Abstract
Localized malignant pleural mesothelioma (LMM) is a rare subset of malignant pleural mesothelioma. Its epidemiology, biology, natural history and optimal treatment are poorly understood. We report a case of LMM treated aggressively with complete surgical resection and adjuvant radiotherapy, but subsequently complicated by local chest wall recurrence and solitary metastasis to the kidney. This case is examined in the context of a small number of cases of LMM in the literature to emphasize the existence of this rare disease entity, their unusual biological behaviour and the need for further tumour molecular and genomic research.
INTRODUCTION
Localized malignant pleural mesothelioma (LMM) is a rare subset of malignant pleura mesothelioma (MPM). While sharing the same histopathological features as diffuse MPM [1], these tumours are focal and well circumscribed rather than progressively encasing the lung. Progress towards understanding the natural history of LMM has been slow due to its rarity and the historical problems defining its origin and nomenclature [2, 3]. Despite LMM being classified as a separate disease entity in the 2004 World Health Organization (WHO) classification [4], little is known about its natural history and optimal treatment. We present a case of LMM with unusual biological behaviour.
CASE REPORT
A 67-year-old Caucasian male, smoker of 50 pack-years with a history of asbestos exposure 50 years prior, presented with left-sided chest pain preceded by 6 months of recurrent pneumonia, which resolved after antibiotic therapy. Chest radiograph and computed tomography (CT) revealed a new 40-mm left upper lobe lesion abutting the pleura. Fine-needle aspiration biopsy showed atypical cells, suspicious for large cell carcinoma. Staging positron emission tomography (PET) and magnetic resonance imaging (MRI) of the brain showed no regional or distant metastases. He was recommended to undergo surgical resection, but declined and opted for alternative therapy.
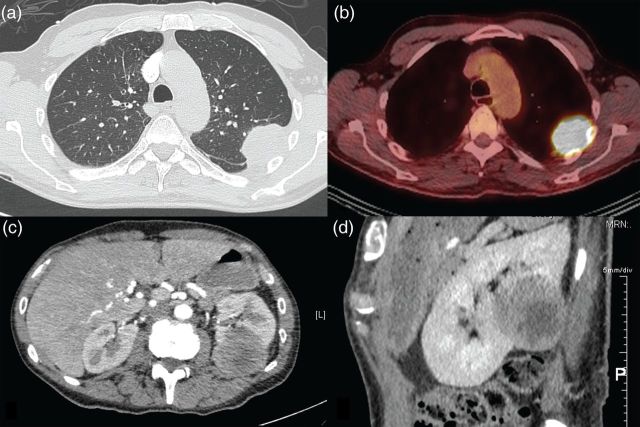
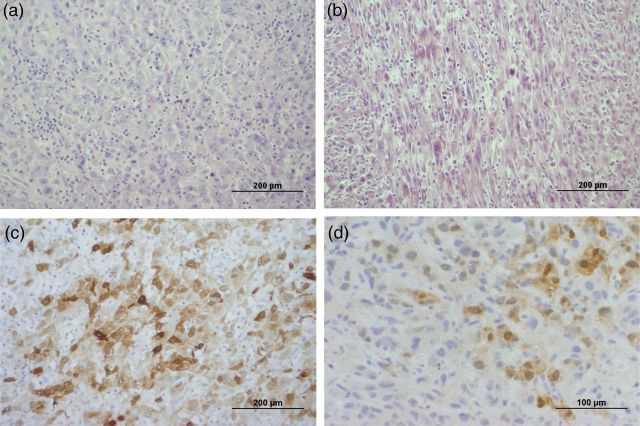
He re-presented 3 months later with weight loss and progressive chest wall pain. CT showed increased tumour size of 80 mm with extension into the left chest wall and an associated pathological rib fracture (Fig. 1a). Restaging PET, MRI brain and endobronchial ultrasound-guided biopsy of mediastinal lymph nodes were negative for metastatic disease (Fig. 1b). He proceeded to a left upper lobectomy, mediastinal lymph node dissection, chest wall resection and reconstruction. Histopathology demonstrated complete excision of a 78-mm Stage III (T3N0M0) poorly differentiate biphasic mesothelioma, comprising pleomorphic epithelioid and spindled (sarcomatoid) cells in approximately equal proportions (Fig. 2a and b). The tumour was positive for calretinin (Fig. 2c) and Wilms' Tumour 1, and negative for cytokeratin 5/6. The tumour had invaded the visceral and parietal pleura as well as the chest wall. The surgical margins and sampled lymph nodes were uninvolved. He received postoperative adjuvant radiotherapy using 60 Gy in 30 fractions external beam to the tumour bed and left posterior chest wall. He declined adjuvant chemotherapy.
Figure 1:
(a) CT chest showing pleural-based left upper lobe lung mass, (b) PET showing hypermetabolic pleural-based left upper lobe lung mass, (c, d) CT abdomen showing left renal metastasis (c) axial view and (d) sagittal view.
Figure 2:
(a, b) Haematoxylin and eosin stain showing (a) epithelioid and (b) sarcomatoid component of resected pleural mesothelioma. (c, d) Calretinin stain of (c) resected pleural mesothelioma and (d) renal metastasis.
Five months later he presented with pneumonia, and a CT scan incidentally found a new 47-mm isolated left renal cortical lesion with no evidence of thoracic local recurrence or other metastases (Fig. 1c and d). Renal biopsy confirmed metastatic sarcomatoid mesothelioma, morphologically identical to the sarcomatoid component of the primary pleural mesothelioma with positive staining for calretinin (Fig. 2d) and Wilms' Tumour 1, and negative staining for cytokeratin 5/6. He received two cycles of carboplatin and pemetrexed palliative chemotherapy, which he tolerated poorly due to myelosuppression. Two months later, he re-presented with respiratory sepsis. CT showed recurrence of LMM at the left pleura and extension of the renal metastasis into the posterior ribs.
The patient functionally declined with complex chest wall pain and delirium. He was palliated and died at home, 14 months after primary diagnosis and 4 months after diagnosis of metastatic disease.
DISCUSSION
While the term localized mesothelioma has been used historically to describe a variety of tumours including solitary fibrous tumours, the first true case series of LMM was published by Crotty et al. [1] in 1994. After clarification of its origin and nomenclature, LMM was defined as a distinct disease entity among pleural tumours by the WHO in 2004 [4]. LMM are rare tumours, and one surgical series from the UK reported 10 cases among 218 cases of MPM over 8 years [5]. In the largest published series of 23 cases of LMM from the United States-Canadian Mesothelioma Reference Panel, the mean and the median age of diagnosis were 62 and 64 years, respectively [3]. Similar to diffuse MPM, LMM is more commonly diagnosed in men, and the most commonly reported histological subtype is epithelioid, although sarcomatoid and biphasic LMM have also been reported as in this case [3, 5–7]. However, unlike diffuse MPM, the association between asbestos exposure and LMM remains unclear with documented exposure in 3 of 6, 4 of 23 and 9 of 10 cases in three separate series [1, 3, 5].
The available literature suggests that the natural history of LMM varies but may involve an initial presentation of locally invasive disease followed by haematogenous metastases [1, 6]. Most reported cases underwent surgical resection with varying uses of adjuvant radiotherapy and chemotherapy [6]. Disease recurrence after surgical resection can be in the local chest wall as well as a variety of distant sites including lung parenchyma, mediastinal and abdominal lymph nodes, stomach, small bowel, axial skeleton, skin and brain [1–3, 5, 8, 9]. Our case is the first report of LMM with renal metastasis. While surgical resection for early stage disease has been advocated, the uses of radiotherapy and chemotherapy are poorly described and their roles undefined, with only one case report describing the use of specific chemotherapeutic agents, cisplatin and pemetrexed [5, 6, 9].
A pooled analysis of available data showed that LMM has an overall better prognosis than diffuse MPM, with a median survival of 29 months in LMM compared with 8–14 months in diffuse MPM [7]. While a small number of cases of LMM may be curable by surgical resection, the survival of patients with metastatic LMM varies between 6 months and 3 years [1, 3, 8, 9]. However, LMM remains difficult to prognosticate using clinical and pathological parameters. TNM staging does not appear to accurately describe prognosis, as patients with local disease (T1-2N0, Stage I–II) have a median survival of 4.8 years, whereas those with locally advanced disease extending into the chest wall (T3-4, Stage III–IV) have a mean survival of 4.7 years [3, 5]. Furthermore, no prior study has found a correlation between histological subtype or tumour size and survival [10].
In conclusion, LMM is a rare and poorly understood disease entity. To our knowledge, this is the first case of LMM with renal metastasis in the literature, highlighting an unpredictable course of disease and the need for further research. Multicentre collaboration in tumour molecular and genomic studies may help improve understanding of the biology and optimal treatment of this disease entity.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Crotty TB, Myers JL, Katzenstein AL, Tazelaar HD, Swensen SJ, Churg A. Localized malignant mesothelioma: a clinicopathologic and flow cytometric study. Am J Surg Pathol. 1994;18:357–63. [PubMed] [Google Scholar]
- 2.Tanzi S, Tiseo M, Internullo E, Cacciani G, Capra R, Carbognani P, et al. Localized malignant pleural mesothelioma: report of two cases. J Thorac Oncol. 2009;4:1038–40. doi: 10.1097/JTO.0b013e3181a8c874. [DOI] [PubMed] [Google Scholar]
- 3.Allen TC, Cagle PT, Churg AM, Colby TV, Gibbs AR, Hammar SP, et al. Localized malignant mesothelioma. Am J Surg Pathol. 2005;29:866–73. doi: 10.1097/01.pas.0000165529.78945.dc. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Müller-Hermelink H, Harris CC. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. [Google Scholar]
- 5.Nakas A, Martin-Ucar AE, Edwards JG, Waller DA. Localised malignant pleural mesothelioma: a separate clinical entity requiring aggressive local surgery. Eur J Cardiothorac Surg. 2008;33:303–6. doi: 10.1016/j.ejcts.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 6.Gelvez-Zapata SM, Gaffney D, Scarci M, Coonar AS. What is the survival after surgery for localized malignant pleural mesothelioma? Interact CardioVasc Thorac Surg. 2013;16:533–7. doi: 10.1093/icvts/ivs542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyland RA, Ware S, Johnson AR, Yates DH. Incidence trends and gender differences in malignant mesothelioma in New South Wales, Australia. Scan J Work Environ Health. 2007;33:286–92. doi: 10.5271/sjweh.1145. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Cheng Y-J, Chen H-P, Hwang J-C, Chang P-C. Multiple bowel intussusceptions from metastatic localized malignant pleural mesothelioma: a case report. World J Gastroenterol. 2010;16:3984. doi: 10.3748/wjg.v16.i31.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi H, Notohara K, Yoshioka H, Matsuoka T, Ikeda H, Kagawa K, et al. Localized malignant pleural mesothelioma showing a thoracic mass and metastasizing to the stomach. Inter Med. 2009;49:671–75. doi: 10.2169/internalmedicine.49.2592. [DOI] [PubMed] [Google Scholar]
- 10.Ismail-Khan R, Robinson LA, Williams CC, Garrett CR, Bepler G, Simon GR. Malignant pleural mesothelioma: a comprehensive review. Chest. 2006;13:255. doi: 10.1177/107327480601300402. [DOI] [PubMed] [Google Scholar]