Chronic EtOH exacerbates S. aureus skin infection, increases bacterial dissemination, and causes PMN and T cell defects in infected skin, and skin-draining LNs.
Keywords: PMNs, T cells, IL-17
Abstract
Alcoholics are at increased risk of Staphylococcus aureus skin infection and serious sequelae, such as bacteremia and death. Despite the association between alcoholism and severe S. aureus skin infection, the impact of EtOH on anti-S. aureus cutaneous immunity has not been investigated in a model of chronic EtOH exposure. To test the hypothesis that EtOH enhances the severity of S. aureus skin infection, mice were fed EtOH for ≥12 weeks via the Meadows-Cook model of alcoholism and inoculated with S. aureus following epidermal abrasion. Evidence of exacerbated staphylococcal disease in EtOH-fed mice included: skin lesions that were larger and contained more organisms, greater weight loss, and increased bacterial dissemination. Infected EtOH-fed mice demonstrated poor maintenance and induction of PMN responses in skin and draining LNs, respectively. Additionally, altered PMN dynamics in the skin of these mice corresponded with reduced production of IL-23 and IL-1β by CD11b+ myeloid cells and IL-17 production by γδ T cells, with the latter defect occurring in the draining LNs as well. In addition, IL-17 restoration attenuated S. aureus-induced dermatopathology and improved bacterial clearance defects in EtOH-fed mice. Taken together, the findings show, in a novel model system, that the EtOH-induced increase in S. aureus-related injury/illness corresponds with defects in the IL-23/IL-17 inflammatory axis and poor PMN accumulation at the site of infection and draining LNs. These findings offer new information about the impact of EtOH on cutaneous host-defense pathways and provide a potential mechanism explaining why alcoholics are predisposed to S. aureus skin infection.
Introduction
S. aureus is a ubiquitous, gram-positive bacterium that asymptomatically colonizes the skin and mucosa of nearly 30% of healthy individuals [1, 2]. Despite its capacity to inhabit human skin as a commensal symbiont, S. aureus is also a potent pathogen that causes more skin and soft-tissue infections than any other single infectious agent [2]. To underscore the seriousness of these infections, S. aureus can disseminate from sites of incipient infection (typically skin) and cause fatal invasive diseases (e.g., bacteremia, endocarditis, and sepsis). In fact, S. aureus is the leading cause of infection-related fatality in the United States, causing more deaths each year than HIV, hepatitis virus, and influenza virus combined [3]. Already a global epidemic, the recent increase in S. aureus infections caused by highly virulent antibiotic-resistant strains portends a rising human health toll and highlights the need to complement and/or supplant the use of inefficacious antibiotics with new anti-S. aureus immunotherapies [2]. Such interventions would be of particular benefit for populations with congenital or acquired vulnerability to severe and/or recurrent S. aureus infections. Accumulating clinical evidence suggests that individuals who chronically abuse alcohol represent one such population and contribute substantially to the global burden of S. aureus-induced illness [4–7].
According to the World Health Organization, there are ∼140 million alcoholics worldwide, and these individuals are at heightened risk of S. aureus skin infection and fatal complications resulting from them [4–8]. An inference from the association between alcoholism and severe S. aureus skin infection is that immune functions conferring protection against this pathogen are suppressed by alcohol. Whereas depressed delayed-type hypersensitivity responses in alcoholic patients are well documented and clearly point out the sensitivity of the cutaneous immune system to chronic EtOH exposure, the consequences of such exposure on anti-S. aureus cutaneous host defense have yet to be pursued experimentally [9]. Given the prevalence of chronic EtOH abuse and the growing threat of S. aureus outbreaks, an improved understanding of the immunologic lesions underlying impaired anti-S. aureus immunity among alcoholic populations could facilitate the development of strategies to protect these individuals better and prevent disease transmission to the broader community as well. In light of the fact that the majority of S. aureus infections originate in the skin, a thorough evaluation of the impact of EtOH on protective anti-S. aureus cutaneous host defense is critical [2].
Given that clinical manifestations of immune deficiency typically present after several decades of alcohol abuse, it is of interest to use experimental models that not only reproduce clinical EtOH-induced immune dysfunction but also do so with relatable kinetics [10]. A major advantage of the Meadows-Cook EtOH-in-water model is its proven ability to reproduce a state of chronic EtOH consumption without excessive mortality, and the immune suppression characteristic of human alcoholism [11, 12]. With the use of this model system, mice can be administered 20% EtOH w/v in water for several months without provoking confounding pathologic conditions (e.g., wasting and corticosterone-induced stress) [13]. Additionally, the Meadows-Cook protocol has been used productively to study the cellular and molecular basis of EtOH-induced cutaneous immune deficiency. With the use of this protocol, we reported recently that chronic EtOH feeding adversely impacts the immune parameters that confer protection against S. aureus skin infection. Specifically, the production of IL-17 by skin resident γδ T cells was diminished following ex vivo anti-CD3 stimulation [14]. Although the significance of compromised γδ T cell-mediated IL-17 responses observed in EtOH-fed mice has not been investigated previously within the context of S. aureus infection, this EtOH-induced immune lesion would be anticipated to undermine cutaneous host defense by limiting PMN accumulation at sites of pathogen invasion.
Both clinical and experimental findings point toward an indispensable role for PMNs as mediators of primary defense against S. aureus skin infection [3]. Abundant in the circulation, PMNs spearhead protective anti-S. aureus cutaneous immune responses by extravasating proximal to a cutaneous bacterial nidus, where they accumulate, phagocytose, and ultimately clear S. aureus organisms [15]. In contrast to steady-state skin, which harbors a relatively small population of sparsely scattered PMNs, neutrophilic abscess formation is a hallmark of S. aureus skin infection and is required for bacterial clearance [3, 16–19]. In association with an increased output of endogenous and pathogen-derived chemoattractants, PMN accumulation in S. aureus-infected wounds is driven by the IL-23/IL-17 inflammatory axis. Myeloid cell-derived IL-23 serves as a potent upstream activator of IL-17 production by skin γδ T cells, which in turn, incite an inflammatory milieu favoring PMN recruitment, survival, and differentiation [3, 16, 20, 21]. Mirroring the consequences of PMN depletion, cutaneous challenge with S. aureus in mice deficient in IL-17 or γδ T cells (the skin’s predominant cellular source of IL-17) results in larger lesions, reduced PMN accumulation, and higher bacterial counts when compared with wild-type controls [18, 22, 23]. Taken together, local induction of cytokine responses leading to PMN accumulation in the vicinity of S. aureus-infected skin is needed for pathogen clearance; thus, any EtOH-induced effects interfering with these pathways would predictably impair anti-S. aureus host defense.
In the present study, the Meadows-Cook model was used to study the effects of chronic EtOH consumption on the outcome of S. aureus skin infection. The results show for the first time that chronic EtOH feeding exacerbates features of clinical disease, including weight loss, skin ulceration size, and bacterial burden at local and disseminated (extracutaneous) sites. Importantly, the EtOH-induced increase in staphylococcal illness corresponded with significant alterations in the composition and function of leukocyte populations within S. aureus-infected skin. The EtOH-induced reduction in PMN accumulation at sites of infection occurred alongside diminished local IL-23 and IL-17 cytokine production by myeloid cells and γδ T cells, respectively. With the demonstration of the significance of the latter defect, administration of rIL-17 reduced significantly lesion size and bacterial burden in EtOH-exposed skin following S. aureus challenge. These findings provide a potential mechanism by which chronic EtOH consumption predisposes to severe S. aureus infections by suppressing inflammatory circuits that mediate PMN accumulation and ultimately, bacterial clearance in the skin. This information could be used to design interventions that better protect alcoholic populations from severe, disseminated S. aureus infections.
MATERIALS AND METHODS
Mice and EtOH administration
Six- to 7-wk-old female C57Bl⁄6 mice were purchased from the U.S. National Institutes of Health National Cancer Institute (Fort Detrick, MD, USA). Mice were housed in specific pathogen-free facilities with all animal protocols approved by the Animal Care and Use Committee at the University of Iowa (Iowa City, IA, USA). At ∼8 wk of age, mice were divided randomly into control and EtOH-consuming groups. EtOH (Pharmco-AAPER Alcohol, Shelbyville, KY, USA) was administered in the drinking water at 10% w/v for 2 days, 15% w/v EtOH for 5 days, and 20% w/v on day 7 and thereafter. Total duration of 20% EtOH feeding was between 12 and 16 wk, with the exception of experiments measuring PMN expression of TLR2 and CRAMP, where mice received EtOH for a duration of 16–24 wk. Age-matched control mice were maintained on the same source of double-distilled drinking water as EtOH-consuming mice. In all cases, mice were permitted ad libitum access to rodent chow and the appropriate source of drinking liquid. This model has been described previously in more detail [13, 14, 24].
Preparation of S. aureus
A USA 100, methicillin-sensitive, spa-type 002 S. aureus clinical isolate (347) was grown in TSB medium overnight at 37°C in a shaking incubator set to 200 rpm. Midlogarithmic-phase bacteria were obtained after 2.5 h subculture of a 1:100 dilution of the overnight culture in TSB. Bacterial cells were pelleted and resuspended in DPBS. Spectrophotometric readings of absorbance at 600 nm were used to estimate bacterial concentration, and 20 μl S. aureus containing 5 × 107 CFU was used for inoculations. For FITC labeling, midlogarithmic growth phase S. aureus was washed twice with 0.9% NaCl, resuspended in 0.1 mg/ml FITC (Sigma Chemical, St. Louis, MO, USA) in PBS, and incubated for 1 h at 37°C with shaking. Before infection, bacteria were washed twice with 0.9% NaCl and resuspended in DPBS with 100 mg/l MgCl2 and 100 mg/l CaCl2 (Invitrogen, Carlsbad, CA, USA). Infectious dose was confirmed by plating serial dilutions of inoculum on TSA and counting ensuing colonies after overnight culture. Each mouse (control or EtOH fed) was infected with the same volume of S. aureus suspension from the same tube, making the inoculum size equivalent between experimental groups.
S. aureus skin infections
Mice were anesthetized with isoflurane, abdominal skin was shaved with an Accu-Edge microtome blade (CardinalHealth, Dublin, OH, USA), and exposed skin was stroked gently 15 times with 200 grit sandpaper. Bacterial suspension (20 µl) or DPBS was applied to this surface, and a gentle stream of air was aimed over the inoculation site until the skin appeared wet but absent of any standing volume from the inoculum suspension. Finally, infected skin sites were covered with the adhesive end of an adhesive bandage for ∼1 h. For rIL-17 administration, 2 doses of rIL-17 in sterile DPBS (1000 ng/50 µl; BioLegend, San Diego, CA, USA) or DPBS alone were injected i.d. at the site of the infection. The 1st dose was given immediately after infection, and the 2nd was given 2 days after infection.
Determination of lesion size and tissue CFUs
Baseline weights of mice were recorded before infection and every day thereafter until euthanasia. For determination of lesion size, digital photos of skin lesions were taken with a Canon Rebel T2i and analyzed by use of ImageJ software (National Institutes of Health Research Services Branch, Bethesda, MD, USA). With the use of a millimeter ruler (captured in photo frame) as a scale bar and the freehand selection tool, tracings were made along the edges of skin ulcers, and the corresponding area measurements were calculated with ImageJ. Calculation of immune cell density (see Figs 2A, 3A, 4, and 5A; day 3): skin lesions were photographed and excised carefully. The total cell recovery of various populations was divided by the area of lesional skin (measured with ImageJ software), and the resulting quotient was represented as cells/cm2. For calculation of cutaneous IL-17-producing cells, 7 days after infection (see Fig. 5A), a 1 cm2 piece of skin that encompassed the remaining skin lesion was excised carefully, and the total IL-17-producing cells from these preparations were represented as cells/cm2. For skin CFUs, 2 mm biopsy punches obtained from the center of skin ulcerations were homogenized in sterile DPBS, and serial dilutions were cultured on TSA plates. To estimate total bacterial load in skin, CFU values from biopsy punches were adjusted to reflect the total area of the skin lesions. For kidney CFUs, kidneys were homogenized in 1 ml sterile DPBS with 100 mg/l MgCl2 and mg/l CaCl2, and serial dilutions were cultured on TSA plates.
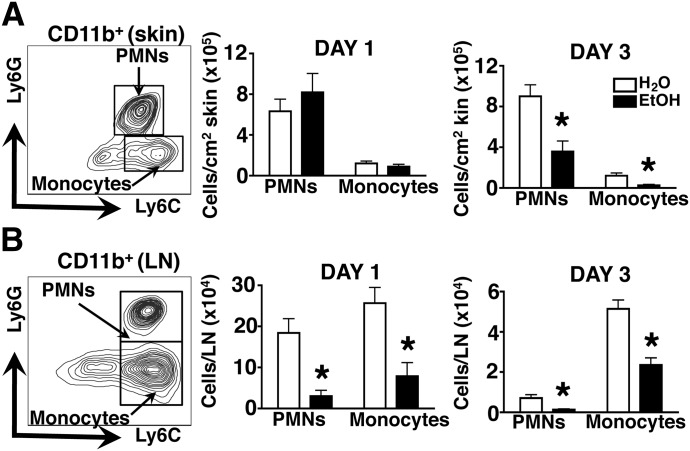
Figure 2. PMN and monocyte accumulation in infected skin rapidly declines, whereas PMN infiltration into the draining LNs is reduced persistently in EtOH-fed mice.
(A and B, left) Flow cytometric gating strategy for PMNs and monocytes from a representative H2O control mouse is shown for skin (A) and LN (B). (Middle and right) Graphs indicate density (A) or number (B) of infiltrating cells in these tissues from EtOH-fed or age-matched H2O controls at 1 and 3 days post-S. aureus skin infection. Density was calculated as described in Materials and Methods. Day 1 versus day 3 EtOH mice, P < 0.01. Despite the significant growth of skin lesions from day 1 to day 3 in EtOH-fed mice (P < 0.01; Fig. 1A), there was a decrease in the density of PMNs in skin preparations from EtOH-fed mice, P < 0.01. Error bars represent sem. Post-test, *P ≤ 0.05; n ≥ 8 mice/treatment group.
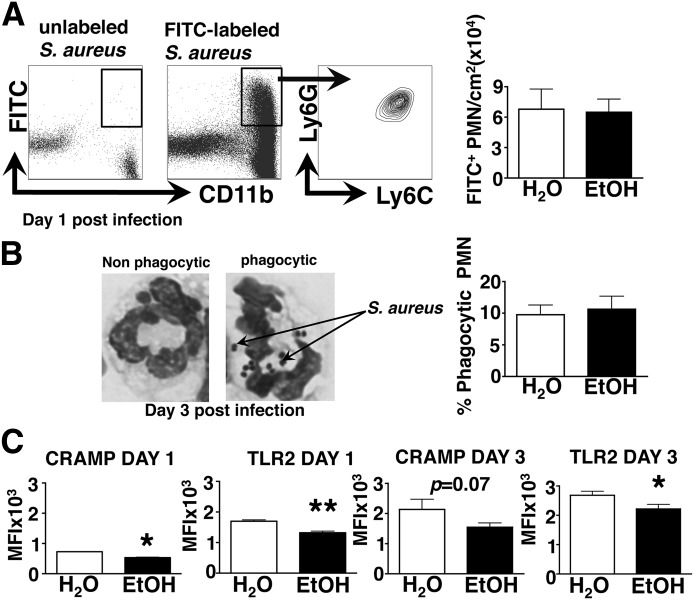
Figure 3. Chronic EtOH feeding selectively decreases PMN function by reducing TLR2 and CRAMP expression, leaving in vivo phagocytosis of S. aureus intact.
(A, left) Gating strategy for evaluating phagocytosis of FITC-labeled S. aureus by PMNs from a representative H2O control mouse. (Right) Density of phagocytic PMNs isolated from skin lesions of EtOH-fed or H2O controls, 1 day postinfection. Error bars represent sem; n ≥ 8 mice/treatment group. (B, left) Representative images of nonphagocytic and phagocytic PMNs. Internalized S. aureus organisms are labeled by arrows. (Right) The percentage of phagocytic PMNs within cytospin preparations from EtOH-fed or H2O controls, 3 days postinfection. Error bars represent sem; n ≥ 8 mice/treatment group. (C) CRAMP and TLR2 mean fluorescence intensity (MFI) values for Ly6C+ Ly6G+ PMNs (gated as in Fig. 2A) in lesional skin preparations from EtOH-fed or H2O controls, 1 and 3 days postinfection. Error bars represent sem. Post-test, *P ≤ 0.05; **P ≤ 0.01. Data are representative of 2 independent experiments; n ≥ 4 mice/treatment group (day 1), and n ≥ 6 mice/treatment group (day 3).
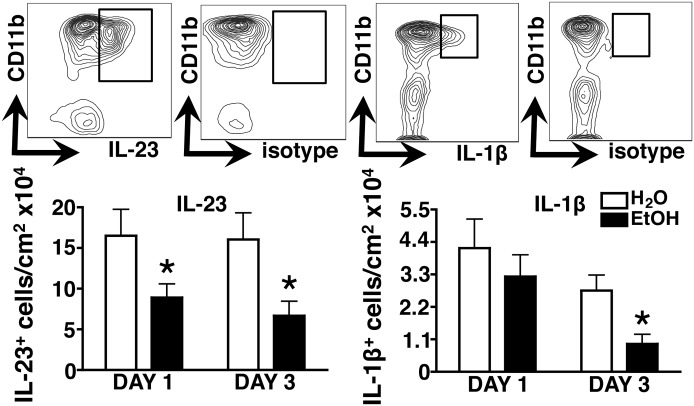
Figure 4. EtOH impairs early IL-23 and IL-1β responses at the site of infection.
Skin cell suspensions were generated from S. aureus-infected EtOH-fed or age-matched H2O controls, and IL-23 and IL-1β were assessed by intracellular cytokine staining. Gating strategies from a representative H2O control mouse are shown in the contour plots. Bar graphs show the density of cytokine-producing myeloid cells, 1 and 3 days postinfection. Error bars represent sem. Post-test, *P ≤ 0.05; n ≥ 6 mice/treatment group.
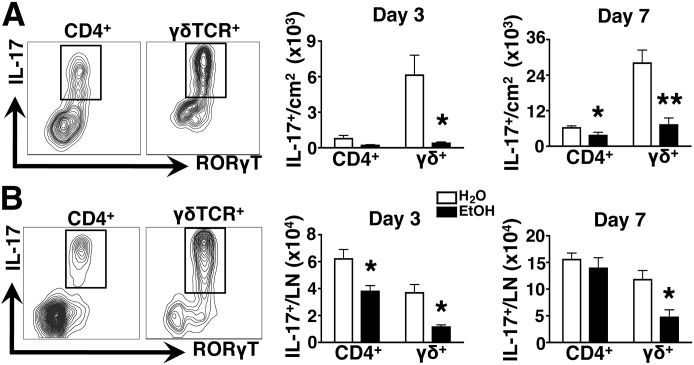
Figure 5. EtOH impairs T cell-mediated IL-17 responses in the skin and draining LN.
Skin and skin draining LN cell suspensions were generated from EtOH-fed or age-matched H2O controls, and IL-17 production by CD4 and γδ T cells was assessed by intracellular cytokine staining and RORγt expression. Gating strategies from a representative H2O control mouse are shown in the contour plots. Bar graphs show the density (A) and number (B) of cytokine-producing cells in the skin (A) and LN (B) at 3 and 7 days post-infection. Error bars represent sem. Post-test, *P ≤ 0.05; **P ≤ 0.01; n ≥ 8 mice/treatment group.
Antibodies
Five-color flow cytometric analyses were performed by use of the following antibodies: rabbit anti-mouse CRAMP was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-Ly6G (AI8), -CXCR2 (TG11/CXCR2), and -γδ TCR (GL3) were purchased from BioLegend. Anti-CD4 (RM4-5) was purchased from BD Biosciences (San Jose, CA, USA). Anti-Ly6C (HK1.4), -IL-23 p19 (fc23cpg), -IL-1β pro form (NJTEN3), -TLR2 (CX5), -CD11b (M1/70), -IL-17A (ebio17B7), -CD45 (30-F11), and -RORγt (B2D) were purchased from eBioscience (San Diego, CA, USA).
Cytokine measurements from tissue homogenates
Biopsy punches (4 mm), obtained from the center of skin ulcerations, were homogenized with Wheaton tissue grinders (CardinalHealth) in sterile RPMI. The supernatants from these preparations were incubated with a multiplex bead array for IL-17A, GM-CSF, KC (CXCL1), and CXCL2. Data were collected on a Luminex 100 (Luminex, Austin, TX, USA) and analyzed with BeadView software (Upstate Cell Signaling Solutions, Lake Placid, NY).
Ex vivo cytokine production in skin and LN
For IL-17, skin or LN cells were cultured in the presence of brefeldin A (10 µg/ml) + PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4 h. For IL-23 and IL-1β, cells were cultured in the presence of brefeldin A (10 µg/ml) without additional stimulation for 4 hrs. All preparations were then fixed, permeabilized and stained.
Cell-suspension preparations
Single-cell suspensions from infected skin were prepared by incubating excised skin in DPBS containing 0.6% trypsin for 90 min at 37°C. Trypsin-digested skin was then chopped in small pieces and incubated in RPMIc (Invitrogen) containing 500 μg/ml collagenase type II (Gibco-BRL, Grand Island, NY, USA) for 90 min at 37°C and dissociated by passing through 16, 18, and 20 gauge needles. The resulting cell suspensions were incubated in RPMIc for an additional 90 min at 37°C. For cytospin preparations, single-cell suspensions were resuspended in DPBS at 2 × 105 cells/ml. Aliquots (300 μl) were pelleted on coated Shandon cytoslides (Thermo Electron, Waltham, MA, USA) and subjected to Wright-Giemsa stain. For LN suspensions, inguinal LNs were cut into small pieces, incubated in RPMIc, and dissociated further by passing through 18 and 20 gauge needles. All samples were passed through a 70 μm filter before staining for identification of immune cells.
Flow cytometric analysis
Following surface staining, cells were fixed in FACS lysing solution (BD Biosciences) and permeabilized by use of 0.5% saponin (Sigma Chemical). To block nonspecific binding, cells were incubated with rat anti-mouse CD16/32 FcγRIII/II (2.4G2) and rat sera before staining. In all experiments, cells were collected on a FACSCanto II (BD Biosciences) by use of Diva acquisition software and analyzed by use of FlowJo software (TreeStar, Ashland, OR, USA). Dead cells were excluded by low forward-scatter and side-light scatter. Spectral overlaps between fluorochrome channels were corrected by automated compensation on singly stained, positive controls for each fluorochrome. In general, 100,000 cells were collected/tube.
Statistics
The P values for EtOH versus H2O comparisons were calculated by use of an unpaired Student’s t-test by use of InStat software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Chronic EtOH consumption increases S. aureus-associated illness
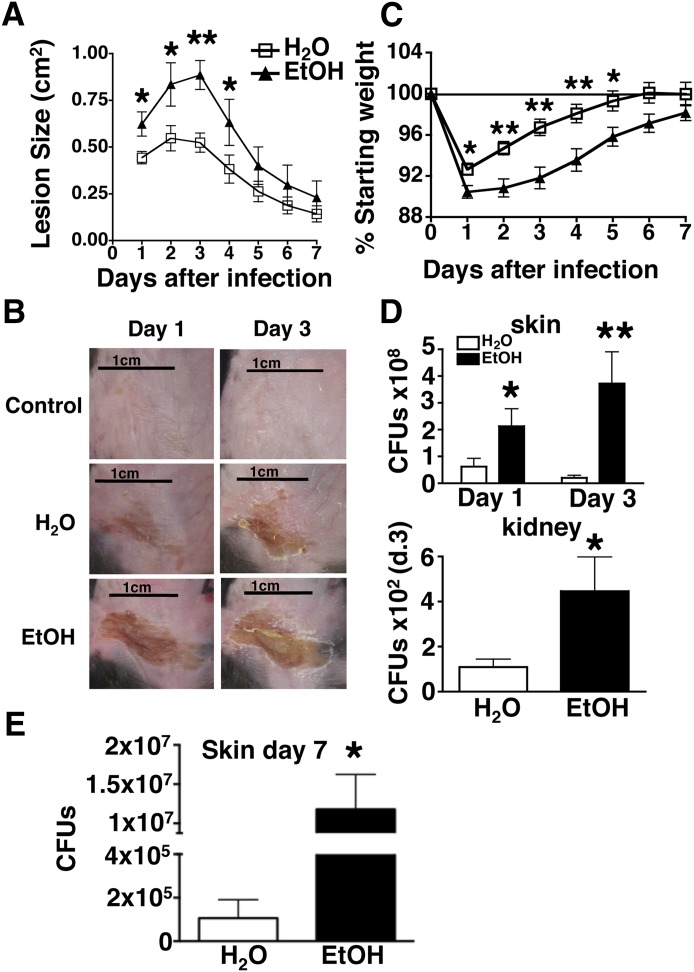
The overall goal of the present study was to establish an experimental system that is well suited to investigate the impact of chronic EtOH consumption on skin infection outcome, as well as important parameters of anti-S. aureus host defense. The Meadows-Cook EtOH-in-drinking-water mouse model of alcoholism offers an experimental system permitting long-term EtOH administration without inducing demonstrable stress [13]. Likewise, inoculation with infectious agents following stratum corneum disruption offers an established method of skin infection that does not bypass the epidermal immune system [25]. This inoculation method is well suited for the purpose of the present study, given that the two principal cellular components of the epidermis (i.e., γδ T cells and Langerhans cells) orchestrate innate and adaptive responses against S. aureus, respectively, and both are numerically reduced and functionally compromised as a result of chronic EtOH exposure [23–27]. Thus, epicutaneous infection of mice maintained on the Meadows-Cook regimen was used to investigate the impact of chronic EtOH consumption on anti-S. aureus host defense. Mice were administered EtOH for ≥12 wk and infected with 5 × 107 organisms onto skin sites following stratum corneum disruption with sandpaper [25]. The area of resultant skin ulcerations was then measured over the course of 7 days. As shown in Fig. 1, when compared with water controls, mice chronically consuming EtOH developed larger skin lesions that contained more organisms at time-points proximal to infection (days 1 and 3). Unlike the S. aureus infection procedure, application of control DPBS after sandpaper treatment produced little erosion and crusting (Fig. 1B), indicating that the injury occurring in infected mice is largely the result of S. aureus infection rather than the epidermal abrasion process preceding it. In addition to exacerbated skin lesions, EtOH-fed mice experienced a more severe and protracted period of weight loss (Fig. 1C). It was possible that the increased morbidity observed in infected EtOH-fed mice relative to infected control mice might be a result of greater systemic dissemination of S. aureus. Indeed, S. aureus CFUs recovered from the kidneys of infected EtOH-fed mice were significantly greater than water-fed controls (Fig. 1D). To assess better the impact of EtOH on the lesional organism burden at a time-point near the resolution of skin infection, the number of S. aureus CFUs was examined at day 7 postinfection. When compared with water controls, the skin lesions of EtOH-fed mice contained ∼100-fold more organisms at this time-point (Fig. 1E). Together, these data demonstrate that chronic EtOH feeding in mice increases the severity of S. aureus infections by worsening cutaneous injury, systemic organism dissemination, and prolonged presence of high cutaneous bacterial load, thereby making it a robust representation of the alcoholic individual infected with S. aureus [4–7].
Figure 1. Chronic EtOH feeding increases the severity of S. aureus-induced illness.
EtOH-fed or H2O control mice were infected with 5.0 × 107 S. aureus organisms. (A) Lesion area following S. aureus skin infection. (B, top) Representative images of H2O control mouse receiving mock infection via sandpaper and DPBS on days 1 and 3 after treatment. (Middle and bottom) Representative images of skin lesions in H2O control mice and EtOH-fed mice (indicated on the left) on days 1 and 3 after infection. (C) Weight loss following S. aureus skin infection. (D) Bacterial load of skin lesions, 1 and 3 days postinfection (calculated as described in Materials and Methods) and bacterial load in kidney, 3 days postinfection. (E) Bacterial load of skin lesions, 7 days postinfection. Error bars represent sem. Post-test, *P ≤ 0.05; **P ≤ 0.01; n ≥ 8 mice/treatment group.
Chronic EtOH consumption impairs PMN accumulation in S. aureus-infected skin and draining LNs
Given the explosive growth potential of skin-invading S. aureus, effective pathogen control and clearance demand that PMNs amass rapidly and mediate antimicrobial effector responses at the site of cutaneous insult [15]. Recent work has also shown that PMN infiltration into inflamed LNs plays a critical role in limiting the systemic dissemination of lymph-borne bacteria [28, 29]. To investigate the impact of chronic EtOH feeding on the accumulation of PMNs in infected skin and draining LNs, S. aureus skin lesions and inguinal LNs were collected, and the PMNs therein were enumerated by flow cytometry (Fig. 2). Not surprisingly, both sites contained substantial numbers of CD11b+ Ly6C+ Ly6G− monocytes, which are early responders to skin infection and potent sources of inflammatory cytokines. Similar analysis of skin and LN preparations from preimmune mice revealed that Ly6G+ PMNs were not detectable in either location at baseline in EtOH-fed or control mice (data not shown). As shown in Fig. 2A, chronic EtOH feeding did not alter the accumulation of PMNs or monocytes in lesional skin, 1 day after infection, relative to water controls. However, a dramatic reduction in the density of both populations was observed 3 days after infection in EtOH-fed mice, a time-point when bacterial burden and lesion size were greatly increased in these mice. Despite the significant growth of skin lesions from day 1 to day 3 in EtOH-fed mice (P < 0.01; Fig. 1A), the total number of PMNs recovered from these lesions remained equivalent (thus, the density of PMNs decreased in lesions from EtOH-fed mice). By comparison, in control mice, PMN numbers increased from day 1 to day 3 (data not shown) without a significant increase in lesion size (Fig. 1A), thus the numbers and density of PMNs increased in control mice. These findings suggest that amid the expansion of S. aureus-driven skin injury, EtOH-fed mice are unable to mount a commensurate increase in cutaneous PMN numbers. In contrast to infected skin, the draining LNs of infected EtOH-fed mice showed a profound reduction in the accumulation of PMNs and monocytes at days 1 and 3 postinfection (Fig. 2B). Taken together, these results suggest that chronic EtOH feeding suppresses the establishment and maintenance of robust PMN (and monocyte) responses in the skin and skin draining LNs after S. aureus skin infection.
Chronic EtOH consumption does not affect PMN phagocytosis but does impair TLR2 and CRAMP expression
To begin to understand better if chronic EtOH feeding provoked functional changes in S. aureus-responding PMNs, their in vivo phagocytic capacity was assessed within infected, EtOH-exposed or control skin. To this end, EtOH-fed and control mice were infected with 5 × 107 FITC-labeled S. aureus organisms. The ensuing skin lesions were collected, and FITC+ PMNs therein were measured by flow cytometry. As shown in Fig. 3A, equivalent densities of FITC+ phagocytes, which were virtually all Ly6C+ Ly6G+ PMNs, were detected in the skin of EtOH-fed and control mice at day 1 following infection. Next, the impact of EtOH on S. aureus phagocytosis, 3 days after infection, was evaluated. At this time-point, cutaneous PMN accumulation is decreased, and skin injury is increased in EtOH-fed mice (Figs. 1 and 2). To avoid issues stemming from loss of fluorescent signal on dividing microbes, 3 days after FITC labeling, unlabeled S. aureus-bearing PMNs were counted after Wright-Giemsa staining of cytospin preparations from cell suspensions of infected skin. With the use of this approach, phagocytic PMNs can be readily identified by the characteristic spherical morphology of S. aureus organisms, as depicted in Fig. 3B. Results from this analysis revealed that despite the EtOH-induced increase in size of cutaneous injury, 3 days after infection, the frequency of phagocytic PMNs was again not different between EtOH-fed and control animals at this time-point (Fig. 3B). However, considering that EtOH-exposed skin lesions contain fewer PMNs, 3 days after infection (Fig. 2A), the equivalent fraction of phagocytic PMNs between H2O control and EtOH-fed mice reveals a reduced density of phagocytic PMNs in the latter population.
Despite the normal phagocytic ability of PMNs in the skin of EtOH-fed infected mice, it remained possible that the antimicrobial activity of PMNs in these mice might be suboptimal. To investigate further the activation status of PMNs within infected skin, the expression of TLR2 and CRAMP was measured. These molecules promote PMN priming and staphylococcal killing, respectively [30, 31]. Flow cytometric analysis of PMN phenotypes showed a significant EtOH-induced reduction in the expression of TLR2 and CRAMP at day 1, and in TLR2 at day 3 postinfection (Fig. 3C). Taken together, these results suggest that whereas EtOH does not impair the capacity of individual PMNs to phagocytose S. aureus, reduced expression of TLR2 and CRAMP may compromise the ability of these cells to execute important antimicrobial effector functions.
Chronic EtOH consumption impairs myeloid cell production of IL-23 and IL-1β within infected skin
By promoting vigorous IL-17 responses, local induction of IL-23 and IL-1β plays a fundamental, protective role in the orchestration of anti-S. aureus PMN responses [23]. To examine the impact of EtOH on IL-23 and IL-1β responses at the site of infection, lesional skin cell suspensions were subjected to short-term ex vivo culture in the presence of Brefeldin A, followed by flow cytometric analysis of cytokine production. CD11b+ myeloid cells were found to be the major source of IL-23 and IL-1β (data not shown), and EtOH feeding differentially impacted the production of these cytokines (Fig. 4). Whereas the density of IL-23-producing cells was persistently reduced at days 1 and 3, a similar reduction in IL-1β-producing cells occurred on day 3 alone in EtOH-fed mice.
Chronic EtOH consumption impairs production of IL-17 within the skin and draining LNs
The EtOH-induced decrease in IL-23 and IL-1β responses in S. aureus-infected skin indicated the potential for downstream effects leading to decreased IL-17 production. To investigate this possibility, lesional skin cell suspensions were stimulated ex vivo with PMA/ionomycin. CD4+ and γδ TCR+ T cells that coexpressed RORγt were evaluated for their production of IL-17 at days 3 and 7 postinfection. As predicted by the EtOH-induced suppression of IL-23 and IL-1β responses, total T cell production of IL-17 shortly after infection (day 3) was blunted in preparations from EtOH-fed mice. This was overwhelmingly a result of a severe defect in cytokine production by γδ T cells, as very little IL-17 was produced by CD4+ T cells at this time-point (Fig. 5A). By day 7, an EtOH-induced defect in skin Th17 (CD4+) and γδ T cell-mediated IL-17 responses was observed. Similar to EtOH-exposed skin, IL-17 responses by γδ T cells in the skin draining LNs were similarly reduced at days 3 and 7 postinfection. However, unlike infected skin, the EtOH-induced reduction in IL-17 responses from LN Th17 cells occurred early after infection but corrected by day 7 (Fig. 5B). These data show an EtOH-induced reduction in innate and adaptive IL-17 responses in the skin and corresponding draining LNs after S. aureus infection.
To confirm the inhibitory effects of EtOH on cutaneous IL-17 production following S. aureus challenge, cytokine production was measured in homogenized lesional skin, 3 days postinfection. In agreement with the flow cytometric findings, the output of cutaneous IL-17 was decreased significantly in preparations from EtOH-fed mice (Table 1). Consistent with this, a corresponding decrease was also observed in the production of GM-CSF, an IL-17 target that promotes PMN differentiation, survival, and key effector functions, including chemotaxis [32–36]. Interestingly, the EtOH-induced decrease in IL-17 and GM-CSF did not occur alongside a decrease in the PMN-attracting chemokines CXCL2 and KC, suggesting that the induction of these chemoattractants occurs through alternative mechanisms that are not suppressed by EtOH exposure. Overall, these findings indicate that the poor accumulation and dysfunction of cutaneous PMN in EtOH-exposed skin correspond with impaired induction of proinflammatory cytokines needed for protection against S. aureus (Figs. 2 and 3).
TABLE 1.
IL-17 and GM-CSF are reduced in lesional skin of EtOH-fed mice
| Cytokine/chemokine | H2O mice | EtOH mice | P |
|---|---|---|---|
| IL-17 | 34 ± 8.9a | 6 ± 1.1 | 0.01 |
| GM-CSF | 299 ± 41.2 | 149 ± 42.1 | 0.04 |
| KC | 7976 ± 892 | 8976 ± 1974 | 0.55 |
| CXCL2 | 40,493 ± 10,296 | 39,734 ± 15,474 | 0.88 |
All values reported as pg/ml.
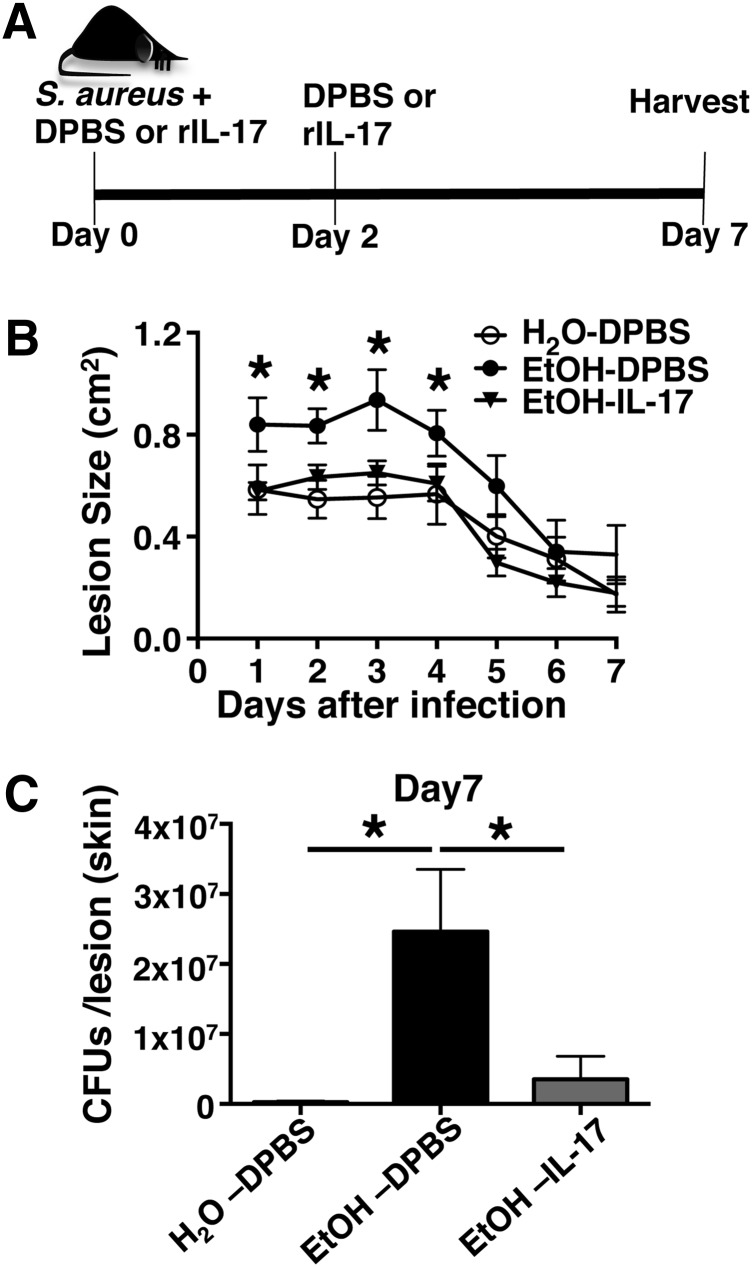
i.d. Administration of rIL-17 improves S. aureus clearance
Given the critical role of IL-17 in anti-S. aureus cutaneous host defense, restoration with exogenous rIL-17 was tested as a potential means to improve cutaneous bacterial clearance in EtOH-fed mice [23]. To this end, EtOH-fed or age-matched controls were delivered an i.d. dose of rIL-17, immediately following infection, as well as a 2nd dose, 2 days after infection (Fig. 6A). When compared with EtOH-fed mice receiving a DPBS injection, administration of IL-17 significantly reduced cutaneous injury and bacterial burden after infection (Fig. 6B and C). Thus, these data indicate that the decreased production of IL-17 is an integral means by which EtOH impairs cutaneous clearance of S. aureus.
Figure 6. i.d. Administration of rIL-17 improves lesion size and S. aureus clearance.
(A) Diagram of the rIL-17 intervention strategy (see Materials and Methods for further details). (B) Lesion area following S. aureus skin infection following indicated treatments. Statistical significance corresponds to comparisons of EtOH-fed mice receiving rIL-17 administration versus vehicle alone (DPBS). (C) Bacterial load of skin lesions, 7 days postinfection from EtOH-fed and age-matched H2O controls receiving rIL-17 administration or vehicle alone (DPBS). Error bars represent sem. *P < 0.05. Data are representative of 2 independent experiments; n ≥ 4 mice/treatment group.
DISCUSSION
Cutaneous immune deficiency commonly occurs in chronic alcoholics, which is reflected by their predisposition to skin infections caused by a wide range of microbes, including S. aureus [4–7, 37, 38]. In experiments with preimmune mice, we showed previously that chronic EtOH consumption alters the composition and function of the cutaneous immune system [14, 24, 26]. Foremost among these changes is subset loss and hyporesponsiveness in skin dendritic cells and T cells implicated in anti-S. aureus host defense (e.g., IL-17-producing γδ T cells) [14, 24]. With the use of a mouse model of chronic alcoholism, in association with cutaneous S. aureus challenge, the significance of these EtOH-induced alterations was examined within the context of infection. The results show, for the first time in a murine model of alcoholism, that chronic EtOH consumption enhances the severity of S. aureus skin infection. This finding builds on a previous report by Di Luzio and Williams [39], which demonstrated that chronic EtOH consumption causes extensive hepatic and renal pathology and markedly elevates mortality following i.v. S. aureus infection. The present study reiterates the deleterious effects of chronic EtOH exposure on anti-S. aureus host defense and provides new information about the pathophysiological consequences of such exposure in the skin, where most S. aureus infections are initiated [2]. In our model, manifestations of the EtOH-induced exacerbation of local and systemic staphylococcal disease include greater skin injury and cutaneous bacterial burden, as well as increased morbidity (weight loss) and bacterial dissemination (Fig. 1). Thus, like their human counterparts, mice chronically consuming EtOH experience exacerbated disease following S. aureus infections.
The recruitment of PMNs to sites of cutaneous S. aureus infection is critical for pathogen clearance [15]. The present study demonstrates that chronic EtOH feeding alters the dynamics of PMN accumulation at infected loci (Fig. 2A). The equivalent density of PMNs observed in the infected skin of EtOH-fed and control mice at day 1 postinfection is not altogether surprising, given that the baseline number of circulating PMNs is unaltered by chronic EtOH feeding [13]. Furthermore, the similar magnitude of PMN infiltration suggests that the immediate availability of chemotactic cues attracting PMNs at the cutaneous focus of infection is unaltered by EtOH. Given the increase in cutaneous bacterial load at day 1 postinfection, it likely that S. aureus-derived chemotactic factors, such as fMLP, are more abundant in EtOH-exposed skin than in control skin at this time-point [15]. However, at 3 days after infection, the increase in S. aureus CFUs occurred alongside decreased density of skin PMNs in EtOH-fed mice (Figs. 1D and 2A), suggesting that bacterial products alone are not sufficient to mediate enduring, cutaneous PMN responses. (The expected increase in cutaneous PMN accumulation and decreased bacterial burden were evident in the skin of control mice.) Thus, whereas similar numbers of blood PMNs are poised to respond to cutaneous infection in control and EtOH-fed mice, the maintenance of robust PMN responses in the latter group of animals is profoundly impaired.
Mature PMNs accumulate in S. aureus skin wounds as a result of accelerated granulopoietic activity, not only in the bone marrow but also at the site of infection [21, 40]. The latter process (that accounts for ∼20% of the cutaneous anti-S. aureus PMN response) involves the in situ differentiation of mature PMNs from hematopoietic progenitor cells that have trafficked directly into the S. aureus abscess environment [21, 40]. Therefore, an EtOH-induced impairment in granulopoiesis in cutaneous wound beds could also contribute to the inability of EtOH-fed mice to manufacture sustained PMN responses in infected skin. Another factor contributing to persistent PMN activity within S. aureus skin wounds is the prolongation of PMN survival, which is conferred by the milieu of the wound bed itself. Compared with their circulating counterparts, the half-life of PMNs that enter into S. aureus-infected skin is extended from 6 to 8 h to several days, raising the possibility that altered dynamics of cell survival contribute to the EtOH-induced decline in PMN densities in the skin lesions at day 3 postinfection [40].
Given that all of the aforementioned potential mechanistic causes of reduced density of cutaneous PMNs in EtOH-fed mice (i.e., recruitment, differentiation, survival) depend on the local induction of inflammation, the impact of EtOH upon cytokine production at the site of infection was explored [23, 40, 41]. Beginning with intracellular cytokine staining after ex vivo culture, the density of IL-1β− and IL-23-producing CD11b+ myeloid cells was shown to be severely reduced in EtOH-exposed skin, particularly at day 3 after infection (Fig. 4). The EtOH-induced inhibition of myeloid cell-derived IL-23 is congruent with previous work, showing the suppressive effects of EtOH upon IL-23 induction following pulmonary bacterial challenge [42]. Consistent with the diminished capacity of myeloid cells to produce IL-23 and IL-1β, the production of IL-17 by γδ T cells was blunted in EtOH-fed mice. Importantly, the inhibitory effects of EtOH upon IL-17 induction by skin T cells following PMA/ionomycin stimulation (Fig. 5) were corroborated by direct measurements of IL-17 in lesional skin homogenates (Table 1), 3 days after infection. Together, depressed inflammatory cytokine responses in the skin correlate well with the onset of increased lesion size in the EtOH-fed mice.
By providing further insight into the decreased density of PMNs in EtOH-exposed skin at day 3 after infection, the IL-17-inducible cytokine, GM-CSF, was reduced significantly (Table 1). GM-CSF has been shown to augment fMLP-directed chemotaxis, prolong PMN survival, and promote granulopoiesis [32–36]. Interestingly, the EtOH-induced decrease in IL-17 did not occur alongside a decrease in the PMN-attracting chemokines CXCL2 and KC nor did EtOH impact the expression of the receptor of these chemokines (i.e., CXCR2) on cutaneous PMNs (Supplemental Fig. 1). Taken together, the reduced density of PMNs in EtOH-exposed skin, 3 days postinfection, correlates with an altered inflammatory milieu within S. aureus-infected wounds. Notable among these alterations is the reduced induction of GM-CSF, which would have clear implications for PMN differentiation and survival at the site of infection [32–36].
Unlike the cutaneous site of infection, where the abundance of pathogen- and tissue damage-derived signals may be sufficient to orchestrate prompt PMN responses, LNs are removed from the focus of bacterial growth and tissue injury and thus, are more dependent on the active orchestration of cytokine/chemokine cues to mediate PMN accumulation in this site [29]. Perhaps not surprisingly then, in contrast to infected skin where PMN responses are poorly sustained, in skin draining LNs, such responses are poorly initiated (Fig. 2B). The influx of PMNs into LNs is also mediated by the induction of inflammatory cytokines [29, 43]. IL-17, in particular, plays a pivotal role in the orchestration of PMN accumulation in the LNs following sterile and infection-derived inflammation. Consistent with the EtOH-induced reduction in IL-17 production by LN T cells, reduced PMN accumulation in skin draining LNs of EtOH-fed mice was also observed after S. aureus infection (Figs. 2B and 5B). Given the importance of LNs as strategic sites of PMN-mediated antimicrobial responses, the dearth of PMNs in this location is congruent with increased bacterial dissemination occurring in EtOH-fed mice (Fig. 1 and see ref. [29]).
Successful defense against S. aureus skin infection is highly dependent on effective PMN function. Highly specialized for microbial killing, PMNs possess an array of cytotoxic compounds (e.g., reactive oxygen species and antimicrobial peptides) that work synergistically to kill extracellular pathogens, such as S. aureus [15]. In general, the efficacy of these weapons depends on their ability to be concentrated together within bacteria-laden phagosomes [3, 15, 31]. Thus, this study first evaluated the impact of EtOH on PMN function by assessing phagocytosis. Although no significant differences were observed in this parameter between control and EtOH-fed mice (Fig. 3), the reduced density of PMNs in the skin of EtOH-fed mice, 3 days after infection, implies a compromised ability of the entire PMN network to capture and kill skin-invading S. aureus at this time-point.
Although the capacity of PMNs to ingest S. aureus is unaltered by EtOH, the ultimate fate of these organisms may still vary considerably between EtOH-exposed and control PMNs. A hypothesis derived from the observed EtOH-elicited increase in bacterial burden is that defective bacterial killing in EtOH-exposed PMNs underlies the inability of EtOH-fed mice to control and clear infection (Fig. 1). Given that the direct assessment of S. aureus killing by purified cutaneous PMNs is hampered by poor cell recovery following isolation procedures, the impact of EtOH on phenotypic aspects of PMNs implicated in antimicrobial function was examined.
Evidence that EtOH adversely impacts the capacity of extravasated PMNs to kill S. aureus is supported by the EtOH-induced reduction in CRAMP expression at day 1 postinfection (Fig. 3C). CRAMP is a prominent cationic antimicrobial peptide in murine PMNs, conferring microbicidal activity against S. aureus [31, 44]. With the demonstration of its importance to cutaneous host defense, CRAMP-deficient mice experience larger skin lesions and exacerbated invasive disease following cutaneous challenge with Group A Streptococcus [45]. Through its recruitment to pathogen-containing phagosomes, CRAMP participates in the nonoxidative degradation of phagocytosed S. aureus [31]. As such, it is likely that the EtOH-induced reduction in the expression of this antimicrobial compound weakens the ability of EtOH-exposed PMNs to destroy ingested microbes.
Additionally, EtOH feeding decreased PMN expression of TLR2 (Fig. 3C), which mediates the recognition of S. aureus-derived products (e.g., lipopetides, peptidoglycan) [3, 46]. The loss of TLR2 corresponds with heightened vulnerability to S. aureus after skin and systemic challenge [19, 23, 47]. As a PMN effector molecule, TLR2 facilitates a broad range of functional responses. For instance, stimulation of human PMNs with palmitoyl-3-cysteine-serine-lysine-4 (a TLR2 agonist) in the presence of GM-CSF triggers IL-8 production and superoxide-priming responses [30]. Therefore, a likely consequence of the EtOH-induced reduction in TLR2 expression is a diminished capacity of PMNs to instruct additional PMN recruitment and mediate oxidative killing mechanisms. Recently, TLR2 was also demonstrated to be required for DNA-neutrophil extracellular trap (netosis) responses mediating bacterial containment [48]. Thus, reduced TLR2 expression in EtOH-exposed PMNs would be expected to facilitate the systemic spread of skin-invading S. aureus. The reduced expression of TLR2 and CRAMP on PMNs recovered from the infected skin of EtOH-fed mice suggests that chronic EtOH feeding impairs key PMN functions involved in controlling and clearing S. aureus.
TLR2 expression is increased in GM-CSF-stimulated PMNs. IL-17 induces production of this growth factor; thus, reduced TLR2 expression among cutaneous PMNs from EtOH-fed mice may be mediated, in part, by the diminished capacity of skin T cells from EtOH-fed mice to produce IL-17 (Fig. 5B). Taken together, the reduced expression of TLR2 and CRAMP on PMNs recovered from the infected skin of EtOH-fed mice suggests that chronic EtOH feeding impairs key PMN functions involved in controlling and clearing S. aureus. Demonstrating that the local inhibition of IL-17 responses represents an integral mechanism by which EtOH impairs host defense, cutaneous wound resolution and bacterial clearance were improved significantly following administration of rIL-17 (Fig. 6).
In conclusion, herein, we show for the first time that chronic EtOH consumption increases the severity of local injury and invasive illness following S. aureus cutaneous challenge. Exacerbated S. aureus pathogenicity in EtOH-fed mice corresponded with multiple signs of local immune suppression, including reduced PMN accumulation at cutaneous sites of infection and poor IL-17 induction. Importantly, local treatment with rIL-17 corrected the increased lesion size and cutaneous bacterial load elicited by EtOH feeding.
That skin ulcers were ultimately able to heal in EtOH-fed mice is likely a reflection of the selective and incomplete suppression of anti-S. aureus immune responses as a result of EtOH exposure. For example, phagocytosis is not impaired in neutrophils from EtOH-fed mice nor was a defect observed in CXCL2 or KC induction. Nonetheless, the state of cutaneous immune deficiency elicited by chronic EtOH feeding is not trivial, as it alters the pathologic course of S. aureus infection by enhancing acute injury and allowing for increased bacterial dissemination to distant sites. Such alterations would likely have serious and potentially life-threatening implications for alcoholic patient populations who frequently present with additional comorbidities, e.g., liver cirrhosis, which would further hamper immune homeostasis and host defense [49, 50]. Taken together, this skin infection model in the presence of chronic EtOH feeding will provide many additional opportunities to increase understanding of mechanisms underlying skin-infection risk and resultant septicemia in alcoholics and ultimately, devise rational immunotherapies for these illnesses.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health National Institute on Alcohol Abuse and Alcoholism Grant R01 AA019568 and the Carver College of Medicine Department of Pathology. The authors thank Drs. Kevin Legge and David Meyerholz for their critical reading of this manuscript. The authors also thank the Comparative Pathology Laboratory at the University of Iowa for technical assistance with cytospin image preparation, Michael Shay for his technical assistance with the collection cytokine measurements from infected skin, and members of the University of Iowa Hematopathology Laboratory for their assistance with Wright-Giemsa staining.
Glossary
- CRAMP
cathelicidin-related antimicrobial peptide
- DPBS
Dulbecco’s PBS
- EtOH
ethanol
- KC
keratinocyte chemokine
- LN
lymph node
- PMN
polymorphonuclear neutrophil
- RORγt
retinoid-related orphan receptor γt
- RPMIc
RPMI with 10% FBS, L-glutamine-penicillin-streptomycin, and 2-ME
- TSA
tryptic soy agar
- TSB
tryptic soy broth
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
AUTHORSHIP
C.P.P. designed and performed experiments, analyzed data, and wrote the paper. J.S.K. characterized the S. aureus clinical isolate, provided technical support, and edited the manuscript. A.R.H. provided reagents and tools and edited the manuscript. A.J.S. designed experiments and wrote the paper. All authors discussed the results and implications of the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Miller L. G., Diep B. A. (2008) Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 46, 752–760. [DOI] [PubMed] [Google Scholar]
- 2.Chambers H. F., Deleo F. R. (2009) Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7, 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller L. S., Cho J. S. (2011) Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 11, 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capdevila O., Grau I., Vadillo M., Cisnal M., Pallares R. (2003) Bacteremic pneumococcal cellulitis compared with bacteremic cellulitis caused by Staphylococcus aureus and Streptococcus pyogenes. Eur. J. Clin. Microbiol. Infect. Dis. 22, 337–341. [DOI] [PubMed] [Google Scholar]
- 5.Forsblom E., Ruotsalainen E., Mölkänen T., Ollgren J., Lyytikäinen O., Järvinen A. (2011) Predisposing factors, disease progression and outcome in 430 prospectively followed patients of healthcare- and community-associated Staphylococcus aureus bacteraemia. J. Hosp. Infect. 78, 102–107. [DOI] [PubMed] [Google Scholar]
- 6.Kaech C., Elzi L., Sendi P., Frei R., Laifer G., Bassetti S., Fluckiger U. (2006) Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin. Microbiol. Infect. 12, 345–352. [DOI] [PubMed] [Google Scholar]
- 7.Skiest D. J., Brown K., Cooper T. W., Hoffman-Roberts H., Mussa H. R., Elliott A. C. (2007) Prospective comparison of methicillin-susceptible and methicillin-resistant community-associated Staphylococcus aureus infections in hospitalized patients. J. Infect. 54, 427–434. [DOI] [PubMed] [Google Scholar]
- 8.Adewale A., Ifudu O. (2014) Kidney injury, fluid, electrolyte and acid-base abnormalities in alcoholics. Niger. Med. J. 55, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacGregor R. R. (1986) Alcohol and immune defense. JAMA 256, 1474–1479. [PubMed] [Google Scholar]
- 10.Song K., Coleman R. A., Zhu X., Alber C., Ballas Z. K., Waldschmidt T. J., Cook R. T. (2002) Chronic ethanol consumption by mice results in activated splenic T cells. J. Leukoc. Biol. 72, 1109–1116. [PubMed] [Google Scholar]
- 11.Meyerholz D. K., Edsen-Moore M., McGill J., Coleman R. A., Cook R. T., Legge K. L. (2008) Chronic alcohol consumption increases the severity of murine influenza virus infections. J. Immunol. 181, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurung P., Young B. M., Coleman R. A., Wiechert S., Turner L. E., Ray N. B., Waldschmidt T. J., Legge K. L., Cook R. T. (2009) Chronic ethanol induces inhibition of antigen-specific CD8+ but not CD4+ immunodominant T cell responses following Listeria monocytogenes inoculation. J. Leukoc. Biol. 85, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook R. T., Schlueter A. J., Coleman R. A., Tygrett L., Ballas Z. K., Jerrells T. R., Nashelsky M. B., Ray N. B., Haugen T. H., Waldschmidt T. J. (2007) Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion—absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol. Clin. Exp. Res. 31, 1746–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parlet C. P., Waldschmidt T. J., Schlueter A. J. (2014) Chronic ethanol feeding induces subset loss and hyporesponsiveness in skin T cells. Alcohol. Clin. Exp. Res. 38, 1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spaan A. N., Surewaard B. G., Nijland R., van Strijp J. A. (2013) Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu. Rev. Microbiol. 67, 629–650. [DOI] [PubMed] [Google Scholar]
- 16.Oyoshi M. K., He R., Li Y., Mondal S., Yoon J., Afshar R., Chen M., Lee D. M., Luo H. R., Luster A. D., Cho J. S., Miller L. S., Larson A., Murphy G. F., Geha R. S. (2012) Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity 37, 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng L. G., Qin J. S., Roediger B., Wang Y., Jain R., Cavanagh L. L., Smith A. L., Jones C. A., de Veer M., Grimbaldeston M. A., Meeusen E. N., Weninger W. (2011) Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J. Invest. Dermatol. 131, 2058–2068. [DOI] [PubMed] [Google Scholar]
- 18.Mölne L., Verdrengh M., Tarkowski A. (2000) Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect. Immun. 68, 6162–6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller L. S., O’Connell R. M., Gutierrez M. A., Pietras E. M., Shahangian A., Gross C. E., Thirumala A., Cheung A. L., Cheng G., Modlin R. L. (2006) MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24, 79–91. [DOI] [PubMed] [Google Scholar]
- 20.Myles I. A., Fontecilla N. M., Valdez P. A., Vithayathil P. J., Naik S., Belkaid Y., Ouyang W., Datta S. K. (2013) Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat. Immunol. 14, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granick J. L., Falahee P. C., Dahmubed D., Borjesson D. L., Miller L. S., Simon S. I. (2013) Staphylococcus aureus recognition by hematopoietic stem and progenitor cells via TLR2/MyD88/PGE2 stimulates granulopoiesis in wounds. Blood 122, 1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mölne L., Corthay A., Holmdahl R., Tarkowski A. (2003) Role of gamma/delta T cell receptor-expressing lymphocytes in cutaneous infection caused by Staphylococcus aureus. Clin. Exp. Immunol. 132, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho J. S., Pietras E. M., Garcia N. C., Ramos R. I., Farzam D. M., Monroe H. R., Magorien J. E., Blauvelt A., Kolls J. K., Cheung A. L., Cheng G., Modlin R. L., Miller L. S. (2010) IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parlet C. P., Schlueter A. J. (2013) Mechanisms by which chronic ethanol feeding impairs the migratory capacity of cutaneous dendritic cells. Alcohol. Clin. Exp. Res. 37, 2098–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igyártó B. Z., Haley K., Ortner D., Bobr A., Gerami-Nejad M., Edelson B. T., Zurawski S. M., Malissen B., Zurawski G., Berman J., Kaplan D. H. (2011) Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ness K. J., Fan J., Wilke W. W., Coleman R. A., Cook R. T., Schlueter A. J. (2008) Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcohol. Clin. Exp. Res. 32, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouchi T., Kubo A., Yokouchi M., Adachi T., Kobayashi T., Kitashima D. Y., Fujii H., Clausen B. E., Koyasu S., Amagai M., Nagao K. (2011) Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 208, 2607–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chtanova T., Schaeffer M., Han S. J., van Dooren G. G., Nollmann M., Herzmark P., Chan S. W., Satija H., Camfield K., Aaron H., Striepen B., Robey E. A. (2008) Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29, 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kastenmüller W., Torabi-Parizi P., Subramanian N., Lämmermann T., Germain R. N. (2012) A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 150, 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurt-Jones E. A., Mandell L., Whitney C., Padgett A., Gosselin K., Newburger P. E., Finberg R. W. (2002) Role of Toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood 100, 1860–1868. [PubMed] [Google Scholar]
- 31.Jann N. J., Schmaler M., Kristian S. A., Radek K. A., Gallo R. L., Nizet V., Peschel A., Landmann R. (2009) Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J. Leukoc. Biol. 86, 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang P., Quinton L. J., Gamble L., Bagby G. J., Summer W. R., Nelson S. (2005) The granulopoietic cytokine response and enhancement of granulopoiesis in mice during endotoxemia. Shock 23, 344–352. [DOI] [PubMed] [Google Scholar]
- 33.Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. (2007) IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852. [DOI] [PubMed] [Google Scholar]
- 34.Khajah M., Millen B., Cara D. C., Waterhouse C., McCafferty D. M. (2011) Granulocyte-macrophage colony-stimulating factor (GM-CSF): a chemoattractive agent for murine leukocytes in vivo. J. Leukoc. Biol. 89, 945–953. [DOI] [PubMed] [Google Scholar]
- 35.Kumaratilake L. M., Ferrante A., Jaeger T., Rzepczyk C. (1996) GM-CSF-induced priming of human neutrophils for enhanced phagocytosis and killing of asexual blood stages of Plasmodium falciparum: synergistic effects of GM-CSF and TNF. Parasite Immunol. 18, 115–123. [DOI] [PubMed] [Google Scholar]
- 36.Fossati G., Mazzucchelli I., Gritti D., Ricevuti G., Edwards S. W., Moulding D. A., Rossi M. L. (1998) In vitro effects of GM-CSF on mature peripheral blood neutrophils. Int. J. Mol. Med. 1, 943–951. [DOI] [PubMed] [Google Scholar]
- 37.Skogberg K., Simonen H., Renkonen O. V., Valtonen V. V. (1988) Beta-haemolytic group A, B, C and G streptococcal septicaemia: a clinical study. Scand. J. Infect. Dis. 20, 119–125. [DOI] [PubMed] [Google Scholar]
- 38.Smith K. E., Fenske N. A. (2000) Cutaneous manifestations of alcohol abuse. J. Am. Acad. Dermatol. 43, 1–16, quiz 16–18. [DOI] [PubMed] [Google Scholar]
- 39.Di Luzio N. R., Williams D. L. (1980) Enhancement of host susceptibility to Staphylococcus aureus infection by chronic ethanol ingestion—modification by glucan immunostimulation. Alcohol. Clin. Exp. Res. 4, 254–260. [DOI] [PubMed] [Google Scholar]
- 40.Kim M. H., Granick J. L., Kwok C., Walker N. J., Borjesson D. L., Curry F. R., Miller L. S., Simon S. I. (2011) Neutrophil survival and c-kit(+)-progenitor proliferation in Staphylococcus aureus-infected skin wounds promote resolution. Blood 117, 3343–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatnagar N., Hong H. S., Krishnaswamy J. K., Haghikia A., Behrens G. M., Schmidt R. E., Jacobs R. (2010) Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood 116, 1308–1316. [DOI] [PubMed] [Google Scholar]
- 42.Happel K. I., Odden A. R., Zhang P., Shellito J. E., Bagby G. J., Nelson S. (2006) Acute alcohol intoxication suppresses the interleukin 23 response to Klebsiella pneumoniae infection. Alcohol. Clin. Exp. Res. 30, 1200–1207. [DOI] [PubMed] [Google Scholar]
- 43.Brackett C. M., Muhitch J. B., Evans S. S., Gollnick S. O. (2013) IL-17 promotes neutrophil entry into tumor-draining lymph nodes following induction of sterile inflammation. J. Immunol. 191, 4348–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisenhauer P. B., Lehrer R. I. (1992) Mouse neutrophils lack defensins. Infect. Immun. 60, 3446–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nizet V., Ohtake T., Lauth X., Trowbridge J., Rudisill J., Dorschner R. A., Pestonjamasp V., Piraino J., Huttner K., Gallo R. L. (2001) Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457. [DOI] [PubMed] [Google Scholar]
- 46.Fournier B. (2013) The function of TLR2 during staphylococcal diseases. Front. Cell. Infect. Microbiol. 2, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi O., Hoshino K., Akira S. (2000) Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165, 5392–5396. [DOI] [PubMed] [Google Scholar]
- 48.Yipp B. G., Petri B., Salina D., Jenne C. N., Scott B. N., Zbytnuik L. D., Pittman K., Asaduzzaman M., Wu K., Meijndert H. C., Malawista S. E., de Boisfleury Chevance A., Zhang K., Conly J., Kubes P. (2012) Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H. J., Gao B., Zakhari S., Nagy L. E. (2012) Inflammation in alcoholic liver disease. Annu. Rev. Nutr. 32, 343–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson S., Kolls J. K. (2002) Alcohol, host defence and society. Nat. Rev. Immunol. 2, 205–209. [DOI] [PubMed] [Google Scholar]