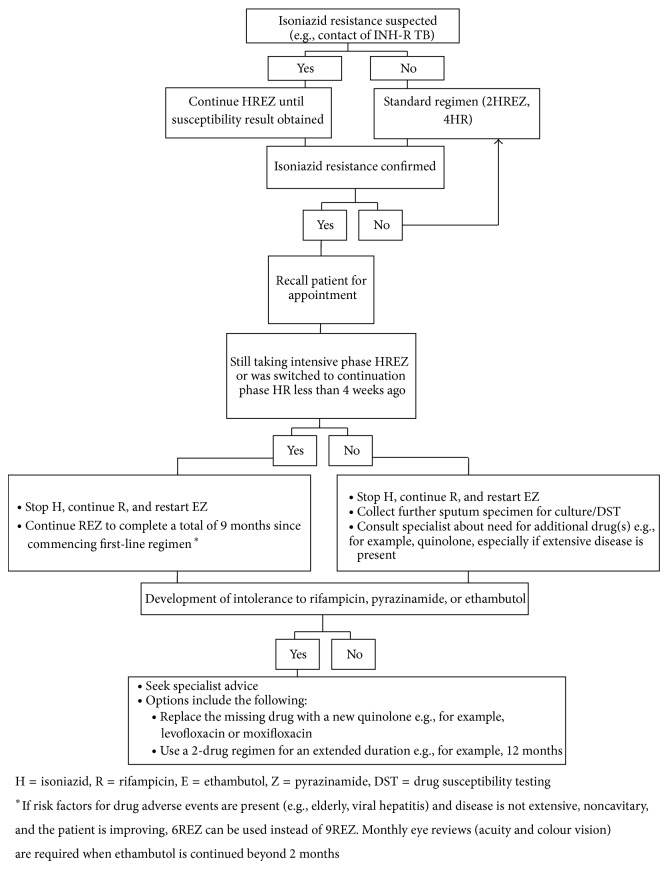
We identified substantial challenges in managing INH-resistance, particularly in the transition from protocol-based standardised care to tailored care. Despite Malaysian guidelines now recommending baseline cultures in all cases of suspected TB, routine susceptibility results are generally unavailable; usual practice in our setting is to undertake sputum mycobacterial cultures only for persistent smear-positivity after two to three months of treatment. Multifactorial delays occurred in implementing regimen changes, comprising the delay in specimens reaching the reference laboratory, the time taken to notify of the treating doctor, patient recall, and dispensing of the new regimen. A specific challenge in this context was that susceptibility results were frequently reported after completion of intensive phase treatment, when the routine switch to HR had already been made. Arising from these findings, we therefore developed an algorithm for managing this situation (Figure 3), based on the evidence summarised in Table 1. Such an algorithm does not replace clinical judgement guided by individual patient needs but provides guidance for nonspecialists in the local health centre settings where most outpatient TB is managed.
Figure 3.
Algorithm for managing isoniazid resistance.
NTM was detected in 7 of 191 people (outpatients and inpatients) with suspected TB. This finding illustrates the problem of NTM colonisation or infection being mistaken for TB, especially where smear microscopy is the diagnostic mainstay. NTM infections have been noted to cause a substantial burden of disease in our clinical practice and elsewhere in Malaysia [32]. Rising rates have also been reported elsewhere in Asia [33, 34]. This disease has been relatively neglected compared with TB due to lower incidence and lack of associated public health risk, yet diagnosis and management can be far more challenging than TB and outcomes poor. More research is required in Sabah to better understand and optimise management of local NTM infections. Greater local access to mycobacterial culture is needed to aid clinicians in distinguishing NTM from TB, especially in patients in whom NTM is more likely, that is, those with underlying chronic lung disease.
A limitation of the study is that findings from the outpatient setting cannot be extrapolated to all TB cases in Sabah and are not representative of the inpatient setting; we have presented results from the selected group of hospital patients to illustrate this, but more data are required on inpatient drug resistance rates, as well as treatment outcomes.
In conclusion, current rates of MDR-TB in the community in Sabah are low, reflecting well on local TB case management, but INH-resistance poses challenges. This must be addressed to avoid additional stepwise acquired resistance. Improvement in local resourcing to ensure that diagnostics such as liquid mycobacterial culture or molecular tests are available in a timely, clinically useful fashion would be a major advance in TB management in Sabah, which has Malaysia's highest TB burden. In the current scenario of delayed access to susceptibility results, our proposed management algorithm can provide guidance here and in settings facing similar issues globally.
Acknowledgments
The authors are very grateful to Kelly Nestor, Ann Wee, Thecla Tasius, Florena Maidik, and Sharon Nestor for enrolling the participants; all the staff at Luyang Outpatient Clinic particularly Dr. Eman Farhad, Dr. Maryam Amaleena, Suzie Taylor, Jenny Omar, and Dr. Elissa Soh; also Justine Peh Woei Ling at the Singapore General Hospital Mycobacteriology Laboratory; Ms. Dawn Carmel Paul for supporting the project; and Dr. Abdul Razak Mutalif (Institute of Respiratory Medicine and Head of Respiratory Services, Ministry of Health, Malaysia) for reviewing and endorsing the paper. This study was funded by the National Health and Medical Research Council (NHMRC) of Australia (Grant 605806). Anna Ralph is supported by NHMRC fellowship 1016567.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Rundi C. Understanding tuberculosis: perspectives and experiences of the people of Sabah, East Malaysia. Journal of Health, Population and Nutrition. 2010;28(2):114–123. doi: 10.3329/jhpn.v28i2.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dony J. F., Ahmad J., Tiong Y. K. Epidemiology of tuberculosis and leprosy, Sabah, Malaysia. Tuberculosis. 2004;84(1-2):8–18. doi: 10.1016/j.tube.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Tuberculosis Report 2014. 2014. (WHO Library Cataloguing-in-Publication Data, WHO/HTM/TB/2014.08). [Google Scholar]
- 4.Ministry of Health Malaysia. Management of Tuberculosis. Clinical Practice Guidelines. 3rd. MOH/P/PAK/258.12(GU); 2012. [Google Scholar]
- 5.Paul D. C., Chew A., Yau N. L., et al. Multidrug resistant Mycobacterium tuberculosis complex: a laboratory perspective. Proceedings of the 7th Public Health Colloquium; November 2012; Kota Kinabalu, Malaysia. Sabah State Health Department; [Google Scholar]
- 6.Jalleh R. D., Kuppusamy I., Soshila R., Aziah A. M., Faridza M. Y. A study of primary drug resistance in pulmonary tuberculosis in West Malaysia 1984–1987. Medical Journal of Malaysia. 1993;48(2):113–116. [PubMed] [Google Scholar]
- 7.Hamzah Z., Min L. J., Ali I. A., et al. Drug resistance pattern of Mycobacterium tuberculosis complex isolated for Perak State (2006–2008). Proceedings of the 1st AMDI International Biohealth Science Conference: Infectious Diseases—Current Challenges (IBSC '10); November-December 2010; Penang, Malaysia. Ipoh Public Health Laboratory Data, Ministry of Health; p. p. 172. [Google Scholar]
- 8.Espinal M. A., Kim S. J., Suarez P. G., et al. Standard short-course chemotherapy drug-resistant tuberculosis: treatment outcomes in 6 countries. The Journal of the American Medical Association. 2000;283(19):2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 9.Farah M. G., Tverdal A., Steen T. W., Heldal E., Brantsaeter A. B., Bjune G. Treatment outcome of new culture positive pulmonary tuberculosis in Norway. BMC Public Health. 2005;5(article 14) doi: 10.1186/1471-2458-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gegia M., Cohen T., Kalandadze I., Vashakidze L., Furin J. Outcomes among tuberculosis patients with isoniazid resistance in Georgia, 2007–2009. International Journal of Tuberculosis and Lung Disease. 2012;16(6):812–816. doi: 10.5588/ijtld.11.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menzies D., Benedetti A., Paydar A., et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Medicine. 2009;6(9) doi: 10.1371/journal.pmed.1000150.e1000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoopes A. J., Kammerer J. S., Harrington T. A., Ijaz K., Armstrong L. R. Isoniazid-monoresistant tuberculosis in the United States, 1993 to 2003. Archives of Internal Medicine. 2008;168(18):1984–1992. doi: 10.1001/archinte.168.18.1984. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. The WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance, 2002–2007: WHO/HTM/TB/2008.394. World Health Organization; 2008. Anti-tuberculosis drug resistance in the world fourth global report. [Google Scholar]
- 14.Pablos-Méndez A., Raviglione M. C., Laszlo A., et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. World Health Organization—International Union against tuberculosis and lung disease working group on anti-tuberculosis drug resistance surveillance. The New England Journal of Medicine. 1998;338(23):1641–1649. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 15.Fox W., Ellard G. A., Mitchison D. A. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council Tuberculosis Units, 1946–1986, with relevant subsequent publications. International Journal of Tuberculosis and Lung Disease. 1999;3:S231–S279. [PubMed] [Google Scholar]
- 16.Nolan C. M., Goldberg S. V. Treatment of isoniazid-resistant tuberculosis with isoniazid, rifampin, ethambutol, and pyrazinamide for 6 months. International Journal of Tuberculosis and Lung Disease. 2002;6(11):952–958. [PubMed] [Google Scholar]
- 17.World Health Organisation. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: Emergency Update. Geneva, Switzerland: WHO; 2008. (WHO/HTM/TB/2008.402). [Google Scholar]
- 18.Blumberg H. M., Burman W. J., Chaisson R. E., et al. American thoracic society/centers for disease control and prevention/infectious diseases society of America: treatment of tuberculosis. The American Journal of Respiratory and Critical Care Medicine. 2003;167(4):603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 19.Centre for Disease Control. Darwin Guidelines for the Control of Tuberculosis in the Northern Territory. Northern Territory Government Department of Health and Community Services; 2002. http://health.nt.gov.au/library/scripts/objectifyMedia.aspx?file=pdf/25/05.pdf&siteID=1&str_title=Tuberculosis. [Google Scholar]
- 20.Pinto L., Menzies D. Treatment of drug-resistant tuberculosis. Infection and Drug Resistance. 2011;4(1):129–135. doi: 10.2147/IDR.S10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reves R., Heilig C. M., Tapy J. M., et al. Intermittent tuberculosis treatment for patients with isoniazid intolerance or drug resistance. International Journal of Tuberculosis and Lung Disease. 2014;18(5):571–580. doi: 10.5588/ijtld.13.0304. [DOI] [PubMed] [Google Scholar]
- 22.Cattamanchi A., Dantes R. B., Metcalfe J. Z., et al. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clinical Infectious Diseases. 2009;48(2):179–185. doi: 10.1086/595689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munsiff S. S., Nilsen D., Fujiwara P. I. Tuberculosis Clinical Policies and Protocols. 4th. New York, NY, USA: Department of Health and Mental Hygiene, Bureau of Tuberculosis Control; 2008. [Google Scholar]
- 24.Chang K. C., Leung C. C., Grosset J., Yew W. W. Treatment of tuberculosis and optimal dosing schedules. Thorax. 2011;66(11):997–1007. doi: 10.1136/thx.2010.148585. [DOI] [PubMed] [Google Scholar]
- 25.Chang K. C., Leung C. C., Yew W. W., Chan S. L., Tam C. M. Dosing schedules of 6-month regimens and relapse for pulmonary tuberculosis. The American Journal of Respiratory and Critical Care Medicine. 2006;174(10):1153–1158. doi: 10.1164/rccm.200605-637oc. [DOI] [PubMed] [Google Scholar]
- 26.Hadorn D. C., Baker D., Hodges J. S., Hicks N. Rating the quality of evidence for clinical practice guidelines. Journal of Clinical Epidemiology. 1996;49(7):749–754. doi: 10.1016/0895-4356(96)00019-4. [DOI] [PubMed] [Google Scholar]
- 27.Rashid Ali M. R. S., William T., Parameswaran U., et al. Mycobacterial viability: a prospective study in pulmonary tuberculosis in Malaysian Borneo using refrigerated, unpreserved sputum batched for up to 8 weeks. International Journal of Tuberculosis and Lung Disease. 2015;19(5) doi: 10.5588/ijtld.14.0938. [DOI] [PubMed] [Google Scholar]
- 28.William T., Parameswaran U., Lee W. K., Yeo T. W., Anstey N. M., Ralph A. P. Pulmonary tuberculosis in outpatients in Sabah, Malaysia: advanced disease but low incidence of HIV co-infection. BMC Infectious Diseases. 2015;15(1, article 32) doi: 10.1186/s12879-015-0758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashid Ali M. R. S., Ralph A. P., William T. Individualized second line antituberculous therapy for an extensively resistant pulmonary tuberculosis (XDR PTB) case in east Malaysia. Malaysian Medical Journal, Accepted. [PubMed] [Google Scholar]
- 30.World Health Organization. Multidrug and Extensively Drug-Resistant TB (M/XDR-TB) 2010 Global Report on Surveillance and Response. 2010. (WHO Library Cataloguing-in-Publication Data, WHO/HTM/TB/2010.3). [Google Scholar]
- 31.Simpson G., Coulter C., Weston J., et al. Resistance patterns of multidrug-resistant tuberculosis in Western Province, Papua New Guinea. International Journal of Tuberculosis and Lung Disease. 2011;15(4):551–552. doi: 10.5588/ijtld.10.0347. [DOI] [PubMed] [Google Scholar]
- 32.Kasthoori J. J., Liam C. K., Wastie M. L. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Medical Journal. 2008;49(2):e47–e49. [PubMed] [Google Scholar]
- 33.Park Y. S., Lee C. H., Lee S. M., et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. International Journal of Tuberculosis and Lung Disease. 2010;14(8):1069–1071. [PubMed] [Google Scholar]
- 34.Chen C.-Y., Chen H.-Y., Chou C.-H., Huang C.-T., Lai C.-C., Hsueh P.-R. Pulmonary infection caused by nontuberculous mycobacteria in a medical center in Taiwan, 2005–2008. Diagnostic Microbiology and Infectious Disease. 2012;72(1):47–51. doi: 10.1016/j.diagmicrobio.2011.09.009. [DOI] [PubMed] [Google Scholar]