Cocaine’s effect on HIV-1 integration is facilitated by its ability to enhance preintegration complex mediated integration activity.
Keywords: preintegration complex, Raltegravir, mass spectrometry
Abstract
Epidemiologic studies suggest that cocaine abuse worsens HIV-1 disease progression. Increased viral load has been suggested to play a key role for the accelerated HIV disease among cocaine-abusing patients. The goal of this study was to investigate whether cocaine enhances proviral DNA integration as a mechanism to increase viral load. We infected CD4+ T cells that are the primary targets of HIV-1 in vivo and treated the cells with physiologically relevant concentrations of cocaine (1 µM–100 µM). Proviral DNA integration in the host genome was measured by nested qPCR. Our results illustrated that cocaine from 1 µM through 50 µM increased HIV-1 integration in CD4+ T cells in a dose-dependent manner. As integration can be modulated by several early postentry steps of HIV-1 infection, we examined the direct effects of cocaine on viral integration by in vitro integration assays by use of HIV-1 PICs. Our data illustrated that cocaine directly increases viral DNA integration. Furthermore, our MS analysis showed that cocaine is able to enter CD4+ T cells and localize to the nucleus-. In summary, our data provide strong evidence that cocaine can increase HIV-1 integration in CD4+ T cells. Therefore, we hypothesize that increased HIV-1 integration is a novel mechanism by which cocaine enhances viral load and worsens disease progression in drug-abusing HIV-1 patients.
Introduction
In the United States, an estimated 1.2 million people are living with HIV [1]. Although ART has dramatically reduced HIV/AIDS-related mortality [2], the latest U.S. Centers for Disease Control and Prevention report underscores that only ∼25% of the HIV-infected patients have their virus under control, and ∼18% of the infected individuals are unaware that they carry the virus [1]. The problem of HIV/AIDS is accentuated further by substance abuse that serves as a powerful cofactor for increased transmission, higher viral load, and enhanced disease progression [3–6]. Cocaine, a commonly abused drug among HIV patients [7], has been associated with accelerated HIV disease and AIDS-related mortality even among ART-adherent patients [8–17]. Although several mechanisms have been proposed, increased viral load has been suggested to play a key role for the accelerated disease seen among cocaine-abusing HIV patients. This paradigm is supported by a body of literature that shows cocaine’s potentiating effect on HIV-1 replication in vitro and animal studies. For example, we [18] and others [19] have shown that cocaine enhances HIV-1 replication in the pure cultures of CD4+ T cells. In addition, it has been demonstrated that cocaine increases HIV-1 replication in the cultures of human PBMCs [20, 21], macrophages [22], astrocytes [23], and DCs [24]. Notably, cocaine has also been shown to increase HIV-1 replication in humanized mice models [25, 26]. Even though the immunomodulatory effects of cocaine are well established [27], the mechanism by which cocaine potentiates HIV-1 replication in vitro and in vivo is not clearly understood.
Published data suggest that cocaine modulates entry and postentry steps of HIV-1 infection. Cocaine has been shown to down-regulate HIV-suppressing chemokines [28, 29] and up-regulate the HIV-1 entry coreceptors [28]. As these cellular regulators modulate virus entry, it has been proposed that cocaine can increase HIV-1 replication by enhancing entry of HIV-1 into target cells. Furthermore, proteomics studies have suggested that cocaine can regulate an array of cellular proteins in astrocytes [23]. Although the direct effects of these factors on HIV-1 infection remain unclear, the authors suggested that these cellular factors may influence HIV-1 replication. We have demonstrated previously that cocaine enhances HIV-1 protein translation in CD4+ T cells by down-regulating the cellular miRNA “miR-125b” [18], which belongs to a family of anti-HIV cellular miRNAs that inhibit HIV-1 by reducing viral protein translation by binding to viral transcripts [30]. Recently, it has been described that cocaine can enhance replication of R5 tropic HIV-1 virions in quiescent CD4+ T cells by increasing reverse transcription of the viral RNA genome [19]. Collectively, these studies suggest that cocaine modulates entry and postentry steps of HIV-1. However, cocaine’s effect on HIV-1 integration has not been elucidated.
HIV-1 integration involves insertion of the reverse-transcribed proviral dsDNA into the host genome [31–33]. This intricate postentry step is carried out by the virally encoded integrase enzyme in a multistep enzymatic process that involves 3′ processing of the proviral dsDNA and strand transfer activity [31]. Given that HIV-1 integration is one of the critical steps in HIV-1 infection and replication, we examined the effects of cocaine on HIV-1 integration in CD4+ T cells that serve as primary targets of HIV-1 in vivo [33]. Data presented in this report demonstrate that cocaine increases HIV-1 infection in a dose-dependent manner. Notably, nested qPCR analysis revealed that cocaine treatment enhances HIV-1 integration in a concentration-dependent manner. A direct effect of cocaine on HIV-1 integration is elucidated by carrying out an in vitro integration assay by use of HIV-1 PICs. MS analysis illustrates that cocaine can enter the cytoplasm and localize to the nucleus of CD4+ T cells. In sum, our results provide strong evidence that cocaine can increase HIV-1 integration in CD4+ T cells and may explain the enhancement of HIV-1 replication in vitro and in vivo.
MATERIALS AND METHODS
Reagents
Cocaine hydrochloride and Benzoylecgonine solution was purchased from Sigma Chemical (St. Louis, MO, USA). HIV-1 p24 antibody KC57-FITC was obtained from Beckman Coulter (Brea, CA, USA). Raltegravir was obtained from NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID. ACH-2 and TZM-bl cells were obtained from NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID: “SupT1” cells were obtained from American Type Culture Collection (Manassas, VA, USA).
Isolation of PBMCs, purification of CD4+ T cells, and cell culture
Blood from healthy donors was purchased from the New York Blood Center, per the Meharry Medical College Institutional Review Board. PBMCs from human blood were isolated by Ficoll-based reagents, per our published methods [18, 34, 35]. In brief, human blood was diluted 1:2 with PBS, and 25 ml diluted blood was overlaid on 12.5 ml Ficoll-Paque premium reagent (GE Healthcare Life Sciences, Pittsburgh, PA, USA) in a 50 ml conical tube and centrifuged at 750 g. Thereafter, the interphase cells (PBMCs) were transferred carefully to a new 50 ml tube and were centrifuged several times and washed with PBS to remove unwanted cell types. The resulting cell pellet was resuspended in PBS, followed by isolation of CD4+ T lymphocytes by magnetic bead-based negative selection, as described previously [18, 34, 35]. The purity of CD4+ T cells was measured by flow cytometry. CD4+ T cells with purity >95% were activated by PHA (5 µg/ml) for 48 h and maintained in RPMI with 20% heat-inactivated FBS, 2 mM l-glutamine, antibiotics, and IL-2 (20 U/ml; Sigma Chemical). The CD4+ T cell line SupT1 was maintained in complete RPMI medium containing 10% heat-inactivated FBS, 2 mM l-glutamine, and antibiotics. Given that cocaine can be degraded by serum esterases and other enzymes, heat-inactivated serum was used to inactivate the enzymes, including esterases in the media.
Virus production
For generating infectious HIV-1 virions, we used the supernatants of chronically infected ACH-2 cells that produce the X4 tropic HIV-1 LAI virions, per our published methods [18, 34, 35]. In brief, PMA- and TNF-α-activated ACH-2 cells were plated, and the virus containing supernatant was collected. We generated VSV-G-pseudotyped HIV-1 by transfecting the pNL4.3 molecular clone of HIV-1 and pVSV-G into 293T cells by use of Lipofectamine (Life Technologies, Carlsbad, CA, USA). After 48 h, culture supernatant containing the VSV-G-pseudotyped virions was collected, centrifuged, and filtered through a 0.45 µM membrane. Concentration of the virions was determined by p24 ELISA assay, and infectivity was measured by luciferase reporter assay by use of TZM-bl cells, which harbor a firefly luciferase reporter gene under the control of the HIV-1 promoter, as described [36].
HIV-1 infection
Cells were treated with cocaine at concentrations ranging from 1 to 100 µM to cover the wide range of concentrations reported in the plasma of cocaine users [37–42]. Activated primary CD4+ T cells (2 × 106 cells) and PBMCs (5 × 106 cells) were infected with HIV-1 LAI, and SupT1 cells were infected with HIV-1 LAI or VSV-G-pseudotyped HIV-1 by spinoculation in the presence of polybrene (Sigma Chemical), as described before [18, 34, 35]. Productive infection was measured by FACS by detecting intracellular HIV-1 p24 protein, per our published methods [18, 34, 35].
Measurement of HIV-1 integration
Integration of HIV-1 proviral DNA in infected cells was measured by nested real-time PCR analysis by use of a previously described method [43] with some modifications. In brief, genomic DNA from infected cells was isolated by QIAamp genomic DNA isolation kit (Qiagen, Valencia, CA, USA). The first-round PCR reaction was carried out by use of primers that target the host Alu sequence and viral LTR sequences designed to amplify the junctions of the integrated viral DNA but not the unintegrated viral DNA [43, 44]. For the second-round PCR, the primer sets were designed to enrich the viral LTR regions from the PCR products of the first round [43, 44]. Integrated viral DNA copy numbers were determined by plotting values derived from a standard curve by use of the molecular clone of HIV “pNL4.3” plasmid as the standard. All reactions were performed in triplicates. Data were analyzed with the CFX software (Bio-Rad Laboratories, Hercules, CA, USA).
PIC preparation and integration assay
HIV-1 PICs were generated by use of a published method [45]. For preparing PICs, we produced virions by transfecting the molecular clone pNL4.3 into human embryonic kidney 293 T cells by use of PolyFect (Qiagen). After 48 h of transfection, the supernatant containing the HIV-1 virions was collected, centrifuged, and filtered through a 0.45 μm filter. Thereafter, the virion preparation was treated with 40 U/ml Turbo DNase (Life Technologies) for 1 h at 37°C. SupT1 cells (4 × 107) were infected with DNase-treated virions by spinoculation for 2 h, and then the cells were incubated for an additional 5 h at 37°C. For isolating PICs, the infected SupT1 cells were harvested at 300 g for 10 min. The cell pellet was washed twice with buffer K−/− (20 mM HEPES, pH 7.6, 150 mM KCl, 5 mM MgCl2) at room temperature. Subsequently, the pellet was resuspended in 1 ml ice-cold buffer K+/+ (20 mM HEPES, pH 7.6, 150 mM KCl, 5 mM MgCl2, 1 mM DTT, 20 µg/ml aprotinin, 0.025% w/v digitonin), transferred to a 1.5 ml microcentrifuge tube, and rocked on a Nutator platform for 10 min at room temperature. Then, the cytoplasmic fractions were separated from the nuclear fractions by centrifugation and treated with RNase A (20 µg/ml) to degrade cellular mRNA. This cytoplasmic fraction was used as the source of HIV-1 PICs for in vitro integration assay.
In vitro integration assay was performed by use of a modified version of the protocol described previously [45]. We used a 2.0 kb DNA fragment as the target that has been used previously for in vitro integration assays by use of PICs [44]. This DNA target was PCR amplified from the ΦX174 genome by use of specific primers pairs: forward primer, 5′CGC TTC CAT GAC GCA GAA GTT 3′, and reverse primer, 5′CAC TGA CCC TCA GCA ATC TTA 3′. The amplified PCR product was gel purified. The integration assay was carried out by adding 0.2 ml PICs and 3 μg/ml target DNA in the absence or presence of cocaine (1–100 µM) and or Raltegravir (1 µM). The integration assay was also carried out in the presence of benzoylecgonine (1–100 µM). The PICs and target DNA were incubated at 37°C for 45 min. The reaction was stopped by adding SDS (0.5%), EDTA (8 mM), and proteinase K (0.5 mg/ml) and incubated overnight at 56°C. The DNA from the reaction mixture was precipitated by ethanol, followed by a phenol/chloroform extraction. The precipitated DNA was resuspended in 50 μl deionized water and analyzed by qPCR. In this qPCR experiment, the first-round PCR reaction was carried out by use of primers that target the ΦX174 genome sequence and viral LTR sequences to amplify the junctions of the integrated viral DNA. For the second-round PCR, the viral LTR primers were used as described previously. Integrated viral DNA copy numbers were determined by plotting values derived from a standard curve. All reactions were performed in triplicates and data were analyzed with CFX software (Bio-Rad Laboratories).
MS
To evaluate cellular entry of cocaine, we used LC-MS with ESI experiments. For this assay, we incubated SupT1 cells (5 × 106 cells) with 10 µM and 50 µM cocaine overnight and washed the cells 3 times to remove residual cocaine. These cells were then lysed by homogenization, and the cell debris was removed by high-speed centrifugation. Then, the cytoplasmic fraction was extracted in organic solvents (chloroform-methanol mixture, 2:1 vol/vol) and dried in a SpeedVac, per previously described methods [46]. The dried material was then dissolved in 0.1% formic acid and analyzed by ESI LC-MS. Nuclear lysates of cocaine-treated SupT1 cells were prepared by use of published methods [43]. We isolated nuclear pellets from the cocaine-treated cells and removed the high molecular weight debris by high-speed centrifugation from the nuclear lysates [43]. The nuclear lysates were then extracted with organic solvent, dried, and subjected to MS analysis. We used pure cocaine hydrochloride as the standard in our MS analysis. Relative abundance was calculated by comparing the parent peak of cocaine in the cytoplasmic and nuclear fractions of treated cells with untreated cells.
Cytotoxicity assay
Cells were treated with cocaine at concentrations ranging from 1 to 100 µM. Cellular apoptosis was measured with AV and PI staining, per our published methods [35]. Data were acquired by FACS and analyzed with FlowJo software.
Statistical analysis
Results were expressed as mean ± sd of data obtained from 3 independent experiments. Significance of differences between control and treated samples was determined by Student’s t-test. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Cocaine enhances HIV-1 infection in primary-activated CD4+ T cells
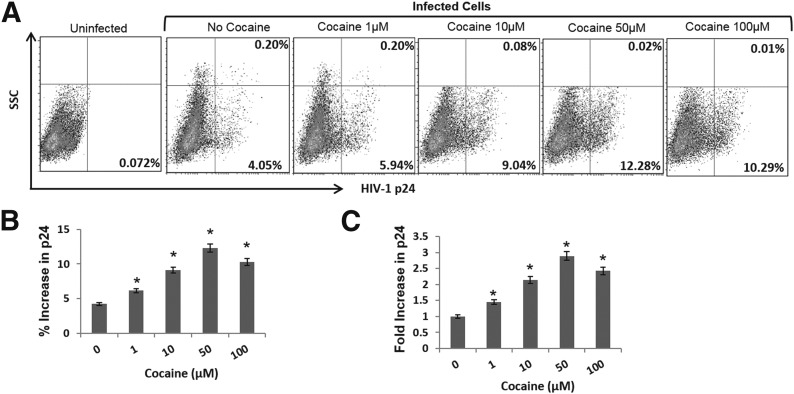
To gain better insights into cocaine’s potentiating effects on HIV-1, first, we measured dose-dependent effects of cocaine on HIV-1 infection in primary-activated CD4+ T cells. For infection studies, X4 tropic virions were used, as CD4+ T cells support robust infection and replication of these virions. In addition X4 tropic virions are predominantly associated with HIV-1 disease progression [47, 48]. We used 1–100 µM cocaine to cover the wide range of concentrations reported in the plasma of cocaine users [37–42]. To carry out these experiments, PBMCs were isolated from fresh human peripheral blood, and CD4+ T cells were enriched from these PBMCs by the negative-selection method [18, 34, 35]. The purity of the CD4+ T cells was measured by flow cytometry, and cells with >95% purity were activated for 48–72 h. The activated CD4+ T cells were infected with X4 tropic HIV-1 virions and treated with cocaine after infection. Productive infection was measured by detecting the intracellular viral p24 antigen by flow cytometry after 48–72 h postinfection (Fig. 1). As illustrated in Fig. 1A and B, cocaine treatment increased the percentage of cells expressing viral p24 compared with untreated, infected cells. For example, the percentage of cells expressing viral p24 was ∼4% after 48 h infection. However, this number was increased to ∼6% when the infected cells were treated with 1 µM cocaine. A maximum increase up to ∼12% cells expressing p24 protein was observed with 50 µM cocaine (Fig 1A and B). However, this number was reduced to ∼10% with 100 µM cocaine treatment. Notably, the potentiating effects of cocaine on HIV-1 infection were consistently observed in CD4+ T cells isolated from 3 different donors (Fig. 1C). Likewise, the MFI values of the infected cells were also increased with cocaine treatment (Supplemental Fig. 1). The increase in MFI suggests that cocaine enhances viral protein translation in infected cells in addition to increasing the number of infected cells.
Figure 1. Cocaine enhances HIV-1 infection in primary CD4+ T cells.
Primary CD4+ T cells with purity >95% were activated by PHA for 48–72 h. These cells were infected with HIV-1 virions and cultured in the absence or presence of increasing concentrations of cocaine (1–100 µM). Productive HIV-1 infection in these cells was measured by flow cytometry-based staining for intracellular viral p24 protein, 48–72 h post infection. (A) Representative p24 data of HIV-1-infected cells isolated from a single donor. SSC, Side-scatter. (B) Relative increase in p24 expression by cocaine from 3 independent experiments by use of cells from a single donor. (C) Fold increase in HIV-1 p24 expression by cocaine in cells isolated from 3 different donors. Results are expressed as mean values, with error bars indicating sd of triplicate measurements. *P < 0.05 is for the comparison of cocaine-treated cells versus untreated cells.
Cocaine increases HIV-1 integration in primary-activated CD4+ T cells
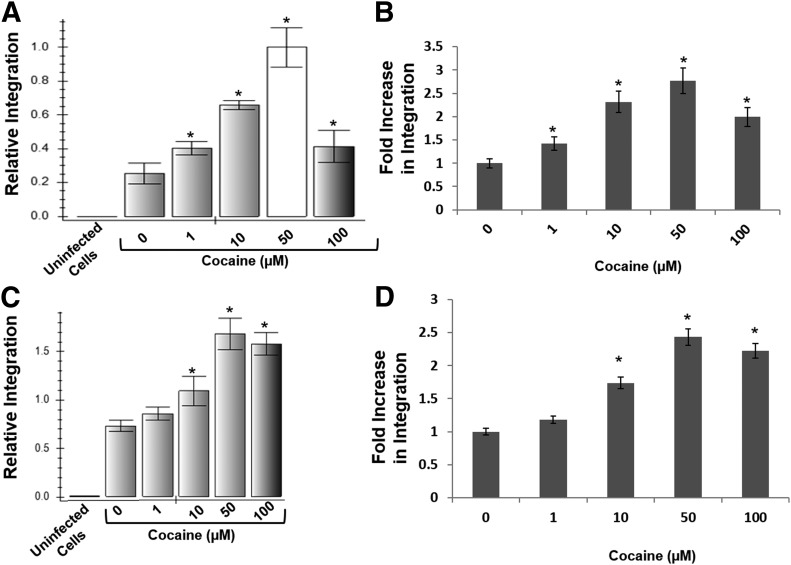
Published data have demonstrated that cocaine modulates entry and postentry steps of HIV-1 infection. However, the effect of cocaine on the viral integration step remains unclear. Therefore, we measured HIV-1 integration in primary-activated CD4+ T cells in the presence of increasing concentrations of cocaine. CD4+ T cells were infected with HIV-1 virions and then cultured overnight in the presence of cocaine (1–100 µM). Proviral DNA integration was measured by isolating genomic DNA from the infected cells and carrying out nested real-time qPCR. The nested qPCR primers sets were designed to amplify the junctions of integrated viral DNA from the target but not the unintegrated viral DNA. Our data revealed that HIV-1 integration in cocaine-treated cells was significantly higher compared with untreated cells (Fig. 2A). Similar to cocaine’s effect on HIV-1 infection, shown in Fig. 1, cocaine treatment increased viral integration in a concentration-dependent manner from 1 µM through 50 µM. A maximum increase in integration of ∼2.5 fold was observed in cells treated with 50 µM cocaine. Interestingly, this increase in integration was reduced in cells treated with 100 µM cocaine compared with that of 50 µM cocaine. The potentiating effects of cocaine on HIV-1 integration were consistently observed in CD4+ T cells from 3 different donors (Fig. 2B). Given that integration is absolutely essential for viral transcription and viral protein translation, we believe increased HIV-1 integration is most likely responsible for the increased viral protein translation in cocaine-treated cells seen in Fig. 1.
Figure 2. Cocaine increases HIV-1 proviral DNA integration in CD4+ T cells.
(A) PHA-activated primary CD4+ T cells were infected with HIV-1 virions and cultured overnight in the absence or presence of increasing concentrations of cocaine (1100 µM). Proviral DNA integration in these cells was measured by isolating genomic DNA and carrying out nested qPCR. The primers used in the first round of PCR are designed to amplify the junctions of integrated viral DNA and the host genomic DNA. The primers in the second round were designed to enrich the viral DNA from the first-round PCR products. Data are represented as mean values, with error bars indicating sd of triplicate measurements. (A) Effects of cocaine on integration in CD4+ T cells isolated from a single donor. (B) Dose -dependent effects of cocaine on HIV-1 integration in CD4+ T cells isolated from 3 different donors. (C) Cocaine increases integration of pseudotyped HIV-1 virions in a CD4+ T cell line. T Lymphocytic SupT1 cells were infected with VSV-G-pseudotyped HIV-1 virions and cultured overnight in the absence or presence of increased concentrations of cocaine (1–100 µM). (D) Fold increase in integration in cocaine-treated cells compared with untreated cells from 3 independent experiments. *P < 0.05 is for the comparison of cocaine-treated cells versus untreated cells.
Given that these data are derived in pure cultures of CD4+ T cells, we also tested the effects of cocaine on HIV-1 infection and integration in human PBMCs. Fresh PBMCs were activated by PHA and infected with HIV-1 virions (X4 tropic). These infected PBMCs were cultured in the presence of increased concentrations of cocaine. Productive infection was measured by intracellular p24 staining (Supplemental Fig. 2A and B). Proviral DNA integration was measured by qPCR, as described in Fig. 2. Similar to the data with purified CD4+ T cells in Fig. 2A and B, cocaine increased viral p24 expression (Supplemental Fig. 2A and B) and proviral DNA integration (Supplemental Fig. 2C) in PBMCs in a dose-dependent manner. These data indicate that the effect of cocaine on HIV-1 infection and integration in CD4+ T cells is not dependent on the presence of other immune cells. Furthermore, integration assays with cocaine metabolite “benzoylecgonine” also showed increased proviral integration in CD4+ T cells (Supplemental Fig. 2D).
To test whether the decrease in HIV-1 infection (Fig. 1) and integration (Fig. 2) at 100 µM cocaine was a result of cytotoxicity, we measured cocaine-induced cytotoxicity of CD4+ T cells. Cytotoxicity was measured by flow cytometry-based staining with AV as an early apoptosis marker and PI as the late apoptotic marker. Data in Supplemental Fig. 3A illustrated that cocaine has no toxicity toward uninfected, activated CD4+ T cells at a concentration from 1 to 100 µM. Similar data were also observed in infected cells with cocaine from 1 to 50 µM concentrations. However, at 100 µM, cocaine showed modest cytotoxicity in infected cells, as shown by the higher percentage of AV + PI-stained cells (Supplemental Fig. 3B). This may, in part, explain the reduction in HIV-1 infection and integration with 100 µM cocaine seen in Figs. 1 and 2.
Cocaine enhances integration of pseudotyped HIV-1 virions
Cocaine has been demonstrated to enhance HIV-1 entry by up-regulating the coreceptors [28] and/or by regulating cellular factors that modulate viral entry. Therefore, it is plausible that the increased integration in cocaine-treated cells could be a result of enhanced viral entry. To test this, we measured cocaine’s effect on integration of VSV-G-pseudotyped HIV-1 virions. Pseudotyped HIV-1 virions do not depend on the cellular receptor (CD4) and coreceptors (CXCR4 and CCR5) for entering the target cells [49]. Instead, the pseudotyped virions enter the target cells via endocytic pathways [49]. Therefore, we infected the CD4+ T cell line SupT1 with VSV-G-pseudotyped HIV-1 virions, per our published methods [18, 37, 38], and cultured the cells in the presence of cocaine. Similar to the results obtained with infectious virions (Fig. 2A and B), cocaine treatment significantly increased integration of pseudotyped HIV-1 in a dose-dependent manner (Fig. 2C and D). Notably, a maximum increase in integration was also observed in cells that were treated with 50 µM cocaine. The cocaine-induced increase in integration of pseudotyped virions is comparable with that of the infectious virions (Fig. 2C and D). Thus, cocaine’s potentiating effect on HIV-1 integration is most likely not dependent on increased viral entry.
Cocaine increases HIV-1 PIC-associated viral DNA integration
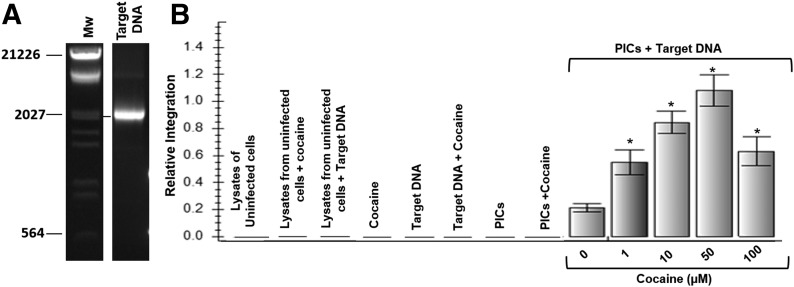
Data in Fig. 2 suggested that cocaine increases HIV-1 integration, and this increase may not depend on higher viral entry. However, there is evidence that cocaine can increase HIV-1 reverse transcription [19]. As increased reverse transcription can enhance viral DNA integration, data in Fig. 2 do not confirm a direct effect on cocaine on HIV-1 integration. Therefore, we measured cocaine’s effect on HIV-1 integration by exploiting the ability of HIV-1 PICs isolated from HIV-1-infected cells to carry out integration of viral DNA into target DNA in vitro [43]. The cytoplasmic fractions of HIV-1-infected SupT1 cells were used as the source of PICs. We used a 2.0 kb DNA as the target that was PCR amplified from the ΦX174 genome. The PCR-amplified product was gel purified (Fig. 3A). An in vitro integration assay was carried out by adding HIV-1 PICs to target DNA in the absence or presence of increasing concentrations of cocaine. Integration was measured by nested qPCR with primer sets that amplified the junctions of viral DNA and the target DNA. Intriguingly, data from this in vitro integration assay illustrated that cocaine treatment increased PICs associated viral DNA integration in a dose-dependent manner from 1 to 50 µM (Fig. 3B). Coincidently, compared with untreated samples, 50 µM cocaine also showed a maximum increase in integration. This is similar to the integration data obtained in the infected cells in Figs. 2 and 3. As predicted, controls, such as PICs without target DNA, target DNA alone, PICs treated with cocaine, and lysates from uninfected cells, did not amplify integrated viral DNA.
Figure 3. Cocaine increases HIV-1 PIC-associated viral DNA integration activity.
(A) PCR amplification and purification of target DNA for HIV-1 PIC-associated in vitro integration assay. A 2.0 kb target DNA fragment was PCR amplified from the ΦX174 genome. The PCR product was detected by agarose gel electrophoresis by use of ethidium bromide staining. Then, the 2.0 kb fragment was purified by the gel extraction method. A portion of the purified PCR product was analyzed by agarose gel electrophoresis for purity. (B) HIV-1 PICs were isolated from HIV-1-infected SupT1 cells, and in vitro integration assays were carried out with use of the 2.0 kb target DNA. Integration of viral DNA into the target was measured by nested qPCR that specifically amplifies the junctions of integrated viral DNA in the target. PIC associated integration assay was performed in the presence of increased concentrations of cocaine (1–100 µM). Controls, such as PICs alone, PICs + cocaine, target DNA alone, cocaine + target DNA, lysates from uninfected cells, lysates from uninfected cells + cocaine, and lysates from uninfected cells + target DNA, showed no integration activity. Cocaine increased PICs associated viral DNA integration in a dose-dependent manner. Data are presented as mean values, with error bars indicating sd of triplicate measurements. *P < 0.05 is for the comparison treated cells versus untreated cells.
To test whether the effect of cocaine on HIV-1 integration is dependent on the use of a specific target DNA, we also used another target DNA in our assay. We amplified a fragment of the mammalian expression plasmid pcDNA and used it in our in vitro integration assay (Supplemental Fig. 4). Data from this assay indicated that the potentiating effect of cocaine is not dependent on target DNA. Therefore, we believe that data in Fig. 3 strongly suggest that cocaine can directly increase HIV-1 DNA integration.
Cocaine enters the cytoplasm and localizes to the nucleus of CD4+ T cells
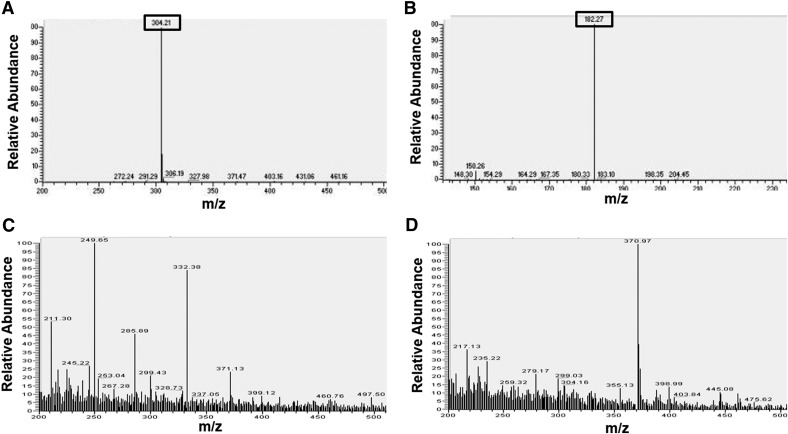
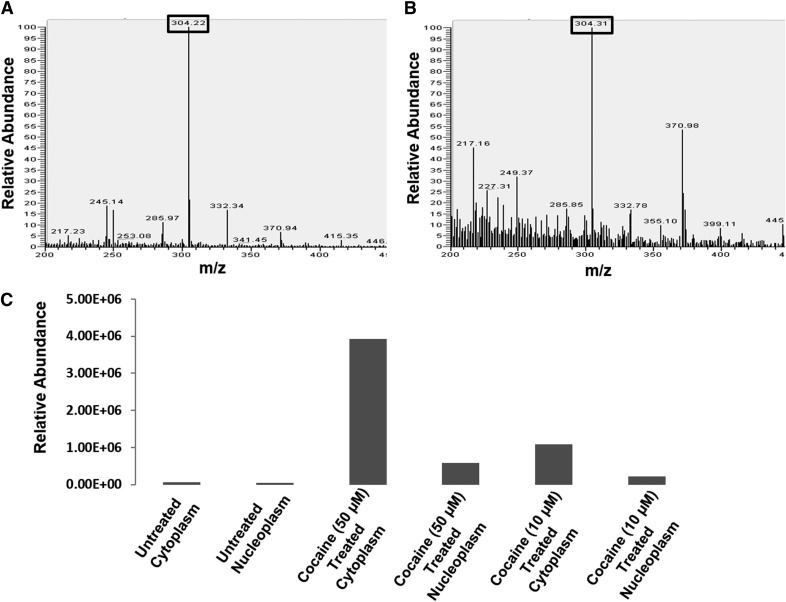
Our in vitro integration data in Fig. 3 provided strong evidence that cocaine can directly increase HIV-1 integration. Therefore, for cocaine to potentiate HIV-1 integration in infected CD4+ T cells, it has to enter the cytoplasm and localize to the nucleus of these cells. However, there are no reports demonstrating that cocaine can localize to the nucleus, as it has been well established that cocaine binds to the plasma membrane-associated cellular transporters and receptors [50]. Moreover, the lack of commercially available, fluorescently labeled cocaine has compounded studies that can address the ability of cocaine to enter cells. Therefore, we conducted ESI-based MS to investigate the ability of cocaine to enter CD4+ T cells. We used cocaine hydrochloride as the standard in our MS experiments. The MS analysis recorded the matching parent peak of cocaine at 304.2 m/z (Figs. 4A and 5) and the main fragment peak at 182.3 m/z by MS/MS analysis (Fig. 4B). We then analyzed the cytoplasmic and nuclear fractions of cocaine-treated SupT1 cells. For preparing these fractions, we treated SupT1 cells with 50 µM cocaine overnight to mimic the conditions for our integration analysis. Then, the cells were harvested and lysed by homogenization. The cytoplasmic fractions were collected after separating high molecular weight cellular debris by high-speed centrifugation. For preparing nuclear fractions, the nuclear pellet was isolated from treated and untreated cells. Then, the nuclear fraction was prepared by homogenizing the nuclear pellet and separating the nuclear debris by high-speed centrifugation. These fractions were then extracted in organic solvents, dried, and redissolved in appropriate solvents before MS analysis. Our MS analysis showed that in the untreated cells, the relative abundance of the parent cocaine peak at 304.2 m/z was negligible in the cytoplasmic (Fig. 4C) and nuclear fractions (Fig. 4D). Interestingly, the parent peak of cocaine could be detected in the cytoplasmic fractions (Fig. 5A) and the nuclear fractions (Fig. 5B) of treated cells. Therefore, these results strongly suggest that cocaine can enter the cytoplasm and localize to the nucleus of SupT1 cells. In addition, these data illustrate that cocaine is not degraded in these experimental conditions.
Figure 4. ESI LC-MS detection of cellular entry of cocaine.
Cocaine hydrochloride was purchased from Sigma Chemical and freshly dissolved in appropriate solvent for MS analysis. (A) The parent peaks of cocaine (304.21 m/z) and (B) the main fragment peak at 182.27 m/z by MS/MS. (C and D) SupT1 cells were cultured in the absence of cocaine overnight. Then, the cells were pelleted and washed 3 times, and the cytoplasmic and nuclear fractions were separated, per published methods [43] and analyzed by ESI LC-MS. Analysis of untreated cells revealed that the parent cocaine peak was not present in the cytoplasmic (C) or in the nuclear (D) fractions of untreated cells.
Figure 5. ESI LC-MS detection of cocaine in the cytoplasmic and nuclear fractions of CD4+ T cells.
SupT1 cells were cultured in the absence or presence of cocaine overnight. Then, the cells were pelleted and washed, and cytoplasmic and nuclear fractions were isolated after removing high molecular weight cellular debris. These fractions were extracted in a chloroform-methanol mixture (2:1, v/v) and analyzed by ESI LC-MS. The parent MS peaks of cocaine (304.2 m/z) were detected in (A) the cytoplasmic and (B) the nuclear fractions of cocaine-treated cells. (C) Relative abundance of cocaine in the cytoplasmic and nuclear fractions of SupT1 cells treated with 10 µM and 50 µM cocaine. Relative abundance was calculated by comparing the detected parent peak of cocaine peak in the cytoplasmic and nuclear fractions of treated cells to untreated cells. These data demonstrated that relative abundance of cocaine in the cytoplasm and nuclear fractions increased in a dose-dependent manner.
Thereafter, we also evaluated whether intracellular levels of cocaine can increase in a concentration-dependent manner. To test this, we treated SupT1 cells with 10 µM and 50 µM cocaine overnight, extracted the cytoplasmic and nuclear fractions, and carried out MS analysis. Comparison of relative abundance of cocaine in the cytoplasmic and nuclear fractions of the treated SupT1 cells showed a dose-dependent increase in cocaine levels (Fig. 5C). Notably, our data also revealed that the amount of cocaine in the cytoplasmic fraction is significantly higher compared with the nuclear fractions for respective cocaine concentrations. These data solidify that cocaine is able to enter CD4+ T cells and localize to the nucleus, the site for viral integration.
The HIV-1 integrase inhibitor “Raltegravir” abrogates cocaine’s potentiating effect on HIV-1 integration
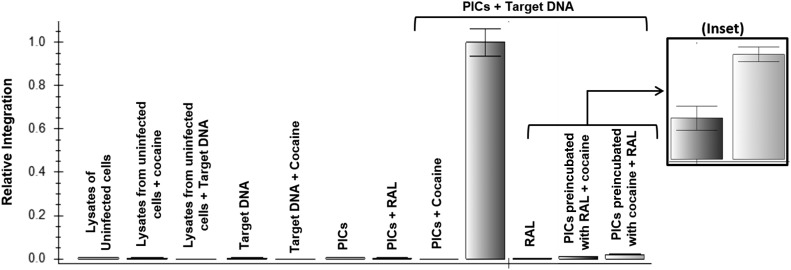
To gain better insights into the mechanism by which cocaine increases HIV-1 integration, we investigated the potentiating effect of cocaine in the presence of Raltegravir, which inhibits HIV-1 integration by binding to the active site of HIV-1 integrase and inhibiting strand transfer activity [51, 52]. To test this, we preincubated HIV-1 PICs with Raltegravir (1 µM) and carried out integration assays in the presence of cocaine (50 µM). As expected, Raltegravir inhibited PIC-associated viral DNA integration significantly (Fig. 6). Interestingly, preincubation of HIV-1 PICs with Raltegravir before addition of cocaine abrogated the potentiating effects of cocaine on HIV-1 integration. Conversely, when the PICs were preincubated with cocaine before Raltegravir addition, the effect of cocaine on integration was also dramatically reduced. Intriguingly, when the data from the preincubated PICs were compared, it indicated that PICs preincubated with cocaine showed higher viral integration compared with Raltegravir preincubated PICs (Fig. 6, inset). These data suggested that either cocaine does not compete with Raltegravir to bind to the active site of HIV-1 integrase or the binding affinity of cocaine is significantly weaker compared with Raltegravir. It is also likely that cocaine may bind to an allosteric site in the HIV-1 integrase enzyme or can modulate integration by binding to other viral or cellular components of HIV-1 PICs.
Figure 6. Raltegravir (RAL) abrogates the potentiating effects of cocaine on HIV-1 integration.
An integration assay with the use of PICs that were preincubated with cocaine (50 µM) or Raltegravir (1 µM) for 15 min on ice. Thereafter, Raltegravir was added to cocaine, preincubated PICs, and cocaine was added to Raltegravir preincubated PICs before the integration assay. Integrated viral DNA copy numbers were determined by plotting values derived from a standard curve. (Inset) Relative integration of preincubated PICs with Raltegravir compared with PICs preincubated with cocaine. Controls, such as PICs alone, PICs + cocaine, PICs + RAL, target DNA alone, cocaine + target DNA, lysates from uninfected cells, lysates from uninfected cells + cocaine, and lysates from uninfected cells + target DNA, showed no integration activity. Data are presented as mean values, with error bars indicating sd of triplicate measurements.
DISCUSSION
The ongoing HIV pandemic have resulted in ∼1.2 million infections in the United States [1] and ∼34 million worldwide [53]. Substance use is a major barrier for combating the global HIV pandemic, as it is associated with HIV transmission, delayed diagnosis, delayed initiation of therapy, and poor adherence to therapy [3–6]. Cocaine is one of the most commonly used illicit drugs among HIV-1-infected individuals [7]. Clinical studies suggest an association between cocaine use and HIV disease progression and AIDS-related mortality, even among adherent ART users [8–10]. Studies also suggest that HIV-positive cocaine users have lower CD4+ T cell counts and accelerated CD4+ T cell decline [11–14] that may contribute to accelerated HIV disease progression [15, 16]. It is important to point out that there are several studies that report lack of association between substance use and HIV/AIDS [54–56]. Furthermore, a direct link between cocaine abuse and HIV-1 disease progression remains contentious, as substance use is often associated with reduced/nonadherence to ART. Nevertheless, the high prevalence of substance use, including that of cocaine among HIV-1-infected individuals [7], warrants molecular details on the synergy between substance use and HIV pathogenesis.
Even though the underlying mechanism remains unclear, it has been suggested that increased HIV-1 infection and replication by cocaine may contribute to the accelerated HIV pathogenesis observed among cocaine abusers. A body of research has demonstrated the potentiating effects of cocaine on HIV-1 infection/replication in monocyte-derived macrophages [22], DCs [24], astrocytes [23], and PBMCs [21]. We [18] and others [19] have also shown that cocaine increases HIV-1 infection/replication in CD4+ T cells that are the primary targets of HIV-1 in vivo [33]. Cocaine has also been shown to increase viral load in a humanized mouse model [25, 26]. In addition, cells from cocaine addicts have been shown to support readily HIV-1 replication and AIDS-related opportunistic infections in contrast to cells from nonusers [10]. Furthermore, cocaine has been described to facilitate transmigration of infected DCs to the CNS [58]. Collectively, these data have demonstrated that cocaine can increase HIV-1 replication, and this increase may, in part, play a role in worsening of HIV disease among cocaine abusers. However, the mechanism by which cocaine enhances HIV-1 replication remains poorly understood.
Accumulating data also indicate that cocaine enhances HIV-1 infection by targeting both entry and postentry steps of the HIV-1 lifecycle. HIV-1 entry into the target cells is facilitated by the binding of viral glycoproteins (HIV-1 gp120 and gp41) to the CD4 receptor and the chemokine coreceptors (CCR5 and CXCR4). Binding of viral glycoprotein leads to membrane fusion and release of viral core into the cytoplasm of target cells [33]. It has been demonstrated that cocaine inhibits expression of HIV-suppressing chemokines in target cells [28, 29]. These include RANTES, MIP-1a, and MIP-1b, known to inhibit binding/fusion/entry of the virus [28, 29]. In addition, it has also been reported that cocaine up-regulates the expression of the entry coreceptors CXCR4 and CCR5 [28, 29]. These findings have been instrumental in depicting that HIV-1 entry is a major target for cocaine. Published data from our laboratory and others also demonstrate that cocaine can modulate postentry steps of the HIV-1 lifecycle. The postentry events of HIV-1 replication can be broadly categorized into reverse transcription, integration, transcription, translation, assembly, and virion-release steps [33]. Our published data have demonstrated that cocaine down-regulates the cellular miRNA, miR-125b, in CD4+ T cells [18]. miR-125b is a member of the of anti-HIV miRNA family that inhibits HIV-1 by binding to the viral transcripts and inhibiting viral protein translation [30]. A recent report has illustrated that cocaine down-regulates the cellular miRNA, “miR-155” [24], which serves as a negative regulator of DC-specific ICAM-3-grabbing nonintegrin [24] that plays a critical role in HIV-1 infection of DCs. Proteomics-based studies have suggested that cocaine regulates an array of cellular proteins in astrocytes that could be implicated in HIV-1 replication [23]. There is also evidence that cocaine’s potentiating effects on HIV-1 are dependent on the sigma 1 receptor that is expressed on the ER [26, 59]. Very recently, it has been described that cocaine enhances reverse transcription of R5 tropic HIV-1 virions in quiescent CD4+ T cells [19]. These authors also suggested that the increased reverse transcription of the viral RNA genome resulted in a higher level of viral integration. Cumulatively, these studies provide strong evidence that cocaine can modulate both entry and postentry steps of HIV-1 infection. However, the direct effects of cocaine on HIV-1 integration remain largely unclear.
The goal of this study is to evaluate whether cocaine can directly modulate HIV-1 integration. To test this, we used 1–100 μM cocaine in our study to cover the wide range of plasma concentrations reported in cocaine abusers. For example, it has been reported that serum levels of cocaine in patients admitted to the Emergency Department averaged ∼1.0 µM [41]. In acute fatal overdose conditions, a mean concentration of cocaine has been reported to 21 µM [43], whereas 3.6 µM has been reported as the mean concentration in cocaine-poisoned patients [37]. There is also evidence that cocaine concentration can go up to 100–1000 µM in the plasma of drug addicts [38, 41]. Although concentration above 100 µM may not be prevalent among cocaine abusers, it is important to point out that the reported concentrations are most likely not the peak concentrations in the plasma of cocaine addicts. This is because the measurements of cocaine concentrations in the plasma are almost always conducted a few hours after cocaine use, and peak cocaine concentrations are achieved within 5 s–60 min, depending on the mode of administrations [39, 40, 60–62]. Therefore, we rationalized that 1–100 μM cocaine may have a high level of physiologic relevance. Given that serum used in the culture media could be a source of enzymes, such as esterases that can degrade cocaine, in all of our experiments, we used heat-inactivated serum. Our MS data showed that cocaine could be detected in the cytoplasmic and nuclear fractions after overnight incubation (Fig. 5), illustrating that in our experimental conditions, most of the cocaine is not degraded.
Our data in Fig. 2 indicated that 1–50 µM cocaine increased HIV-1 integration in CD4+ T cells in a dose-dependent manner. Although cells treated with 100 µM cocaine showed increased HIV-1 integration compared with the untreated cells, there was a significant decline in integration compared with cells treated with 50 µM cocaine with primary CD4+ T cells. This may be attributed to cytotoxicity effects of cocaine at 100 µM, as shown in Supplemental Fig. 3, and our published data on cocaine induced cytotoxicity CD4+ T cells at 100 µM [35]. Surprisingly, integration assays with cocaine metabolite benzoylecgonine also showed increased proviral DNA integration in CD4+ T cells (Supplemental Fig. 2D). Therefore, further studies are required to confirm whether the observed cytotoxicity is the cause for reduction in integration in cells treated with 100 µM cocaine.
Even though data in Fig. 2 described the potentiating effects of cocaine on HIV-1 integration, they do not confirm a direct role of cocaine on viral integration. This is because higher integration could be a result of an increase in other early postentry steps that could enhance integration. These include uncoating, reverse transcription, nuclear targeting, and/or transport of PICs. This is supported by published data that describe that increased integration of R5 HIV-1 in quiescent CD4+ T cells was mainly a result of increased viral reverse transcription [19]. In addition, increased HIV-1 integration in cocaine-treated cells can be achieved by the regulation of cellular factors that could facilitate viral integration. It is also likely that cocaine treatment may alter the composition of HIV-1 PICs in infected cells that may render efficient viral integration. Given that many early postentry steps can influence HIV-1 integration, the establishment of the direct effect of cocaine on HIV-1 integration in infected cells remains highly challenging. To elucidate a direct effect of cocaine on viral DNA integration, we used HIV-1 PICs and carried out in vitro integration assays by use of a PCR-amplified and -purified target DNA. HIV-1 PICs are large nucleoprotein complexes that contain key viral and cellular factors and can carry out integration of viral DNA into target DNA [32, 63]. Data presented in Fig. 3 showed that cocaine significantly increased HIV-1 PICs associated viral DNA integration. The potentiating effect of cocaine increased in a dose-dependent manner that is similar to its effect on HIV-1 replication. Intriguingly, 50 µM cocaine showed a maximum increase that was reduced at 100 µM to some extent. Although our data do not explain the mechanism for this observation, we can speculate that binding of cocaine in excess of 100 µM may alter the conformation of the HIV-1 PICs or destabilize the functional stability of this high molecular weight nucleoprotein complex. However, further studies are needed to explain the reduced HIV-1 integration at 100 µM cocaine. In addition, elucidating the specific binding targets of cocaine on this nucleoprotein complex is highly challenging, as the composition of HIV-1 PICs remains largely unclear. Therefore, we cannot rule out that cocaine could bind to other cellular/viral factors of the PICs to increase viral DNA integration. Nevertheless, we believe increased HIV-1 integration is most likely responsible for the increased viral protein translation in cocaine-treated cells, as proviral DNA integration is essential for viral transcription and viral protein translation,.
HIV-1 integration is carried out in 2 steps, by: 1) the 3′ processing step that removes the CA dinucleotide from the 3′ end of the reverse-transcribed viral DNA and 2) the strand transfer step that involves insertion of the proviral DNA into the host genome [31]. The 3′ processing step is carried out in the cytoplasm of the host cell, whereas the strand transfer step is conducted in the nucleus. Therefore, after 3′ processing, the PICs localize to the nucleus, where the PIC-associated HIV-1 integrase enzyme carries out integration of the viral DNA into the host genome [32, 63]. Our PIC-associated in vitro integration assay (Fig. 3) provided evidence that cocaine modulates the strand transfer step of HIV-1 integration. Notably, our preincubation studies of HIV-1 PICs (Fig. 6) suggested that cocaine’s potentiating effects on the strand transfer activity are abrogated significantly by Raltegravir. This could be explained by envisioning that: 1) cocaine binds to viral and cellular factors associated with HIV-1 PICs other than HIV-1 integrase that could facilitate integration, 2) cocaine binds to an allosteric site of HIV-1 integrase enzyme and does not compete with Raltegravir, or 3) the affinity of cocaine binding to an HIV-1 integrase active site is significantly lower than Raltegravir. Given that the compositions of HIV-1 PICs are not clearly identified, further studies are necessary to confirm the binding partners of cocaine on HIV-1 PICs and better understand the mechanism by which cocaine modulates strand transfer activity. However, further studies are necessary to confirm the binding partners of cocaine on HIV-1 PICs and better understand the mechanism by which cocaine modulates strand transfer activity. Furthermore, cocaine binding was able to rescue Raltegravir’s inhibitory effect to some extent. Therefore, it is plausible that binding of cocaine induces conformational changes in the integrase enzyme that may reduce binding affinity of Raltegravir. Biochemical studies that use purified HIV-1 integrase are warranted to decipher the binding pockets of cocaine. We are also cognizant that our data do not address the effect of cocaine on the 3′ processing step of viral integration. In addition, based on the evidence that cocaine can bind to DNA aptamers [64–68], we cannot rule out that cocaine can facilitate integration by binding to the viral or target DNA and altering the nucleic acid geometry that is more favorable for viral DNA integration. Nevertheless, the data presented here provide strong evidence that cocaine could directly increase HIV-1 integration.
Given that our in vitro data demonstrated that cocaine enhances HIV-1 integration, the next logical question is whether cocaine can directly modulate HIV-1 integration in infected cells. To enhance directly the strand transfer step in infected cells, cocaine has to enter the cell and localize to the nucleus—the site of viral DNA integration. Unfortunately, an extensive search of literature yielded no data describing the ability of cocaine to localize to the nucleus. This is because cocaine’s addictive effects have been attributed to its ability to bind to the plasma membrane-associated DAT [69, 70]. Nevertheless, cocaine is known to bind to several cellular proteins [40], including the sigma 1 receptors. Sigma receptors are unique ER proteins that are directly involved in modulating intracellular calcium mobilization and immune functionality [71, 72]. Although the mechanism by which cocaine binds to the intracellular ER-associated sigma 1 receptor remains elusive, another psychostimulant, “Methamphetamine,” is known to enter target cells by binding to DAT [50]. Therefore, the possibility that cocaine can enter CD4+ T cells is plausible. Therefore, we investigated the ability of cocaine to enter the cytoplasm and nucleus of CD4+ T cells by ESI-based LC-MS. Our data in Fig. 6 showed that cocaine can be detected in the cytoplasmic and nuclear fractions of CD4+ T cells that were treated with cocaine. We acknowledge that these fractions may contain cocaine that is bound to the membrane-associated cellular receptors, and experiments with purified cytoplasmic and nuclear PICs are needed to pinpoint nuclear entry of cocaine. Nevertheless, our dose-dependent studies indicate that cocaine has the ability to localize to the nucleus of CD4+ T cells. However, our data do not pinpoint the cellular receptors or transporters that are used by cocaine for cellular entry. It is important to point out that although human PBMCs have been reported to express DAT [73], the expression of DAT on CD4+ T cells remains to be determined. Therefore, further investigation is necessary to delineate the cellular pathways responsible for entry of cocaine into CD4+ T cells.
Collectively, our data provide strong evidence that cocaine can directly enhance HIV-1 integration. As we used physiologically relevant cocaine concentrations, our data may, in part, explain the potentiating effects of cocaine on HIV-1 infection and replication in vitro and in vivo (Fig. 7). Our data also highlight that increased HIV-1 integration could lead to increased viral transcription and viral protein production (Fig. 1 and Supplemental Fig. 1). In addition, there is evidence that cocaine can activate proviral expression from latently infected cell lines [22]. Therefore, it is plausible that increased HIV-1 integration by cocaine may have implications in viral transcription and latency. Most importantly, our data may explain the deleterious effects of cocaine abuse on accelerated CD4+ T cell apoptosis in HIV-1 patients. This is because a recent study shows that increased viral integration leads to CD4+ T cell apoptosis [74]. Given that viral load and CD4+ T cell death are important diagnostics of HIV-1 disease progression, our data highlight a potentially novel mechanism by which cocaine can accentuate HIV-1 disease progression in drug-abusing patients (Fig. 7).
Figure 7. A schematic highlighting the effects of cocaine abuse on HIV-1 disease progression.

Accumulating evidence suggests that cocaine use can contribute to accelerated HIV-1 disease progression via several mechanisms. First, studies show that cocaine enhances viral entry and postentry steps of HIV-1 infection, including viral protein translation. Data in this manuscript emphasize that cocaine enhances proviral DNA integration. Increased entry and postentry steps can increase HIV-1 infection that can contribute to increased viral load. Second, cocaine abuse has also been linked to accelerated CD4+ T cell apoptosis in HIV-1-infected individuals. We have reported previously that cocaine synergies with HIV-1 virions to induce CD4+ T cell apoptosis ex vivo [38]. Intriguingly, a recent report suggests that increased viral integration can enhance CD4+ T cell apoptosis [74]. Therefore, our data, demonstrating increased integration by cocaine, suggest a potentially novel mechanism by which cocaine can accentuate CD4+ T cell death. Given that viral load and CD4+ T cell death are important diagnostics of progression to AIDS, these complex viral, cellular, and molecular alterations can contribute to the worsening of disease among cocaine-abusing, HIV-1-infected individuals.
ACKNOWLEDGMENTS
This work is partly supported by Grants DA024558, DA30896, and DA033892 and the Diversity-Promoting Institutions Drug Abuse Research Program Grant R24DA021471 from the National Institute on Drug Abuse, U.S. National Institutes of Health (NIH; to C.D.). The authors also acknowledge Research Centers in Minority Institutions Grant G12MD007586; the Vanderbilt CTSA Grant UL1RR024975; the Meharry Translational Research Center (MeTRC) CTSA Grant U54 RR026140 from the National Center for Research Resources, NIH; the U54 Grant MD007593 from the National Institute on Minority Health and Health Disparities, NIH; and the DA029506 grant from that established the patient samples.
Glossary
- ART
antiretroviral therapy
- AV
Annexin V
- CTSA
Clinical and Translational Science Award
- DAT
dopamine transporter
- DC
dendritic cell
- ER
endoplasmic reticulum
- ESI
electrospray ionization
- LC
liquid chromatography
- LTR
long-terminal repeat
- m/z
mass-to-charge ratio
- MFI
mean fluorescence intensity
- miRNA
microRNA
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- NIAID
National Institute of Allergy and Infectious Diseases
- PI
propidium iodide
- PIC
preintegration complex
- qPCR
quantitative PCR
- VSV-G
vesicular stomatitis virus G
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
AUTHORSHIP
A.B.A. carried out the infection and integration experiments. J.P. performed flow cytometry experiments. C.K.M. assisted in real-time assay. V.P. and S.P. conducted the MS analysis. C.D. designed and directed the entire study. J.P. and C.D. wrote the manuscript. All authors reviewed the manuscript.
DISCLOSURES
The authors declare no competing financial and nonfinancial interests.
REFERENCES
- 1.Centers for Disease Control and Prevention. (2012) HIV Surveillance Report. Available at: http://wwwcdcgov/hiv/topics/surveillance/resources/22.
- 2.Walensky R. P., Paltiel A. D., Losina E., Mercincavage L. M., Schackman B. R., Sax P. E., Weinstein M. C., Freedberg K. A. (2006) The survival benefits of AIDS treatment in the United States. J. Infect. Dis. 194, 11–19. [DOI] [PubMed] [Google Scholar]
- 3.Kipp A. M., Desruisseau A. J., Qian H. Z. (2011) Non-injection drug use and HIV disease progression in the era of combination antiretroviral therapy. J. Subst. Abuse Treat. 40, 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman H., Pross S., Klein T. W. (2006) Addictive drugs and their relationship with infectious diseases. FEMS Immunol. Med. Microbiol. 47, 330–342. [DOI] [PubMed] [Google Scholar]
- 5.Khalsa J. H., Royal W. (2004) Do drugs of abuse impact on HIV disease? J. Neuroimmunol. 147, 6–8. [DOI] [PubMed] [Google Scholar]
- 6.Cabral G. A. (2006) Drugs of abuse, immune modulation, and AIDS. J. Neuroimmune Pharmacol. 1, 280–295. [DOI] [PubMed] [Google Scholar]
- 7.Pence B. W., Thielman N. M., Whetten K., Ostermann J., Kumar V., Mugavero M. J. (2008) Coping strategies and patterns of alcohol and drug use among HIV-infected patients in the United States Southeast. AIDS Patient Care STDS 22, 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaisson R. E., Bacchetti P., Osmond D., Brodie B., Sande M. A., Moss A. R. (1989) Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA 261, 561–565. [PubMed] [Google Scholar]
- 9.Anthony J. C., Vlahov D., Nelson K. E., Cohn S., Astemborski J., Solomon L. (1991) New evidence on intravenous cocaine use and the risk of infection with human immunodeficiency virus type 1. Am. J. Epidemiol. 134, 1175–1189. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin G. C., Roth M. D., Tashkin D. P. (1998) Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J. Neuroimmunol. 83, 133–138. [DOI] [PubMed] [Google Scholar]
- 11.Baum M. K., Rafie C., Lai S., Sales S., Page B., Campa A. (2009) Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J. Acquir. Immune Defic. Syndr. 50, 93–99. [DOI] [PubMed] [Google Scholar]
- 12.Duncan R., Shapshak P., Page J. B., Chiappelli F., McCoy C. B., Messiah S. E. (2007) Crack cocaine: effect modifier of RNA viral load and CD4 count in HIV infected African American women. Front. Biosci. 12, 1488–1495. [DOI] [PubMed] [Google Scholar]
- 13.Cook J. A., Burke-Miller J. K., Cohen M. H., Cook R. L., Vlahov D., Wilson T. E., Golub E. T., Schwartz R. M., Howard A. A., Ponath C., Plankey M. W., Levine A. M., Grey D. D. (2008) Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS 22, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas G. M., Griswold M., Gebo K. A., Keruly J., Chaisson R. E., Moore R. D. (2006) Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am. J. Epidemiol. 163, 412–420. [DOI] [PubMed] [Google Scholar]
- 15.Arnsten J. H., Demas P. A., Farzadegan H., Grant R. W., Gourevitch M. N., Chang C. J., Buono D., Eckholdt H., Howard A. A., Schoenbaum E. E. (2001) Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin. Infect. Dis. 33, 1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnsten J. H., Demas P. A., Grant R. W., Gourevitch M. N., Farzadegan H., Howard A. A., Schoenbaum E. E. (2002) Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J. Gen. Intern. Med. 17, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook J. A. (2011) Associations between use of crack cocaine and HIV-1 disease progression: research findings and implications for mother-to-infant transmission. Life Sci. 88, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantri C. K., Pandhare Dash J., Mantri J. V., Dash C. C. (2012) Cocaine enhances HIV-1 replication in CD4+ T cells by down-regulating MiR-125b. PLoS ONE 7, e51387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S. G., Jung J. B., Dixit D., Rovner R. Jr., Zack J. A., Baldwin G. C., Vatakis D. N. (2013) Cocaine exposure enhances permissiveness of quiescent T cells to HIV infection. J. Leukoc. Biol. 94, 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagasra O., Pomerantz R. J. (1993) Human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells in the presence of cocaine. J. Infect. Dis. 168, 1157–1164. [DOI] [PubMed] [Google Scholar]
- 21.Peterson P. K., Gekker G., Chao C. C., Schut R., Molitor T. W., Balfour H. H. Jr. (1991) Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor-beta. J. Immunol. 146, 81–84. [PubMed] [Google Scholar]
- 22.Dhillon N. K., Williams R., Peng F., Tsai Y. J., Dhillon S., Nicolay B., Gadgil M., Kumar A., Buch S. J. (2007) Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J. Neurovirol. 13, 483–495. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds J. L., Mahajan S. D., Bindukumar B., Sykes D., Schwartz S. A., Nair M. P. (2006) Proteomic analysis of the effects of cocaine on the enhancement of HIV-1 replication in normal human astrocytes (NHA). Brain Res. 1123, 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Napuri J., Pilakka-Kanthikeel S., Raymond A., Agudelo M., Yndart-Arias A., Saxena S. K., Nair M. (2013) Cocaine enhances HIV-1 infectivity in monocyte derived dendritic cells by suppressing microRNA-155. PLoS ONE 8, e83682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth M. D., Tashkin D. P., Choi R., Jamieson B. D., Zack J. A., Baldwin G. C. (2002) Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J. Infect. Dis. 185, 701–705. [DOI] [PubMed] [Google Scholar]
- 26.Roth M. D., Whittaker K. M., Choi R., Tashkin D. P., Baldwin G. C. (2005) Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J. Leukoc. Biol. 78, 1198–1203. [DOI] [PubMed] [Google Scholar]
- 27.Jeong T. C., Jordan S. D., Matulka R. A., Stanulis E. D., Park S. S., Holsapple M. P. (1996) Immunosuppression induced by acute exposure to cocaine is dependent on metabolism by cytochrome P-450. J. Pharmacol. Exp. Ther. 276, 1257–1265. [PubMed] [Google Scholar]
- 28.Nair M. P., Mahajan S., Chadha K. C., Nair N. M., Hewitt R. G., Pillai S. K., Chadha P., Sukumaran P. C., Schwartz S. A. (2001) Effect of cocaine on chemokine and CCR-5 gene expression by mononuclear cells from normal donors and HIV-1 infected patients. Adv. Exp. Med. Biol. 493, 235–240. [DOI] [PubMed] [Google Scholar]
- 29.Nair M. P., Chadha K. C., Hewitt R. G., Mahajan S., Sweet A., Schwartz S. A. (2000) Cocaine differentially modulates chemokine production by mononuclear cells from normal donors and human immunodeficiency virus type 1-infected patients. Clin. Diagn. Lab. Immunol. 7, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J., Wang F., Argyris E., Chen K., Liang Z., Tian H., Huang W., Squires K., Verlinghieri G., Zhang H. (2007) Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat. Med. 13, 1241–1247. [DOI] [PubMed] [Google Scholar]
- 31.Engelman A., Mizuuchi K., Craigie R. (1991) HIV-1 DNA integration: mechanism of viral DNA cleavage and DNA strand transfer. Cell 67, 1211–1221. [DOI] [PubMed] [Google Scholar]
- 32.Bukrinsky M. I., Sharova N., McDonald T. L., Pushkarskaya T., Tarpley W. G., Stevenson M. (1993) Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc. Natl. Acad. Sci. USA 90, 6125–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telesnitsky A., Goff S. P. (1997) Reverse transcriptase and the generation of retroviral DNA. In Retroviruses (Coffin J. M., Hughes S. H., Varmus H. E., eds.), Cold Spring Harbor Laboratory, New York, 121–160. [PubMed] [Google Scholar]
- 34.Manti C. K., Mantri J., Pandhare J., Dash C. (2014) Methamphetamine inhibits HIV-1 replication in CD4+ T cells by modulating anti-HIV-1 miRNA expression. Am. J. Pathol. 184, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandhare J., Addai A.B., Mantri C. K., Hager C., Smith, R. M., Barnett L., Villatta F., Kalams S. A., Dash C. (2014) Cocaine enhances HIV-1-induced CD4 T-cell apoptosis: implications in disease progression in cocaine-abusing HIV-1 patients. Am. J. Pathol. 184, 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platt E. J., Bilska M., Kozak S. L., Kabat D., Montefiori D. C. (2009) Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J. Virol. 83, 8289–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karch S. B., Stephens B., Ho C. H. (1998) Relating cocaine blood concentrations to toxicity—an autopsy study of 99 cases. J. Forensic Sci. 43, 41–45. [PubMed] [Google Scholar]
- 38.Peretti F. J., Isenschmid D. S., Levine B., Caplan Y. H., Smialek J. E. (1990) Cocaine fatality: an unexplained blood concentration in a fatal overdose. Forensic Sci. Int. 48, 135–138. [DOI] [PubMed] [Google Scholar]
- 39.Van Dyke C., Barash P. G., Jatlow P., Byck R. (1976) Cocaine: plasma concentrations after intranasal application in man. Science 191, 859–861. [DOI] [PubMed] [Google Scholar]
- 40.Heard K., Palmer R., Zahniser N. R. (2008) Mechanisms of acute cocaine toxicity. Open Pharmacol. .J 2, 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blaho K., Logan B., Winbery S., Park L., Schwilke E. (2000) Blood cocaine and metabolite concentrations, clinical findings, and outcome of patients presenting to an ED. Am. J. Emerg. Med. 18, 593–598. [DOI] [PubMed] [Google Scholar]
- 42.Mittleman R. E., Wetli C. V. (1984) Death caused by recreational cocaine use. An update. JAMA 252, 1889–1893. [PubMed] [Google Scholar]
- 43.Engelman A., Oztop I., Vandegraaff N., Raghavendra N. K. (2009) Quantitative analysis of HIV-1 preintegration complexes. Methods 47, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raghavendra N. K., Shkriabai N., Graham R. Lj., Hess S., Kvaratskhelia M., Wu L. (2010) Identification of host proteins associated with HIV-1 preintegration complexes isolated from infected CD4+ cells. Retrovirology 7, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelman A. (2009) Isolation and analysis of HIV-1 preintegration complexes. Methods Mol. Biol. 485, 135–149. [DOI] [PubMed] [Google Scholar]
- 46.Bystrowska B., Adamczyk P., Moniczewski A., Zaniewska M., Fuxe K., Filip M. (2012) LC/MS/MS evaluation of cocaine and its metabolites in different brain areas, peripheral organs and plasma in cocaine self-administering rats. Pharmacol. Rep. 64, 1337–1349. [DOI] [PubMed] [Google Scholar]
- 47.Connor R. I., Sheridan K. E., Ceradini D., Choe S., Landau N. R. (1997) Change in coreceptor use correlates with disease progression in HIV-1—infected individuals. J. Exp. Med. 185, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jekle A., Keppler O. T., De Clercq E., Schols D., Weinstein M., Goldsmith M. A. (2003) In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J. Virol. 77, 5846–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aiken C. (1997) Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71, 5871–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ritz M. C., Lamb R. J., Goldberg S. R., Kuhar M. J. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237, 1219–1223. [DOI] [PubMed] [Google Scholar]
- 51.Hazuda D. J., Young S. D., Guare J. P., Anthony N. J., Gomez R. P., Wai J. S., Vacca J. P., Handt L., Motzel S. L., Klein H. J., Dornadula G., Danovich R. M., Witmer M. V., Wilson K. A., Tussey L., Schleif W. A., Gabryelski L. S., Jin L., Miller M. D., Casimiro D. R., Emini E. A., Shiver J. W. (2004) Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science 305, 528–532. [DOI] [PubMed] [Google Scholar]
- 52.Summa V., Petrocchi A., Bonelli F., Crescenzi B., Donghi M., Ferrara M., Fiore F., Gardelli C., Gonzalez Paz O., Hazuda D. J., Jones P., Kinzel O., Laufer R., Monteagudo E., Muraglia E., Nizi E., Orvieto F., Pace P., Pescatore G., Scarpelli R., Stillmock K., Witmer M. V., Rowley M. (2008) Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J. Med. Chem. 51, 5843–5855. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. (2014) HIV/AIDS, Fact Sheet No 360. Available at: http://www.who.int/mediacentre/factsheets/fs360/en/index.html.
- 54.Chao C., Jacobson L. P., Tashkin D., Martínez-Maza O., Roth M. D., Margolick J. B., Chmiel J. S., Rinaldo C., Zhang Z. F., Detels R. (2008) Recreational drug use and T lymphocyte subpopulations in HIV-uninfected and HIV-infected men. Drug Alcohol Depend. 94, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pezzotti P., Galai N., Vlahov D., Rezza G., Lyles C. M., Astemborski J. (1999) Direct comparison of time to AIDS and infectious disease death between HIV seroconverter injection drug users in Italy and the United States: results from the ALIVE and ISS studies. AIDS Link to Intravenous Experiences. Italian Seroconversion Study. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20, 275–282. [DOI] [PubMed] [Google Scholar]
- 56.Junghans C., Low N., Chan P., Witschi A., Vernazza P., Egger M. (1999) Uniform risk of clinical progression despite differences in utilization of highly active antiretroviral therapy: Swiss HIV Cohort Study. AIDS 13, 2547–2554. [DOI] [PubMed] [Google Scholar]
- 57.Peterson P. K., Gekker G., Chao C. C., Schut R., Verhoef J., Edelman C. K., Erice A., Balfour H. H. Jr. (1992) Cocaine amplifies HIV-1 replication in cytomegalovirus-stimulated peripheral blood mononuclear cell cocultures. J. Immunol. 149, 676–680. [PubMed] [Google Scholar]
- 58.Wu D. T., Woodman S. E., Weiss J. M., McManus C. M., D’Aversa T. G., Hesselgesser J., Major E. O., Nath A., Berman J. W. (2000) Mechanisms of leukocyte trafficking into the CNS. J. Neurovirol. 6 (Suppl 1), S82–S85. [PubMed] [Google Scholar]
- 59.Ramamoorthy J. D., Ramamoorthy S., Mahesh V. B., Leibach F. H., Ganapathy V. (1995) Cocaine-sensitive sigma-receptor and its interaction with steroid hormones in the human placental syncytiotrophoblast and in choriocarcinoma cells. Endocrinology 136, 924–932. [DOI] [PubMed] [Google Scholar]
- 60.Evans S. M., Cone E. J., Henningfield J. E. (1996) Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects. J. Pharmacol. Exp. Ther. 279, 1345–1356. [PubMed] [Google Scholar]
- 61.Jenkins A. J., Keenan R. M., Henningfield J. E., Cone E. J. (2002) Correlation between pharmacological effects and plasma cocaine concentrations after smoked administration. J. Anal. Toxicol. 26, 382–392. [DOI] [PubMed] [Google Scholar]
- 62.Jufer R. A., Walsh S. L., Cone E. J. (1998) Cocaine and metabolite concentrations in plasma during repeated oral administration: development of a human laboratory model of chronic cocaine use. J. Anal. Toxicol. 22, 435–444. [DOI] [PubMed] [Google Scholar]
- 63.Miller M. D., Farnet C. M., Bushman F. D. (1997) Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J. Virol. 71, 5382–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang K., Wang K., Zhu X., Zhang J., Xu L., Huang B., Xie M. (2014) Label-free and ultrasensitive fluorescence detection of cocaine based on a strategy that utilizes DNA-templated silver nanoclusters and the nicking endonuclease-assisted signal amplification method. Chem. Commun. (Camb.) 50, 180–182. [DOI] [PubMed] [Google Scholar]
- 65.Sheng Q., Liu R., Zhang S., Zheng J. (2014) Ultrasensitive electrochemical cocaine biosensor based on reversible DNA nanostructure. Biosens. Bioelectron. 51, 191–194. [DOI] [PubMed] [Google Scholar]
- 66.Wang F., Freage L., Orbach R., Willner I. (2013) Autonomous replication of nucleic acids by polymerization/nicking enzyme/DNAzyme cascades for the amplified detection of DNA and the aptamer-cocaine complex. Anal. Chem. 85, 8196–8203. [DOI] [PubMed] [Google Scholar]
- 67.Wen Y., Pei H., Wan Y., Su Y., Huang Q., Song S., Fan C. (2011) DNA nanostructure-decorated surfaces for enhanced aptamer-target binding and electrochemical cocaine sensors. Anal. Chem. 83, 7418–7423. [DOI] [PubMed] [Google Scholar]
- 68.Ma C., Wang W., Yang Q., Shi C., Cao L. (2011) Cocaine detection via rolling circle amplification of short DNA strand separated by magnetic beads. Biosens. Bioelectron. 26, 3309–3312. [DOI] [PubMed] [Google Scholar]
- 69.Fumagalli F., Gainetdinov R. R., Valenzano K. J., Caron M. G. (1998) Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J. Neurosci. 18, 4861–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang N. Y., Rutledge C. O. (1982) Comparison of the release of [3H]dopamine from isolated corpus striatum by amphetamine, fenfluramine and unlabelled dopamine. Biochem. Pharmacol. 31, 983–992. [DOI] [PubMed] [Google Scholar]
- 71.Maurice T., Martin-Fardon R., Romieu P., Matsumoto R. R. (2002) Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci. Biobehav. Rev. 26, 499–527. [DOI] [PubMed] [Google Scholar]
- 72.Buch S., Yao H., Guo M., Mori T., Su T. P., Wang J. (2011) Cocaine and HIV-1 interplay: molecular mechanisms of action and addiction. J. Neuroimmune Pharmacol. 6, 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amenta F., Bronzetti E., Cantalamessa F., El-Assouad D., Felici L., Ricci A., Tayebati S. K. (2001) Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J. Neuroimmunol. 117, 133–142. [DOI] [PubMed] [Google Scholar]
- 74.Cooper A., García M., Petrovas C., Yamamoto T., Koup R. A., Nabel G. J. (2013) HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature 498, 376–379. [DOI] [PubMed] [Google Scholar]