Abstract
Background
Patients with Pulmonary Tuberculosis (PTB) often develop impairment in pulmonary function due to anatomical changes secondary to the illness. Physiotherapy in the form of pulmonary rehabilitation has been advocated.
Objective
The aim of the study was to determine whether adherence to a six-week home-based pulmonary rehabilitation programme (PRP) improved the baseline measurements of lung function, exercise tolerance and health-related quality of life (HRQoL) in patients receiving out-patient treatment for PTB.
Method
A single blinded randomized control study design was used to assess the effects of a six-week home- based PRP in patients receiving treatment for PTB at a local clinic in Khayelitsha, Western Cape. We evaluated lung function by spirometry (MINATO AUTOSPIRO-model no. AZ-505), exercise tolerance using the 6-min-walk test (6MWT), the Borg exercise exertion scale and HRQoL using the EQ-5 D questionnaire in an intervention group (n=34) and a control group (n=33). The trend of the effects of the PRP on lung function was towards increases, but there was no statistical difference between the intervention and control groups at the end of the sixth week in the values of FVC (p=0.2; 95% CI −0.9 to 0.51) as well as FEV1 (p=0.1; 95% CI −0.07 to 0.51). Similar trend was observed for exercise tolerance, and there was no significant difference in HRQoL (p=0.789).
Conclusion
The outcome of the study provides motivation for further consideration and implementation of a pulmonary rehabilitation programme for patients with PTB.
Keywords: Pulmonary rehabilitation, pulmonary tuberculosis
Introduction
Despite the advances in care of patients with Pulmonary Tuberculosis (PTB) in the acute phase1, these advances have yet to encompass their long term management. Recent literature in the field has shown that pulmonary impairment after PTB is still present after cure,1,2 and patients often complained of inability to cope with physical activities that they previously performed on daily basis3. Internationally pulmonary rehabilitation guidelines have been developed for patients suffering from Chronic Obstructive Airway Disease (COAD)2. Research in this area has shown that pulmonary programmes can improve not only the pulmonary function and cardiovascular fitness of patients but also impact positively on their Health-Related Quality of Life (HRQoL)4,5,. Although PTB has not traditionally been classified into the category of COAD6, recent studies show that chronic obstructive airway disease is present even after six months of microbiological cure7. Thus, it is hypothesised that due to the sequelae patients suffer, they would benefit from pulmonary rehabilitation7.
However, the health seeking behaviour of PTB patients, including those with the comorbidity of HIV, are negatively affected by the current poor health care infrastructure in South Africa3. In addition, there has been little focus on physiotherapy related interventions for patients with PTB, which could address the impairment after PTB cure. The National Tuberculosis Strategic Plan for South Africa (2007–2011)8 encourages community and patient empowerment in order to improve the capacity of patients to better control their own health and assist other patients in improving their own lives. Therefore, the intention of this study was to determine whether a six week home-based Pulmonary Rehabilitation Programme5 (PRP) would assist in the improvement of pulmonary function, exercise tolerance and HRQoL for patients with PTB before the completion of their treatment.
Methodology
Setting
Routinely, PTB patients require hospitalisation until they are well enough to be treated at a clinic or in the community. The study was conducted between June- September 2009 at the Ubuntu TB and HIV Clinic situated at Khayelitsha Site B Community Health Centre in Cape Town, South Africa. Khayelitsha suburb, an under resourced community, had an incidence rate of 1122/100 000 in 20039. In the same year, the healthcare complex in the community serviced a total of 4566 TB cases, most of whom were also HIV positive10. The community has one of the highest prevalence of PTB and HIV infection on a national as well as on international scale10. It must be acknowledged however that South Africa is still dealing with issues of equity in access to healthcare in the post-apartheid era11,12.
Study subjects and design
For inclusion into this study, all adult PTB participants between the ages of 18 and 65 years needed to be ambulant as the home based pulmonary rehabilitation programme includes lower limb exercises. The participants were also required to be contactable by telephone for follow-up appointments, pass the Physical Activity Readiness Questionnaire (Par-Q) assessment, and attend the clinic for four consecutive months to meet eligibility into the study. The sample size was calculated using Statistica Version 8. It was estimated that the treatment group would be able to walk about 340m during the 6 minute walk test and the control group would be able to walk 50m less, with a standard deviation of 77m. A sample size of 31 was therefore required in each group to establish a significant difference at a p level of 0.05 and a power of 80%13.
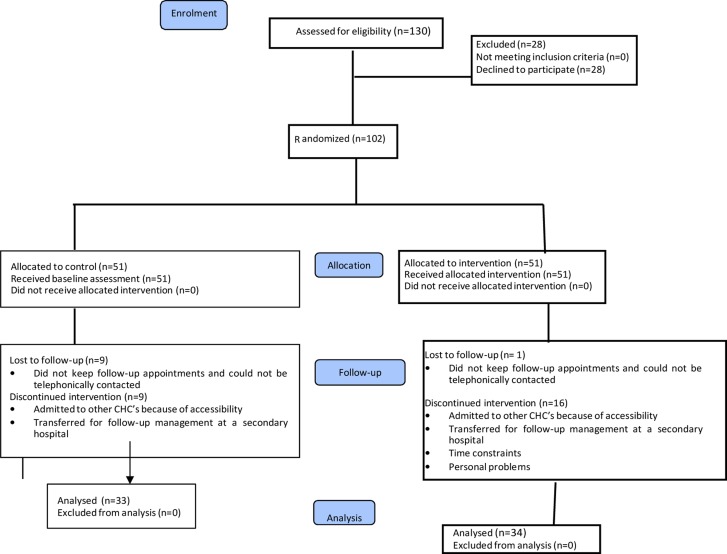
Ethical approval to conduct the study was obtained from both the University of Cape Town (HREC 251/2009) as well as the City of Cape Town. One hundred and thirty patients who agreed to take part in the study were assessed by the principal researcher (first author) using the Par-Q questionnaire which is often used to determine the safety or possible risk of exercising for an individual based upon their answers to specific health history questions. One hundred and two patients were selected to participate in the study (Figure 1).
Figure 1.
Consort flow diagram: demographic profile of participants
The principal researcher then administered a questionnaire specifically designed to obtain demographic information about the patients, the HRQoL questionnaire, and tested lung function and exercise tolerance, to provide base line data for the participants.
Using a single blinded randomized control study design14, the selected participants were then randomly allocated by an independent research assistant into either the intervention group (IG) or the control group (CG). The independent research assistant carried out the intervention programs for the two groups over the period of 6 weeks5,6. The principal researcher, who was blinded to the group allocation, repeated the administration of the HRQoL questionnaire, the lung function test, and the exercise tolerance test at 3 and 6 weeks after the baseline assessment, during follow up visits.
Instrumentation
A detailed explanation of the study and procedure was provided by the principal researcher before the informed consent document was signed and any testing performed. To assess the lung function, an automotive desktop spirometer (MINATO AUTOSPIRO-model no. AZ-505) was used to measure the Forced Expiratory Volume in one second (FEV1) and Forced Vital capacity (FVC) of all participants15,16. Exercise tolerance testing was performed using the six-minute- walk test15,16, during which the distance covered by each participant within the allocated time was recorded. The 15-Grade Modified Borg Scale (MBS)4,16 was used to assess rate of perceived exertion (RPE) after the sixminute walk test. The participants were asked to choose a number between 6 (no exertion) and 20 (maximal exertion) which best described their perceived exertion. Data pertaining to HRQoL was collected using the EQ-5D Questionnaire, which is based on the five domains of HRQoL17.
Intervention Programme
Based on the clinical competency guidelines for Pulmonary Rehabilitation Programme (PRP)14, a home based PRP was designed for the participants in the intervention group (IG) and implemented by the research assistant. The duration of the rehabilitation programme as used in an earlier assessment of HRQoL and exercise tolerance in COAD participants was utilized for cost effectiveness and time management in a clinic setting6.
The program included cardiovascular, low-impact exercises such as upper and lower limb range of movement activities, wall push-ups, repeated sit to stand movements, calf raises, and walking. Participants were advised to “walk faster” every day when doing the walking component of the program. The pulmonary components consisted of pursed-lipped breathing, diaphragmatic breathing, postural correction and the facilitation of coughing. The PRP was thoroughly explained and demonstrated to participants in the IG, each receiving printed illustrated diagrams of the exercises as well as instructions on repetitions, sets, and durations in Afrikaans, English, and Isi Xhosa. Participants in the CG were provided with a health education material, also in Afrikaans, English, and Isi Xhosa, which included information on the symptoms, diagnostic tools, treatment and prevention of PTB. This is one of the practical guidelines of the South African National TB Control program.
Summary of Analysis
IBM SPSS version 21 was used for data analysis. The data was approximately normally distributed. A p value <0.05 was considered as statistically significant. Intergroup changes were tested using a repeated measure ANOVA. Changes over time were tested using a time multiplied by treatment interaction which signified the comparative effect of the intervention versus control, and a p value <0.05 for the interaction indicated statistical significance. Baseline imbalances were accounted for in the analysis. Profile plots were used to establish the direction and trend of the effects. Responses to the EQ-5D questionnaire were in form of a likert scale and, being a non-parametric data, the Mann-Whitney U test was used to compare outcome measures between IG and CG. The Modified Borg Scale reading was also assessed using Mann-Whitney U test.
Results
Only 67 participants completed the six week exercise programme, with 35 participants lost to follow up as shown in Figure 1 (14 transferred to other hospitals, 8 relocated outside the province, 3 had difficulty with travelling to the clinic, and 10 were not reachable). Table 1 shows the demographic profile of those participants who completed the six week programme for both the intervention and control group.
Table 1.
Demographic profile of participants
| Total sample group (n=67) | Control Group (n=33) |
Intervention Group (n=34) |
||
| Gender | Male | 33 (49%) | 16 (49%) | 17 (50%) |
| Female | 34 (51%) | 17 (51%) | 17 (50%) | |
| Occupation | Employed | 17 (25%) | 7 (21%) | 10 (29%) |
| Unemployed | 50 (75%) | 26 (79%) | 24 (71%) | |
| Level of Education | No formal Education | 0 | 0 | 0 |
| Primary School (Gr. 1–7) |
16 (24%) | 11 (33%) | 5 (15%) | |
| High School (Gr. 8–11) |
36 (54%) | 17 (52%) | 19 (56%) | |
| Completed Gr. 12 | 15 (22%) | 5 (15%) | 10 (29%) | |
| Marital status | Married | 22 (33%) | 11 (33%) | 11 (32%) |
| Widowed | 2 (3%) | 0 (0%) | 2 (6%) | |
| Divorced/single | 43 (64%) | 22 (67%) | 21 (62%) | |
| Smoking History | Current smoker | 5 (7%) | 4 (12%) | 1 (3%) |
| Ex-smoker | 29 (43%) | 15 (46%) | 14 (41%) | |
| Never smoked before | 33 (49%) | 14 (42%) | 19 (56%) | |
| Diagnosis | PTB only * | 21 (31%) | 10 (30%) | 11 (32%) |
| PTB + HIV# | 46 (69%) | 23 (70%) | 23 (68%) | |
| ARV'S§ | Yes | 45 (67%) | 22 (67%) | 23 (68%) |
| No | 22 (33%) | 11 (33%) | 11 (32%) | |
| PTB regimen‡ | 1 | 23 (34%) | 14 (42%) | 9 (26%) |
| 2 | 44 (66%) | 19 (58%) | 25 (74%) |
CG - Control Group; IG - Intervention Group;
Pulmonary tuberculosis in the absence of comorbidity;
Human Immunodeficiency Virus;
Anti-retroviral treatment;
PTB regimen 2 is associated with multiple drug-resistant PTB
The hospital notes of the patients were perused for clinical information. Information relating to updated laboratory tests and radiological reports were not readily accessible for all the patients. Most of the participants (69%) were HIV+ and receiving anti-retroviral treatment in addition to TB treatment. Participants with PTB only (31%) were first time cases, and also undergoing treatment. About two thirds of the patients were already experiencing multidrug resistance. No differences were found between the groups when tested for covariate analysis of gender (p=1), ARV treatment (p=1), educational qualification (p=0.61), or smoking history (p=0.74).
Pulmonary function
The mean differences in the lung function at baseline between the control and intervention groups (Table 2) were not statistically significant in the values of FEV1 (p=0.57; 95% CI: −0.22 to 0.39) and FVC (p=0.6; 95% CI: −0.23 to 0.397).
Table 2.
Pulmonary parameters for participants (n=67)
| Pulmonary Function readings at baseline | ||||||
| CG (n=33) | IG (n=34) | |||||
| Mean | Range | SD | Mean | Range | SD | |
| FEV1(L) | 1.47 | 0.50–4.15 | 0.70 | 1.60 * | 0.82–3.11 | 0.56 |
| FVC (L) | 1.54 | 0.53–4.19 | 0.71 | 1.68 * | 0.94–3.32 | 0.57 |
| FEV1/FVC | 0.95 | 0.72–1.00 | 0.07 | 0.96 | 0.77–1.00 | 0.06 |
| Pulmonary Function readings at week six | ||||||
| FEV1 (L) | 1.54 | 0.45–4.10 | 0.66 | 1.77 | 1.07–2.99 | 0.54 |
| FVC (L) | 1.64 | 0.54–4.12 | 0.68 | 1.86 | 1.07–3.30 | 0.58 |
| FEV1/FVC | 0.94 | 0.64–1.00 | 0.07 | 0.96 | 0.79–1.00 | 0.06 |
Statistically significant value (p>0.0
Similarly, there were no significant differences between the two groups in week three in FEV1 (p=0.50; 95% CI: −0.21 to 0.41) and FVC (p=0.50; 95% CI: −0.20 to 0.42). Again there was no statistically significant differences in week six in FEV1 (p=0.1; 95% CI: −0.07 to 0.51) and FVC (p=0.2; 95% CI: −0.9 to 0.51). Intragroup analysis found that both values for FVC (p=0.004; 95% CI: −0.36 to −0.07) and FEV1 (p=0.001; 95% CI: −0.33 to −0.08) (Table 3) were statistically significant when the difference between baseline and week 6 was evaluated in the intervention group.
Table 3.
Pulmonary parameters for PTB only participants (n=21)
| Pulmonary Function readings at baseline | ||||||
| CG (n=10) | IG (n=11) | |||||
| Mean | Range | SD | Mean | Range | SD | |
| FEV1(L) | 1.49 | 0.87–2.24 | 0.50 | 1.67* | 0.87–3.11 | 0.66 |
| FVC (L) | 1.60 | 0.89–2.53 | 0.57 | 1.71* | 0.94–3.32 | 0.70 |
| FEV1/FVC | 0.94 | 0.78–1.00 | 0.75 | 0.98 | 0.93–1.00 | 0.03 |
| Pulmonary Function readings at week six | ||||||
| FEV1 (L) | 1.28 | 0.45–2.41 | 0.62 | 1.98 | 1.23–2.95 | 0.65 |
| FVC (L) | 1.39 | 0.54–2.42 | 0.62 | 2.09 | 1.25–3.17 | 0.69 |
| FEV1/FVC | 0.91 | 0.64–1.00 | 0.11 | 0.95 | 0.79–1.00 | 0.08 |
Statistically significant value (p>0.05)
In the control group, only the FVC was found to be statistically significant (p=0.037; 95% CI: −0.19 to −0.062). Repeated measures of lung function parameters over the six week period, adjusting for PTB regimen (p=0.2) and smoking history, was not statistically significant in FVC (p=0.47) as well as FEV1 (p=0.36).
Exercise tolerance
In Table 4, the difference in distance covered between the participants in the IG and the CG was statistically significant (p=0.01; 95% CI: 12.16 to 110.1) at baseline, at week 3 (p=0.01; 95% CI:13.66 to 103.6), and week six (p=0.007; 95% CI: 15.37 to 92.7).
Table 4.
Distance covered in 6-min walk test and rate perceived exertion for the Intervention and Control groups (n=67)
| Control Group (n=33) | Intervention Group (n=34) | |||||
| Mean | Range | SD | Mean | Range | SD | |
| Distance covered (m) | 340.00 | 160–680 | 104.67 | 401.18 | 200–600 | 96.13 |
| Borg Scale reading | 11.42 | 7–15 | 1.64 | 10.06 | 7–15 | 2.32 |
| WEEK 6 | ||||||
| Distance covered (m) | 356.97 | 190–520 | 78.72 | 411.03 | 235–580 | 79.79 |
| Borg Scale reading | 11.24 | 7–15 | 1.48 | 10.35 | 7–13 | 1.82 |
Similar significant difference was noted in the evaluation of the Modified Borg Scale in week six (p=0.03) between the intervention and control groups. However, adjusting for smoking, PTB treatment, educational level, and ARV treatment, the differences in the exercise tolerance parameters were not statistically significant (P>0.05).
Health-related quality of life
The difference between the IG and CG over the 6 week period was not statistically significant (p=0.789).
Discussion
Pulmonary Tuberculosis (PTB) has been described as a long term disability which has overall detrimental effects on the physical and social aspects of PTB diagnosed patients16. While pulmonary rehabilitation guidelines have been developed for patients with COAD2, similar guidelines are not yet in place in the managing the pulmonary impairment in PTB after cure. In line with the national Tuberculosis Strategic Plan for South Africa which encourages community and patient empowerment8, this study was therefore aimed at determining if a six-week home based pulmonary rehabilitation programme would assist in improving pulmonary function, exercise tolerance, and health-related quality of life in patient with PTB who are still undergoing treatment.
Making inferences from the outcome of this pilot study is limiting due to the small sample size, and the limited clinical parameters of the participants (updated radiological and laboratory reports) which were not readily accessible. The researchers were unable to ascertain the extent of lung destruction with the resultant functional impairment, and the specific duration for which each participant had been on TB medication prior to inclusion in the study. What was assured was that each participant had been receiving treatment at the clinic for four consecutive months to qualify for inclusion in the study. Other information about the patients that were not readily accessible to the researchers pertained to the HIV/AIDS status, namely CD4 count and viral load.
The socio-demographic profile of the participants in the study was consistent with the scenario relating to the historical past of South Africa11,12. Most of the participants presented with comorbidity of HIV+ and PTB diagnosis (69%), while about two-thirds of them were receiving treatment for multidrug resistant TB. The implementation of the home-based rehabilitation programme is in line with international recommendation of increased community involvement in the diagnosis and management of tuberculosis as part of the global plan for TB818.
When covariant analysis is carried out, no statistically significant difference is found in the parameters assessed between the two groups. The differences reported between the two groups may be attributed to chance alone. While the overall difference in lung function parameters between the IG and CG was not statistically significant, the change that occurred within the intervention group from baseline to the 6th week of testing was statistically significant. Similarly, the difference between the IG and CG in the exercise tolerance outcome was statistically at the end of the 6th week. These differences provide some motivation for the consideration and implementation of a pulmonary rehabilitation programme5,15,16 during PTB treatment to limit the pulmonary impairment experienced after microbiological cure1,2, and in turn the long term disability that contribute to the overall detrimental effects on physical and socialaspects of PTB patients16. As no adverse effects or complications were noted during the intervention period, it is recommended that further research be carried out that involves larger sample size in multiple research sites, and test further the hypothesis that PTB patients would benefit from pulmonary rehabilitation.
Disclaimer
We, the authors, hereby state that the views expressed in the submitted article are our own and not an official position of the institution or of the funder. We also declare that there is no conflict of interest.
Sources of Funding and Acknowledgements
National Research Foundation
University of Cape Town
Biostatistics Unit, Centre for Evidence-Based Health Care, Stellenbosch University
References
- 1.Ghosh S, Win BR, Kumar R, David R, Prabhu D. Assessment of Respiratory morbidity among pulmonary tuberculosis patients treated under the Revised National Tuberculosis Control Programme. Pulmonary. 2006;8(2):51–54. [Google Scholar]
- 2.De Valliere S, Barker RD. Residual lung damage after completion of treatment for multi- drug resistant tuberculosis. International Journal of Tuberculosis and Lung Disease. 2004;8(6):767–771. [PubMed] [Google Scholar]
- 3.Loveday M, Thomson L, Ndlela Z, Dudley L. The implementation of the National Tuberculosis Control Programme (NTCP) at a regional/ district hospital and three of its feeder clinics: A case study. Durban: Health Systems Trust; 2007. [Online] Available: http://www.hst.org.za/uploads/files/anrep0607.pdf. (11 April 2010). [Google Scholar]
- 4.Ries AL, Kaplan RM, Limberg TM, Prenitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Annals of Internal Medicine. 1995;122:823–832. doi: 10.7326/0003-4819-122-11-199506010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Tada A, Matsumoto H, Soda R, et al. Effects of pulmonary rehabilitation in patients with pulmonary tuberculosis sequelae. Nihon KokyukiGakkaiZasshi. 2002;40(4):275–281. (Abstract) [PubMed] [Google Scholar]
- 6.Finnerty PJ, Keeping I, Bullough I, Jones J. The effectiveness of outpatient pulmonary rehabilitation in chronic lung disease. Chest. 2001;119:1705–1710. doi: 10.1378/chest.119.6.1705. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich RI, Adams S, Baatjies R, Jeebhay MF. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies [Review article] The International Journal of Tuberculosis and Lung Disease. 2011;15(7):886–891. doi: 10.5588/ijtld.10.0526. [DOI] [PubMed] [Google Scholar]
- 8.South Africa. Department of Health, author. Tuberculosis strategic plan for South Africa, 2007–2011. Department of Health; [Online] Available: http://www.doh.gov.za/tb/docs/stratplan/2007-2011/tb/part1.pdf. (6 December 2010) [Google Scholar]
- 9.Cape Town TB Control Progress Report 1997–2003. Health Systems Trust; City of Cape Town; Metro District Health Services, Provincial Government of the Western Cape; 2004. [Google Scholar]
- 10.Medecins Sans Frontieres, author. Report on the Integration of TB and HIV services in Site B Khayelitsha. 2005. www.msf.org.za/msf-publications/report-on-integration-tb-and-hiv-services-ubuntu-clinic-khayelitsha. [Google Scholar]
- 11.Coovadia H, Jewkes R, Barron P, Sanders D, McIntyre D. The health and health system of South Africa: historical roots of current public health challenges. Lancet. 2009;374:817–834. doi: 10.1016/S0140-6736(09)60951-X. [DOI] [PubMed] [Google Scholar]
- 12.Mayosi BM, Lawn JE, van Niekerk A, Bradshaw D, Abdul Karim SS, Coovadia HM. Health in South Africa: changes and challenges since 2009. Lancet. 2012;380:2029–2043. doi: 10.1016/S0140-6736(12)61814-5. [DOI] [PubMed] [Google Scholar]
- 13.Redelmeier D A, et al. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. American Journal of Respiratory and Critical Care Medicine. 1997;155(4):1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 14.Wedzicha JA, Bestall JC, Garrod R, Garnham R, Paul EA, Jones PW. Randomised controlled trial of pulmonary rehabilitation in severe chronic obstructive disease patients, stratified with the MRC dyspnoea scale. European Respiratory Journal. 1998;12:363–369. doi: 10.1183/09031936.98.12020363. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida N, Yoshiyama T, Asai E, Komatsu Y, Sugiyama Y, Mineta Y. Exercise Training for the Improvement of Exercise Performance of Patients with Pulmonary Tuberculosis Sequelae. Internal Medicine. 2006;45(6):399–403. doi: 10.2169/internalmedicine.45.1505. [DOI] [PubMed] [Google Scholar]
- 16.Maguire GP, Anstey NM, Ardian M, et al. Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. International Journal of Tuberculosis and Lung Disease. 2009;13(12):1500–1506. [PubMed] [Google Scholar]
- 17.Jelsma J, Ferguson G. The determinants of selfreported health-related quality of life in a culturally and socially diverse South African community. Bulletin of the World Health Organisation. 2003;82(3):206–212. [PMC free article] [PubMed] [Google Scholar]
- 18.Community involvement in tuberculosis care and prevention, towards partnerships for health, author. Guiding principles and recommendations based on a WHO review. World Health Organization; 2008. [PubMed] [Google Scholar]