Abstract
Background:
We performed a retrospective study on patients with idiopathic thrombocytopenic purpura (ITP) to evaluate the response to splenectomy in relation to preoperative platelet count.
Materials and Methods:
Two groups of patients operated on with laparoscopic or open splenectomy for ITP, with a platelet count ≤30,000/μL (study group: 22 patients) and >30,000/μL (control group: 18 patients), respectively, were compared. The two groups were homogeneous in relation to age, sex, length of preoperative steroid therapy, and time interval between diagnosis and surgery (Student t test with P > .1). The results of surgery were evaluated at one year after splenectomy. Positive response to surgery, according to the American Society of Hematologic Guidelines, was considered in patients with a postoperative platelet count ≥100,000/μL or in patients with a postoperative platelet count ≥30,000/μL and a twofold increase in platelet count from baseline, in the absence of bleeding.
The postoperative platelet count increase rate was statistically related to preoperative platelet count in both the study and control groups. Statistical analysis was performed using the Student's t test for independent sample and the Pearson correlation in a 2-tailed test.
Results:
No relationship between preoperative platelet count and postoperative platelet percent increase was observed in the control group (r = –0.41; P = .089), whereas a significant negative correlation (r = –0.68; P = .0004) was found in the study group.
Conclusions:
A higher increase of postoperative percent platelet count may be predicted in patients with a low preoperative platelet count.
Keywords: Idiopathic thrombocytopenic purpura, Splenectomy, Laparoscopic splenectomy
INTRODUCTION
Idiopathic thrombocytopenic purpura (ITP) is an autoimmune hematological disorder resulting in low platelet count (diagnosed with the presence of a platelet count <100.000/μL), which may lead to spontaneous bleeding.
Based on ITP's autoimmune pathogenesis, immunosuppressive or immune-modulating therapy has been the standard initial treatment for adults with moderate to severe thrombocytopenic purpura, and splenectomy, performed either by the laparoscopic or traditional approach, has been indicated only in cases refractory to medical therapy.1–6
Complete response after splenectomy has been observed in 66% to 100% of cases.5–9 Response to splenectomy has been related to several predictive factors4–14 but is still uncertain, with no definitive results reported on this issue.
Only few studies have addressed the results of splenectomy in patients with a very low platelet count.15 Preoperative platelet count, however, has never been related to a postsplenectomy platelet increase.
In this study, we retrospectively analyzed the results of splenectomy in patients with ITP, comparing 2 groups of patients with preoperative platelet count, respectively, inferior and superior to 30,000/μL.
MATERIALS AND METHODS
We evaluated retrospectively 40 consecutive patients affected by ITP who underwent laparoscopic or open splenectomy from January 2002 to December 2011. Among these patients, in 22 cases (study group) the preoperative platelet count was ≤30,000/μL (mean 15,954, range 8000–30,000), whereas in the remaining 18 patients (frequency-matched control group), the preoperative platelet count was 30,000/μL (mean 55,833, range 34,000–90,000). Table 1 summarizes data for each group of patients.
Table 1.
Patients' Data
| Study Group (Preoperative Platelet Count ≤30,000/μL) | Control Group (Preoperative Platelet Count >30,000/μL) | |
|---|---|---|
| No. patients | 22 | 18 |
| Mean age, y (range) | 30.18 (19–50) | 29.56 (17–45) |
| Sex ratio (M/F) | 11/11 | 7/11 |
| Open/laparoscopic splenectomies | 8/14 | 12/6 |
| Mean preoperative platelet count, μL (range) | 15,954/μL (8000–30,000) | 55,833/μL (34,000–90,000) |
| Mean postoperative platelet count, μL at 1 year (range) | 300,364/μL (22,000–750,000) | 221,333/μL (24,000–600,000) |
| Rate of response to splenectomy | 90.9% | 72.2% |
The mean age of patients was 30.2 years (range 19–50) in the study group, and 29.6 years (range 17–45) in the control group. The male-to-female ratio was 1.00 (11 males, 11 females) in the study group and 0.64 (7 males, 11 females) in the control group.
Alternative etiologies of thrombocytopenia were accurately ruled out before treatment in all patients.
When surgery was proposed, all patients were symptomatic with various degrees of mucocutaneous bleeding, and menorrhagia in the females. Surgery was indicated for recurrent bleeding episodes or for risks of bleeding resulting from the patients' lifestyle (2 patients in the control group performed heavy sports) or secondary to associated pathologies (2 patients in the control group had, respectively, peptic ulcers and ulcerative colitis).
All patients were previously treated with corticosteroid therapy (prednisone 1–2 mg/kg/d), and the length of presurgical medical treatment ranged from 2 weeks to 28 months (mean 8.45) in the study group and from 2 weeks to 36 months (mean 11.19) in the control group.
Overall, 24 patients (60%) did not respond to steroids (13 in the study group, 11 in the control group), whereas in 16 cases (40%) (9 in the study group, 7 in the control group) corticosteroid therapy was associated with temporary remission but relapse of the disease with the interruption of medical treatment.
The interval between diagnosis and splenectomy ranged from 4 to 60 months (mean 18.9) in the study group and from 4 to 45 months (mean 19.7) in the control group.
Open and laparoscopic splenectomies were performed in the traditional fashion reported elsewhere.16 In 9 cases, cholecystectomy was performed associated with splenectomy using the standard technique.17
All patients were followed up for at least one year after the surgery. Results of the surgery were evaluated at one year after splenectomy through the assessment of blood platelet count and remission of symptoms in both the study and control groups. Based on the recommendations of the International Working Group reported by the American Society of Hematology (ASH),16 a positive response was considered when the postoperative platelet count was ≥100,000/μL or in patients with a postoperative platelet count ≥30,000/μL and a twofold increase in platelet count from baseline, in the absence of bleeding.
The postoperative platelet count increase rate was statistically related to the preoperative platelet count in both the study and control groups.
Statistical analysis was performed using the Student t test for independent sample and the Pearson correlation in a 2-tailed test.
RESULTS
The study and the control groups were statistically homogeneous. The Student t test for independent sample showed a P value > .1, with no significant differences when age, sex, length of preoperative steroid therapy, and diagnosis-to-splenectomy interval were compared between the study and control groups.
Accessory spleens were found and removed in 6 patients (15%): in 2 patients with a preoperative platelet count ≤30,000/μL and in 4 patients with a preoperative platelet count >30,000/μL.
According to the criteria of the International Working Group reported by ASH,18 in this study response to splenectomy was observed in 13 of 18 patients (12 complete responders with a postoperative platelet count >100,000/μL and 1 patient responder with a platelet count ≥30,000 but <100,000/μL and a doubling platelet count from baseline) in the control group (overall rate 72.2%) and in 20 of 22 patients (19 complete responders with a postoperative platelet count >100,000/μL and 1 patient responder with a platelet count ≥30,000 but <100,000/μL and a doubling platelet count from baseline) in the study group (overall rate 90.9%).
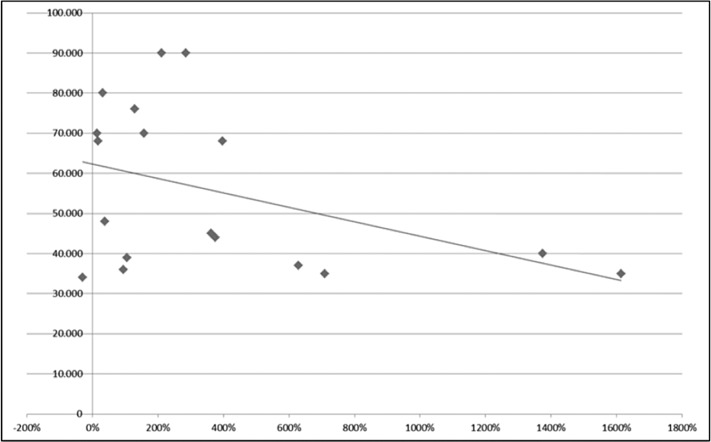
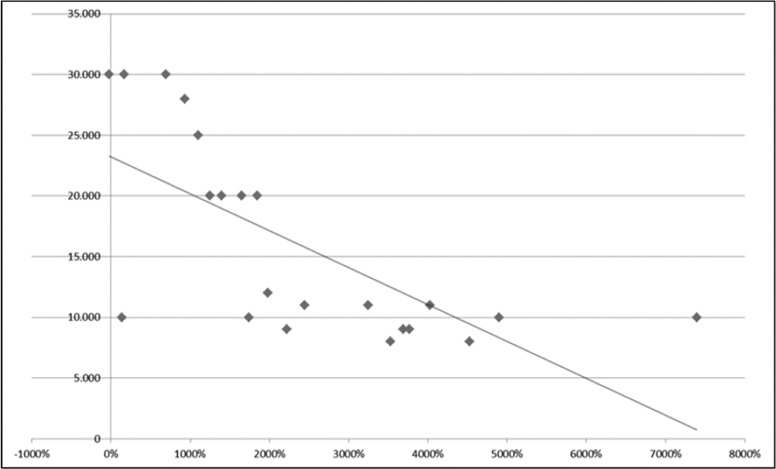
The Pearson correlation test, used to find an association between preoperative platelet count and postoperative platelet percent increase at one year after surgery, showed no relationship in the control group (preoperative platelet count >30,000/μL), with r = –0.41 and P = .089 (Figure 1). In the study group, in which the preoperative platelet count was ≤30,000/μL, a significant negative correlation (r = –0.68; P = .0004) was found between the 2 variables, demonstrating a higher increase in postoperative percent platelet count in patients with a lower preoperative platelet count (Figure 2).
Figure 1.
The correlation between preoperative platelet count and postsplenectomy platelet count increase (percentage of preoperative value) in patients with a preoperative platelet count >30 × 10*9/L (no correlation found: r = –0.41; P = .089).
Figure 2.
The correlation between preoperative platelet count and postsplenectomy platelet count increase (percentage of preoperative value) in patients with a preoperative platelet count ≤30 × 10*9/L (significant negative correlation demonstrating a higher increase of postoperative percent platelet count in patients with a lower preoperative platelet count: r = –0.68; P = .0004).
DISCUSSION
ITP is an autoimmune hematological disease characterized by the destruction of opsonized platelets in the reticulo-endothelial system,6–15,19 particularly in the spleen or by suppression of platelet production,19 resulting in a persistent low platelet count, which may lead to spontaneous bleeding.6
ITP is a relatively common hematological disorder affecting people of all ages, with an incidence of roughly 2 in 100,000 per year and spontaneous remission of 5% to 11% in adult patients.20 Primary ITP was defined by the International Working Group as a platelet count <100,000/μL in the absence of other causes or disorders that may be associated with thrombocytopenia.
There is no evidence in the literature that could allow determination of a threshold platelet count under which a patient with ITP should be treated. According to the 1996 ASH guidelines,1 treatment for newly diagnosed ITP should be indicated for patients with a platelet count <30,000/μL or in patients with a platelet count <50,000/μL with significant bleeding or risk of bleeding (such as in hypertension, peptic ulcer disease, or having a vigorous lifestyle). In the 2011 ASH guidelines, however, it is outlined that there is limited evidence for treatment recommendation based on platelet count in ITP patients.18 Therefore there is currently no evidence on which to base an indication for treatment in relation to platelet count. Several authors9,21–25 suggest that the decision to treat should be made on an individual basis considering severity of bleeding, bleeding risk (such as previous bleeding episodes, hypertension, age, and sex), the patient's preferences, and a lifestyle that increases the risk of bleeding.
The primary aim of ITP treatment is to maintain a stable platelet count at a level that prevents bleeding events.2,18 The International Working Group proposed the definition of complete response when a platelet count >100,000/μL is achieved with treatment. Therapeutic response is also considered in patients with a platelet count >30,000/μL if a doubling platelet count from the baseline is observed in symptomless patients.18 Although it is regarded as the second-line therapy in refractory disease after medical treatment, splenectomy is a well-established modality found in the literature and appears to be the single best option to convert a patient with ITP to a “nonpatient.”26 The percentage of patients who need splenectomy is unclear and is usually reported to be 20% to 45% according to two large series.19,22
Nevertheless, it appears that the popularity of splenectomy has decreased dramatically in the past few years, despite increasing reports of its high rate and durable response with surgery.26
There are discordant opinions, however, about the indication for splenectomy and the timing of surgery. Splenectomy should not be performed as the initial therapy, and it should be reserved for patients who did not respond to medical therapy first.
The British Committee for Standards in Haematology guideline recommended splenectomy with a platelet count <30,000/μL in cases refractory to medical treatment.3 This platelet threshold is considered a trigger for treatment, either medical or surgical with splenectomy, by several clinicians. However, this is not the only criterion, and indications for splenectomy are the same as for first-line medical treatment, which were mentioned earlier.
The timing of performing splenectomy is also still controversial. In 1996, ASH guideline panelists considered indications to splenectomy after 6 weeks from initial diagnosis when the platelet count is <10,000/μL or after 3 months with a platelet count <30,000/μL (with or without bleeding symptoms). In the same consensus conference, the panelists considered splenectomy to be inappropriate in symptomless patients who have had the diagnosis for 6 months and have a platelet count >50,000/μL and low hemostatic risk.1
Other authors27 accordingly, to reduce the duration and morbidity of medical treatment, have recommended early splenectomy in patients who do not respond rapidly to initial glucocorticoid treatment. However, in their 2010 consensus review, Provan et al noted that in most reported series, splenectomy was deferred at least 6 months after diagnosis.2 In our series, we primarily performed splenectomy in patients who did not respond to medical treatment after at least 6 months of therapy.
Despite the high response rate of splenectomy, the number of postsurgical failures remains significant and the knowledge of predictive factors of response to splenectomy could help select patients for surgery, avoiding unnecessary operations.10
Several studies7,11,12,19 have been carried out to evaluate the response to splenectomy in ITP patients according to some hypothetic predictive factors. Predictive factors such as age and sex, length of the disease and time interval between diagnosis and surgery, and length and response to the preoperative corticosteroid therapy have been related to the response to splenectomy.
In some studies, a better response to splenectomy was observed in patients ≤52 years,6–8,12 complete or partial preoperative response to steroid therapy,5,7 in cases with short length of disease,6 and in patients responding with a temporary increase in platelet count to high-dose preoperative intravenous antibody therapy.5,7 Alternately, other investigators failed to demonstrate that age and sex,5,11,13,14 length of the disease and time between diagnosis and splenectomy,5,23 response to medical treatment,5,25 and preoperative platelet count5,13 could predict the success of splenectomy.
All of the studies reported in the literature relating the aforementioned predictive factors of response to splenectomy, however, are not conclusive, and further investigations are needed.
Only a few studies have addressed the response to splenectomy in relation to the preoperative platelet count. Radaelli et al,5 in a series of 65 adult patients with ITP who underwent splenectomy did not find any relationship between response to splenectomy and preoperative platelet count. Balagué et al,13 studying 103 patients treated by splenectomy in their univariate and multivariate statistical analysis, were unable to demonstrate that preoperative platelet count was a predictive factor of response to splenectomy, because there was not a significant difference in the response comparing patients with preoperative platelet counts less or more than 20,000/μL. In this latter study, response was considered when a stable postoperative platelet count ≥50,000/μL was obtained. Duperier et al,12 in their study of 67 patients, observed a successful response to splenectomy in patients with a preoperative platelet count >70,000/μL. In their univariate analysis, a statistical difference with a P value of .005 between patients groups with more or less than 70,000/μL was observed. In their multivariate analysis, younger patients (P = .005) and a higher preoperative platelet count (P = .007) again predicted a successful response to splenectomy.
In the present study, we found that a higher increase in postoperative percent platelet count with a stable positive response can be found in patients with a preoperative platelet count ≤30,000/μL. In one of our previous studies comparing laparoscopic and open splenectomies groups,4 we observed that this greater increase of postoperative platelet count in patients with a lower preoperative platelet count seemed not to be related to the type of surgery. We used the cutoff of 30,000/μL because a recent recommendation in the ASH Guidelines (published in 2011) suggest using this value of platelet count to decide whether to begin a treatment in adult ITP patients.
It is currently difficult to find any explanation for these results. However, the results of this study could be relevant when indication to splenectomy in ITP patients has to be considered. Splenectomy should be better performed in patients who are not responding to medical treatment when the preoperative platelet count is ≤30,000/μL. This cutoff in the indications to splenectomy does not increase surgical morbidity. The risk of bleeding seems not to be augmented, especially when splenectomy is performed laparoscopically, which, according to the literature,15,28,29 could be responsible for hematological benefits, significantly reduce the need for platelets transfusion, and lower the perioperative blood loss and complications when compared with open surgery.
Contributor Information
Rosario Vecchio, Department of Surgery, University of Catania, Italy, Catania, Italy..
Eva Intagliata, Department of Surgery, University of Catania, Italy, Catania, Italy..
Francesco La Corte, Department of Surgery, University of Catania, Italy, Catania, Italy..
Salvatore Marchese, Department of Surgery, University of Catania, Italy, Catania, Italy..
Rossella R. Cacciola, Hematologic Unit, Department of Biomedical Science, University of Catania, Catania Italy..
Emma Cacciola, Hematologic Unit, Department of Biomedical Science, University of Catania, Catania Italy..
References:
- 1. George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3–40. [PubMed] [Google Scholar]
- 2. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. [DOI] [PubMed] [Google Scholar]
- 3. British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol. 2003;120:574–596. [DOI] [PubMed] [Google Scholar]
- 4. Vecchio R, Marchese S, Intagliata E, Swehli E, Ferla F, Cacciola E. Long-term results after splenectomy in adult idiopathic thrombocytopenic purpura: comparison between open and laparoscopic procedures. J Laparoendosc Adv Surg Tech. 2013;23(3):192–198. [DOI] [PubMed] [Google Scholar]
- 5. Radaelli F, Faccini P, Goldaniga M, et al. Factors predicting response to splenectomy in adult patients with idiopathic thrombocytopenic purpura. Haematologica. 2000;85:1040–1044. [PubMed] [Google Scholar]
- 6. Shojaiefard A, Mousavi SA, Faghihi SH, Abdollahzade S. Prediction of response to splenectomy in patients with idiopathic thrombocytopenic purpura. World J Surg. 2008;32:488–493. [DOI] [PubMed] [Google Scholar]
- 7. Elezovíc I, Boskovíc D, Colovíc M, et al. Late results of splenectomy in patients with chronic immune thrombocytopenic purpura. Acta Chir Iugosl. 2002;49(3):29–34. [DOI] [PubMed] [Google Scholar]
- 8. Avinash S, Maulik P, Ramkrishna P, Chetan K, Jijina F. Postsplenectomy response in adult patients with immune thrombocytopenic purpura. Asian J Transfus Sci. 2009;3(1):6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vianelli N, Galli M, de Vivo A, et al. Efficacy and safety of splenectomy in immune thrombocytopenic purpura: long-term results of 402 cases. Haematologica. 2005;90:72–77. [PubMed] [Google Scholar]
- 10. Vecchio R, Cacciola E, Cacciola RR, Palazzo F, Rinzivillo C, Giustolisi R. Predictive factors of response to splenectomy in adult chronic idiopathic thrombocytopenic purpura. Int Surg. 2000;85:252–256. [PubMed] [Google Scholar]
- 11. Todorovíc-Tirnaníc M, Obradovíc V, Rolovíc Z, et al. Prediction of the splenectomy efficacy in chronic immune thrombocytopenic purpura. Glas Srp Akad Nauka Med. 2005;(48):119–135. [PubMed] [Google Scholar]
- 12. Duperier T, Brody F, Felsher J, Walsh RM, Rosen M, Ponsky J. Predictive factors for successful laparoscopic splenectomy in patients with immune thrombocytopenic purpura. Arch Surg. 2004;139:61–66. [DOI] [PubMed] [Google Scholar]
- 13. Balagué C, Vela S, Targarona EM, et al. Predictive factors for successful laparoscopic splenectomy in immune thrombocytopenic purpura: study of clinical and laboratory data. Surg Endosc. 2006;20(8):1208–1213. [DOI] [PubMed] [Google Scholar]
- 14. Zoghlami-Rintelen C, Weltermann A, Bittermann C, et al. Efficacy and safety of splenectomy in adult chronic immune thrombocytopenia. Ann Hematol. 2003;82:290–294. [DOI] [PubMed] [Google Scholar]
- 15. Wu X, Zhou J, Pankaj P, Peng B. Laparoscopic splenectomy for immune thrombocytopenia (ITP) patients with platelet counts lower than 1 × 109/L. Int J Hematol. 2011;94:533–538. [DOI] [PubMed] [Google Scholar]
- 16. Vecchio R, Marchese S, Swehli E, Intagliata E. Splenic hilum management during laparoscopic splenectomy. J Laparoendosc Adv Surg Tech A. 2011;21(8):717–720. [DOI] [PubMed] [Google Scholar]
- 17. Vecchio R, MacFadyen BV. Laparoscopic common bile duct exploration. Langenbecks Arch Surg. 2002;387(1):45–54. [DOI] [PubMed] [Google Scholar]
- 18. Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA; American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. [DOI] [PubMed] [Google Scholar]
- 19. McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44(4 Suppl 5):S3–S11. [DOI] [PubMed] [Google Scholar]
- 20. Fogarty PF, Segal JB. The epidemiology of immune thrombocytopenic purpura. Curr Opin Hemato1. 2007;14:515–519. [DOI] [PubMed] [Google Scholar]
- 21. Bizzoni L, Mazzucconi MG, Gentile M, et al. Idiopathic thrombocytopenic purpura (ITP) in the elderly: clinical course in 178 patients. Eur J Haematol. 2006;76(3):210–216. [DOI] [PubMed] [Google Scholar]
- 22. Daou S, Federici L, Zimmer J, Maloisel F, Serraj K, Andres E. Idiopathic thrombocytopenic purpura in elderly patients: a study of 47 cases from a single reference center. Eur J Intern Med. 2008;19(6):447–451. [DOI] [PubMed] [Google Scholar]
- 23. Neylon AJ, Saunders PWG, Howard MR, Proctor SJ, Taylor PRA. Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population-based cohort of 245 patients. Br J Haematol. 2003;122(6):966–974. [DOI] [PubMed] [Google Scholar]
- 24. Pamuk GE, Pamuk ON, Baslar Z, et al. Overview of 321 patients with idiopathic thrombocytopenic purpura. Retrospective analysis of the clinical features and response to therapy. Ann Hematol. 2002;81:436–440. [DOI] [PubMed] [Google Scholar]
- 25. Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the general practice research database. Br J Haematol. 2009;145(2):235–244. [DOI] [PubMed] [Google Scholar]
- 26. Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP). Blood. 2005;106:2244–2251. [DOI] [PubMed] [Google Scholar]
- 27. Gadenstatter M, Lamprecht B, Klingler A, et al. Splenectomy versus medical treatment for idiopathic thrombocytopenic purpura. Am J Surg. 2002;184:606–609; discussion 609–610. [DOI] [PubMed] [Google Scholar]
- 28. Vecchio R, Cacciola E, Lipari G, et al. Laparoscopic splenectomy reduces the need for platelet transfusion in patients with idiopathic thrombocytopenic purpura. JSLS. 2005;9:415–418. [PMC free article] [PubMed] [Google Scholar]
- 29. Vecchio R, Cacciola E, Martino M, Cacciola RR, MacFadyen BV. Modifications of coagulation and fibrinolytic parameters in laparoscopic cholecystectomy. Surg Endosc. 2003;17(3):428–433. [DOI] [PubMed] [Google Scholar]