Abstract
We describe a case of a patient with recurrent syncopal episodes that ultimately was discovered to be due to ictal bradycardia caused by temporal lobe epilepsy. A diagnostic dilemma was presented by a 55-year-old male who had recurrent syncopal events despite having an atrial pacemaker. The patient was noted to have automatisms and was diagnosed via electrocardiogram/electroencephalogram (EEG/ECG) co-registration to have ictal bradycardia and atrioventricular (AV) block leading to syncope. He was successfully managed with seizure control with the use of levetiracetam. Ictal bradycardia and AV block are uncommon manifestations of epilepsy and can progress to complete heart block and asystole. Diagnosis is best performed with simultaneous ECG and EEG recordings. Definitive management is seizure control with the use of antiepileptic drugs, with the question of pacemaker placement still up for debate.
INTRODUCTION
Epilepsy is the most common serious neurologic disease seen in 3% of the world's population. Sudden unexpected death in epilepsy (SUDEP) accounts for 8–17% of the deaths in epilepsy patients [1]. Autonomic alterations during seizures are associated with a variety of cardiac manifestations that have been postulated to be some of the underlying mechanisms for SUDEP. These manifestations include change in heart rate variability, ictal tachycardias, ictal bradycardia, atrioventricular (AV) block and asystole. Ictal bradycardia is an uncommon presentation and peri-ictal AV conduction block has been rarely reported [2]. Here we describe a patient with recurrent syncopal episodes, diagnosed to have ictal bradycardia and peri-ictal AV block via electrocardiogram/electroencephalogram (EEG/ECG) co-registration.
CASE REPORT
A 55-year-old right-handed male presented to the emergency department after a syncopal episode. He was teaching a karate class when a coworker saw him fall to the ground. He spontaneously regained consciousness after a few seconds but was confused for ∼15–20 min. He had no recollection of the event, and was hemodynamically stable and neurologically intact. He denied illicit drug use. Vitals, physical examination and laboratory studies were within normal limits. ECG showed normal sinus rhythm with normal PR and QT intervals and no ST-T wave changes. He was on no AV blocking agents. Head CT showed no intracranial bleed or mass lesion.
He had a history of five similar episodes in the past. During the last episode, he had been noted to have sinus arrest on telemetry and underwent single atrial lead pacemaker placement. The pacemaker was programmed to AAI mode. AV conduction was tested and was normal. Coronary angiogram showed no evidence of coronary artery disease. EEG had been minimally abnormal with left temporal theta slowing, suggestive of an area of neurophysiologic dysfunction, but no epileptiform activity was noted. He had no history of febrile seizures, central nervous system infection or cranial trauma.
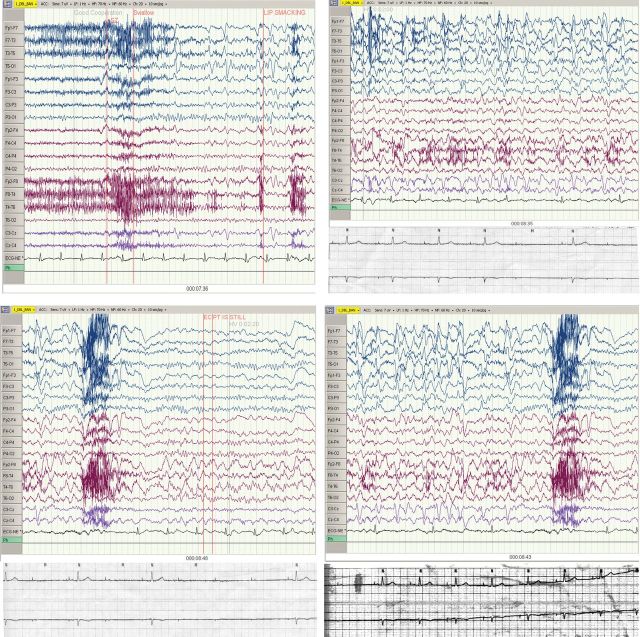
The patient was admitted for further work up. Continuous EEG monitoring was ordered to evaluate for seizure activity. At ∼7 min and 36 s, the patient started to have recurrent automatisms and became unresponsive, with the EEG showing complex partial seizure activity emanating from the left anterior temporal region with continued post-ictal right temporal delta slowing. The seizure lasted ∼1 min. The simultaneous ECG lead demonstrated heart rate slowing toward the end of the seizure episode, with the rhythm changing from sinus to atrially paced at 60 beats min−1 with 1:1 conduction, to atrially paced with 2:1 AV block, followed by complete heart block for 4 s, followed by resumption of the baseline atrially paced rhythm with 1:1 conduction (Fig. 1).
Figure 1:
Top left: Baseline. At 7 min and 36 s into the EEG, there are epileptiform discharges at the end of the strip, and the patient demonstrates lip smacking. There are no changes in the ECG activity at this time, and the patient is in normal sinus rhythm at a rate of 76 beats min−1. Top right: 8 min and 35 s into the EEG. EEG is demonstrating seizure activity. Telemetry strip reveals an atrial paced rhythm at the start, indicating either overdrive suppression of the sinus node or sinus nodal arrest. The atrial paced rhythm with 1:1 AV conduction then changes to an atrial paced rhythm with 2:1 AV block. The bottom panel illustrates this better on a corresponding telemetry strip. Bottom left: 8 min and 43 s into the EEG. 2:1 AV block now progresses to onset of complete heart block. The telemetry strip in the bottom panel illustrates this better, showing changing of 2:1 AV conduction to complete heart block without any escape rhythm for 4 s. Bottom right: 8 min and 48 s into the EEG with cardiac activity returning to normal sinus rhythm. The bottom panel shows the final section of telemetry showing complete heart block reverting back to an atrial paced rhythm with 1:1 AV conduction.
Cardiac electrophysiology recommended treating the seizure episodes before considering upgrade of the pacemaker to a dual-chamber device. The patient was started and subsequently discharged on levetiracetam. More than 15 months later, he has had no seizures or syncopal events.
DISCUSSION
Cardiac arrhythmias are frequently associated with epileptiform seizures. The most frequent cardiac arrhythmia seen during epileptic seizures is sinus tachycardia that occurs in >90% of seizures and is usually of no consequence. Patients with bradycardia and asystole during an epileptic seizure are defined to have ictal bradycardia syndrome [3] which could potentially also include AV block. Ictal bradycardia is considered a rare event that affects <5% of epilepsy patients, while associated asystolic episodes occur less frequently (0.3–0.4%) [4]. However, the incidence of the syndrome may actually be underestimated. Rugg-Gunn et al. [5] demonstrated a much higher incidence of ictal bradycardia (35%) and ictal asystole (16%) by using implantable loop recorders in 19 patients with refractory partial seizures.
Ictal bradycardia and bradyarrhythmia are thought to be caused by an increase in parasympathetic activity or disruption of sympathetic activity resulting from propagative ictal activity in the respective autonomic cortical or subcortical networks [1]. Epileptic stimulation may also affect the heart by direct neural pathways. Cardiac rhythm analysis in our case reveals a progressive decrease in heart rate from normal sinus rhythm to an atrial paced rhythm with subsequent development of AV block. This pattern is highly suggestive of increased vagal activation.
Ictal bradycardia syndrome is reported in association with complex partial seizures involving the temporal lobe, and secondarily the frontal lobe with direct or indirect participation from the insular cortex [6]. Some studies have shown that left hemispheric stimulation leads to bradycardia and right stimulation leads to tachycardia. However, more recent studies have demonstrated a less evident lateralization of cardiovascular effects and seizures may increase or decrease heart rate independent of the side of the epileptiform focus [4].
The gold standard for diagnosis of ictal bradyarrhythmias is simultaneous ECG and video EEG documentation of bradyarrhythmia during an ictal discharge. Ictal bradyarrhythmia usually starts 10–30 s after the seizure onset and apparently after the seizure discharges become bilateral [6]. This feature could help in differentiating epilepsy-induced bradycardia/asystole from convulsive syncope caused by a circulatory disturbance leading to cerebral hypoperfusion. Convulsive syncope always precedes the EEG abnormalities in the EEG/ECG co-registration. The EEG slowing and flattening in convulsive syncope appears after a few seconds and no evidence of ictal EEG activity is found [7].
The undesirable cardiac effects of epilepsy can best be avoided by complete seizure control. However, antiepileptic therapy may also alter autonomic function or produce proarrhythmic effects. Cardiac monitoring using Holter monitor, event monitor and loop recorders is suggested in high-risk epilepsy patients to monitor and treat potential cardiac arrhythmias. However, there are currently no guidelines on who should undergo further cardiovascular investigations. Since antiepileptic drugs may not control seizures in ∼37% of patients with epilepsy [8], some advocate dual-chamber pacemaker placement to prevent bradyarrhythmias and potentially SUDEP. Seizure-related falls, fractures and motor vehicle accidents were shown to be reduced following pacemaker implantation in patients with ictal asystole [9]. However, the effect of pacemaker implantation on mortality in this population has not yet been determined and its role in patients with ictal bradyarrhythmias is still controversial [10]. The patient in our case was started on levetiracetam and has had no recurrent events after more than 15 months into the treatment.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Velagapudi P, Turagam M, Laurence T, Kocheril A. Cardiac arrhythmias and sudden unexpected death in epilepsy (SUDEP) Pacing Clin Electrophysiol. 2012;35:363–70. doi: 10.1111/j.1540-8159.2011.03276.x. [DOI] [PubMed] [Google Scholar]
- 2.Surges R, Scott CA, Walker MC. Peri-ictal atrioventricular conduction block in a patient with a lesion in the left insula: case report and review of the literature. Epilepsy Behav. 2009;16:347–9. doi: 10.1016/j.yebeh.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 3.Reeves AL, Nollet KE, Klass DW, Sharbrough FW, So EL. The ictal bradycardia syndrome. Epilepsia. 1996;37:983–7. doi: 10.1111/j.1528-1157.1996.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 4.Serafini A, Gelisse P, Reana V, Crespel A. Cardiac asystole during a cluster of right temporo-parietal seizures. Seizure. 2011;20:181–3. doi: 10.1016/j.seizure.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Rugg-Gunn FJ, Simister RJ, Squirrell M, Holdright DR, Duncan JS. Cardiac arrhythmias in focal epilepsy: a prospective long-term study. Lancet. 2004;364:2212–9. doi: 10.1016/S0140-6736(04)17594-6. [DOI] [PubMed] [Google Scholar]
- 6.Britton JW, Ghearing GR, Benarroch EE, Cascino GD. The ictal bradycardia syndrome: localization and lateralization. Epilepsia. 2006;47:737–44. doi: 10.1111/j.1528-1167.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 7.Rocamora R, Kurthen M, Lickfett L, Von Oertzen J, Elger CE. Cardiac asystole in epilepsy: clinical and neurophysiologic features. Epilepsia. 2003;44:179–85. doi: 10.1046/j.1528-1157.2003.15101.x. [DOI] [PubMed] [Google Scholar]
- 8.Zarraga IG, Ware DL. Syncope, seizure, or both? An unusual case of complete heart block. J Electrocardiol. 2007;40:493–5. doi: 10.1016/j.jelectrocard.2007.03.244. [DOI] [PubMed] [Google Scholar]
- 9.Moseley BD, Ghearing GR, Munger TM, Britton JW. The treatment of ictal asystole with cardiac pacing. Epilepsia. 2011;52:e16–9. doi: 10.1111/j.1528-1167.2010.02972.x. [DOI] [PubMed] [Google Scholar]
- 10.Kasim S, Hennesey M, Crowley J. Persistent fainting after implantation of a ‘Curative’ pacemaker. N Engl J Med. 2009;360:88–9. doi: 10.1056/NEJMc0807099. [DOI] [PubMed] [Google Scholar]