Abstract
Clinicians of all specialties need to be aware of a recent, nationwide increase in the number of Actinomyces bloodstream infections. We report a case of bimicrobial bloodstream infection with Actinomyces odontolyticus and Escherichia coli in an intravenous drug user. A 36-year-old, male intravenous drug user was admitted with acute-onset pleuritic chest pain, back pain, pyrexia, tachycardia, tachypnoea and hypotension. Chest CT showed multiple, bilateral, cavitating lung lesions, most likely the result of septic emboli originating from an infected deep venous thrombosis (DVT). Blood cultures led to a mixed growth of A. odontolyticus, identified by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF), and E. coli. The rising tide of bloodstream infections with Actinomyces species is likely to continue with the increasing availability of sophisticated molecular identification techniques, including MALDI-TOF. In this case, the results of antimicrobial susceptibility tests were particularly important because the E. coli was susceptible to ciprofloxacin, whereas the A. odontolyticus was resistant.
INTRODUCTION
Actinomyces species are associated with a wide range of infections, including dental caries, abscesses and bloodstream infections [1]. Worryingly, the number of bloodstream infections is on the increase, largely accounted for by a rise in reports of Actinomyces odontolyticus. In 2007, only a single case of bloodstream infection with A. odontolyticus was reported, compared with 12 cases in 2011 [2]. Actinomyces odontolyticus is frequently resistant to a number of commonly used antibiotics [3–6], so clinicians of all specialties need to be alert to the organism. We report a case of bimicrobial bloodstream infection with A. odontolyticus and Escherichia coli in an intravenous drug user.
CASE REPORT
In June 2014, a 36-year-old, male patient was admitted to the hospital by ambulance, complaining of acute-onset sharp, stabbing pleuritic chest pain and back pain. Observations showed a temperature of 38.2°C, heart rate 132 bpm, intermittent tachypnoea and hypotension, with a blood pressure of 96/41.
The patient's complex medical history included: intermittent intravenous drug abuse of 10 years' duration; recurrent femoral vein deep venous thrombotic (DVT) events (usually associated with significant cellulitis) and radiologically confirmed dental abscess in 2007. In August 2011, he was diagnosed with culture-negative discitis causing complete obliteration of the C6-7 interspace. He completed a total of 10 weeks' oral linezolid 600 mg BD and, by February 2012, he was asymptomatic apart from some residual neck stiffness. HIV and hepatitis C tests were negative and he was naturally immune to hepatitis B.
On admission, clinical examination revealed an actively discharging sinus of the patient's left groin. One week prior to admission, the patient had attempted to inject heroin at this site, but accidentally injected the drug into the surrounding tissues. Otherwise, no murmurs were audible on auscultation of the heart; no tenderness was elicited on palpation of the spine and there were no initial stigmata of endocarditis. Full blood count showed a while cell count (WCC) of 9.5 × 109 cells/l, haemoglobin 61 g/l and platelets 267 × 103 cells/l. Biochemical profile showed normal urea, electrolytes, liver function and a C-reactive protein (CRP) of 283 mg/l. Lactate was 0.9 mmol/l. Doppler ultrasound revealed a left ilio-femoral DVT. An initial chest X-ray was unremarkable. He was started on i.v. flucloxacillin 2 g four times a day and received a blood transfusion, denying any haematuria, haemoptysis or melaena.
Three days following admission, the patient remained pyrexial and his CRP had increased to 317 mg/l. At this stage, a positive blood culture set led to the isolation of Gram-negative bacilli after 1 days' incubation. Therefore, the patient's flucloxacillin was switched to i.v. co-amoxiclav 1.2 g three times a day (TDS). A repeat chest X-ray now showed multiple, new, bilateral, ill-defined nodules. CT chest/thorax/abdomen with contrast showed multiple, cavitating lesions throughout both lung fields, suspicious of septic emboli originating from an infected DVT. Transoesophageal echocardiogram showed a patent foramen ovale and a well-functioning trileaflet aortic valve. No vegetations were seen.
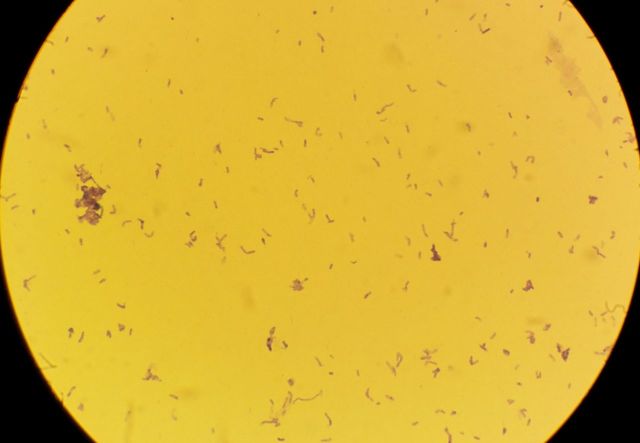
Four days following admission, subculture of the peripheral blood culture set led to the isolation of a lactose-fermenting coliform, identified by Vitek2 as E. coli, which was resistant to co-amoxiclav and susceptible to piperacillin–tazobactam, gentamicin, ciprofloxacin, cefotaxime and trimethoprim. Therefore, the patient's co-amoxiclav was switched to i.v. piperacillin–tazobactam 4.5 g TDS, which was administered via a newly inserted central line. Interestingly, a small colony-form Gram-positive bacillus was also isolated on subculture, which was non-spore-forming and non-branching but slightly curved (Fig. 1).
Figure 1:
Photomicrograph of Gram morphology of A. odontolyticus on subculture on chocolate agar, at 37°C, in CO2, at 24 h' incubation.
The Gram-positive bacillus exhibited fastidious growth requirements, growing slowly on blood and chocolate agar plates after 24 h' incubation in CO2 at 37°C (Fig. 2). The organism was catalase- and esculin-negative. Identification according to Vitek2 was Actinomyces meyeri (profile 6365101010005, excellent identification) and by API Coryne was Erysipelothrix rhusiopathiae (profile 2400040, %ID 96.9, T 0.31).
Figure 2:
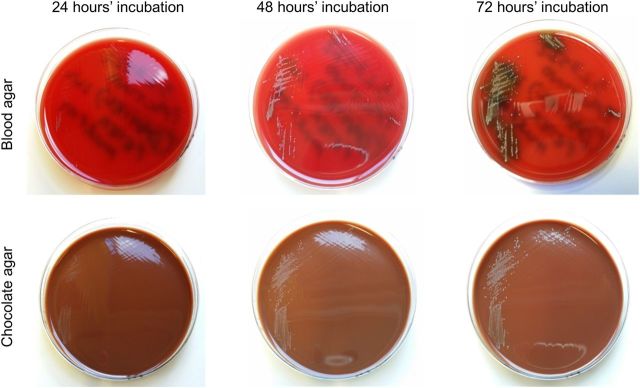
Photographs of growth achieved at 24, 48 and 72 h' incubation of A. odontolyticus, on blood and chocolate agar, at 37°C, in CO2.
The Gram-positive bacillus was sent to Public Health England's (PHE) Antimicrobial Resistance and Healthcare Associated Infections Reference Unit (AMRHAI). MALDI-TOF analysis identified the organism as A. odontolyticus (score 2.327, good identification to species level). Antimicrobial susceptibility test results, and interpretative breakpoints, are given in Table 1.
Table 1:
Minimal inhibitory concentrations (MICs) of selected antibiotics against A. odontolyticus, including interpretations and breakpoints, as reported by the AMRHAI reference unit, PHE Colindale
| Antibiotics | MIC | S/I/R | Breakpoint |
|---|---|---|---|
| Co-amoxiclav | 0.125 | S | 8 and 16 |
| Cefotaxime | 0.25 | S | 8 and 16 |
| Ceftriaxone | 0.25 | S | 8 and 32 |
| Imipenem | 0.064 | S | 2 and 4 |
| Co-trimoxazole | 0.125 | S | 2 |
| Clarithromycin | ≤0.016 | S | 2 and 4 |
| Linezolid | 0.25 | S | 4 |
| Ciprofloxacin | 4 | R | 1 and 2 |
| Moxifloxacin | 2 | I | 1 and 2 |
| Doxycycline | 0.064 | S | 1 and 4 |
| Minocycline | ≤0.016 | S | 1 and 4 |
Three weeks following admission, the patient's central line was removed and i.v. piperacillin–tazobactam was switched to a combination of oral linezolid 600 mg BD and oral ciprofloxacin 750 mg BD. No organisms were isolated from culture of the central line tip. At the time of discharge from hospital, CRP was 38 mg/l. His WCC, urea, electrolytes and bilirubin had been within normal limits throughout the entire admission. Four culture-negative blood cultures were collected on treatment. In addition, mycobacterial culture of three smear-negative sputum specimens was negative at 4 weeks' incubation.
DISCUSSION
Actinomyces odontolyticus was first isolated in 1958 from deep carious dentine [7]. The majority of cases with invasive disease have presented with pulmonary, cardiopulmonary or mediastinal pathology [8]. Infections are most frequently identified in patients with significant underlying risk factors for infection [8, 9]. This is the first reported case of bimicrobial bloodstream infection leading to the isolation of A. odontolyticus in combination with E. coli. The patient's multiple pulmonary abscesses were most likely the result of septic emboli originating from an infected DVT.
This case exemplifies that the isolation and correct identification of Actinomyces species remains a diagnostic challenge. After Vitek2 and API Coryne failed to correctly identify the organism to species level, the isolate's identity was confirmed by MALDI-TOF. In this case, isolation of A. odontolyticus from blood cultures prompted a prolonged course of appropriate antimicrobial therapy, in the range of 6–12 months [10]. The results of antimicrobial susceptibility tests were particularly important because the E. coli was susceptible to ciprofloxacin, whereas the A. odontolyticus was resistant. Furthermore, Actinomyces species are known to exhibit reduced susceptibility to metronidazole [3–5], the aminoglycosides and colistin [3].
In conclusion, clinicians of all specialties need to be aware of the rising tide of bloodstream infections with Actinomyces species. The number of reports is likely to increase with the more widespread availability of sophisticated molecular identification techniques, including MALTI-TOF.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Wade WG, Kononen E. Propionibacterium, Lactobacillus, Actinomyces, and Other Non-Spore-Forming Anaerobic Gram-Positive Rods in Manual of Clinical Microbiology. 10th edn. Washington, DC: ASM Press; 2011. [Google Scholar]
- 2.HPA. Uncommon pathogens involved in bacteraemia: England, Wales and Northern Ireland, 2007–2011. Health Prot Rep. 2011;6:1–24. [Google Scholar]
- 3.Peloux Y, Raoult D, Chardon H, Escarguel J. Actinomyces odontolyticus infections: review of six patients. J Infect. 1985;11:125–9. doi: 10.1016/s0163-4453(85)91979-6. [DOI] [PubMed] [Google Scholar]
- 4.Ray P, Mandal J, Gautam V, Singh K, Gupta D. A case of pulmonary actinomycosis caused by Actinomyces odontolyticus from India. Indian J Med Res. 2005;122:547–8. [PubMed] [Google Scholar]
- 5.LeCorn DW, Vertucci FJ, Rojas MF, Progulske-Fox A, Bélanger M. In vitro activity of amoxicillin, clindamycin, doxycycline, metronidazole, and moxifloxacin against oral Actinomyces. J Endod. 2007;33:557–60. doi: 10.1016/j.joen.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Sofianou D, Avgoustinakis E, Dilopoulou A, Pournaras S, Tsirakidis G, Tsakris A. Soft-tissue abscess involving Actinomyces odontolyticus and two Prevotella species in an intravenous drug abuser. Comp Immunol Microbiol Infect Dis. 2004;27:75–9. doi: 10.1016/S0147-9571(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 7.Batty I. Actinomyces odontolyticus, a new species of actinomycete regularly isolated from deep carious dentine. J Pathol Bacteriol. 1958;75:455–9. doi: 10.1002/path.1700750225. [DOI] [PubMed] [Google Scholar]
- 8.Cone LA, Leung MM, Hirschberg J. Actinomyces odontolyticus bacteremia. Emerg Infect Dis. 2003;9:1629–32. doi: 10.3201/eid0912.020646. [DOI] [PubMed] [Google Scholar]
- 9.Litwin KA, Jadbabaie F, Villanueva M. Case of pleuropericardial disease caused by Actinomyces odontolyticus that resulted in cardiac tamponade. Clin Infect Dis. 1999;29:219–20. doi: 10.1086/520169. [DOI] [PubMed] [Google Scholar]
- 10.Finegold SM, Wexler HM. Present status of therapy for anaerobic infections. Clin Infect Dis. 1996;23(Suppl):9–14. doi: 10.1093/clinids/23.supplement_1.s9. [DOI] [PubMed] [Google Scholar]