Abstract
Background
Systemic lupus erythematosus (SLE) may be characterized by periods of remissions and chronic or acute relapses. The complexity of clinical presentation of the SLE patients leads to incorrect evaluation of disease activity. Mean platelet volume (MPV) has been studied as a simple inflammatory marker in several diseases. There is no study in the literature about MPV levels in adult SLE patients with arthritis.
Objectives
We aimed to investigate the MPV levels in the SLE population with arthritis during and between activations.
Methods
The study consisted of 44 SLE patients with arthritis in activation period (Group 1), the same 44 SLE patients with arthritis in remission period (Group 2) and 44 healthy controls (Group 3). Erythrocyte sedimentation rate (ESR), creactive protein (CRP), white blood cell count, platelet count, and mean platelet volume (MPV) levels were retrospectively recorded from patient files.
Results
The mean ages of the SLE subjects were 42 ± 16 years, while the mean ages of controls was 41 ± 17 years. MPV was significantly lower in Group 1(7.66±0.89fL) than in Group 2 (8.61±1.06 fL) and Group 3(8.62±1.11fL) (p<0.0001). The differences between groups reached statistical significance.
Conclusions
We suggest that MPV levels decrease in patients with arthritis of SLE activation when compared to the same patients in remission and healthy controls.
Keywords: Systemic lupus erythematosus, Arthritis, Mean platelet volume
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease of unknown cause that may affect any organ of the body. Arthritis lupus is typically non-erosive, non-deforming, and frequently appears before other manifestations of SLE. It affects predominantly women, especially in their 20s and 30s. The clinical course of SLE is characterized by periods of remissions and relapses1. The correct evaluation of disease activity is of great importance because of the the complexity of the disease. Common methods including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are not completely in accordance with evaluating activation of SLE with arthritis2,3. Anti-double stranded DNA antibody and complement are used to certain clinical manifestations of the disease, especially nephritis, rather than the activity of the disease itself and arthritis4. Currently, disease activity in SLE can be assessed using composite disease activity indices, such as the SLE Disease Activity Index (SLEDAI) and British Isles Lupus Assessment Group (BILAG)5. However, they could be complex for use in routine clinical practice. Therefore, there is a great amount of interest in the identification of biomarkers that can quantify disease activity, although a single biomarker is unlikely to replace clinical evaluation because of the heterogeneity of SLE6.
Mean platelet volume (MPV) is a parameter detected during routine blood count and to which clinicians do not usually pay much attention. Platelet volume is known to be a marker determined from megakaryocytes during platelet production, which is associated with platelet function and activation. Under normal circumstances, there is an inverse relationship between platelet size and number7,8. MPV has been studied as a simple inflammatory marker in several diseases. Some studies have reported that MPV increases in myocardial infarction and cerebrovascular disease; while in contrast, it decreases in active rheumatologic diseases including rheumatoid arthritis (RA), ankylosing spondylitis and ulcerative colitis9–12. To our knowledge, there is not a study in the literature about MPV levels in adult SLE patients with arthritis. The present study aimed to investigate the MPV levels in an adult SLE population.
Patients and Methods
The data used in the present study were obtained retrospectively from patients who had SLE diagnosis with arthritis complaints based on ACR criteria in August 2011-December 2012 period in Rheumatology Clinic of the School of Medicine of the Cumhuriyet University (Sivas, Turkey). Ethical approval for the study was obtained from the local ethics committee. The study consisted of 44 SLE patients with arthritis in activation period (Group 1), the same 44 SLE patients with arthritis in remission period (Group 2) and 44 healthy controls (Group 3). The age of SLE patients and healthy controls ranged in 18 to 79 years. Participants with a history of smoking, acute or chronic infectious diseases, hemoglobin >16.5 g/dl, thrombocytopenia, anemia, hypertension, angina pectoris, myocardial infarction, diabetes mellitus, anti-phospholipid syndromes, recurrent miscarriage, amyloidosis, thrombosis or chronic renal insufficiency were excluded from the study. ESR, CRP, White Blood Cell (WBC) count, platelet count, and MPV levels were retrospectively recorded from patient files. All blood samples were studied within less than one hour after the sampling. The complete blood count have been performed in the same analyzer, Mindray BC-6800, which is routinely checked every month in the central laboratory of our institution.
Statistical analyses
Data were evaluated using the Statistical Package for Social Sciences 12.0 program for windows and by analyzing descriptive statistics (means and standard deviation), comparing the means of quantitative data for more than two groups with Kruskal-Wallis test and by comparing dual groups using the Student's t-test. P value of 0.05 was considered significant. Correlations between parameters were computed through Pearson's correlation analysis. Variables found to be statistically significant in univariate analyses were entered into multivariate logistic regression analysis. Multivariate logistic regression models were created to identify independent predictors of activation of arthritis in SLE patients.
Results
Demographic and clinical features of SLE patients are given in Table I.
Table I.
Main clinical characteristics of the patients with systemic lupus erythematosus and of the controls*
| SLE patients n=44 |
Controls n=44 |
|
| Sex, no. female/male | 33/11 | 35/9 |
| Median age, years (range) | 42±16 | 41±17 |
| Age at SLE onset, years(range) | 32±8.9 | - |
| Remission duration, days (range) | 12±4.9 | - |
| Butterfly rash | 28/44 | - |
| Discoid lesions | 11/44 | - |
| Photosensitivity | 23/44 | - |
| Mouth ulcers | 14/44 | - |
| Arthralgias/arthritis | 44/44 | - |
| Serositis | 4/44 | - |
| Pleurisy | 4/44 | - |
| Pericarditis | 1/44 | - |
| Renal disease | 12/44 | - |
| Neuropsychiatric manifestations | 4/44 | - |
| Antinuclear antibodies | 41/44 | - |
| Anti—double-stranded DNA | 24/44 | - |
Except where indicated otherwise, values are the number
The mean ages of the SLE subjects were 42 ± 16 years, while the mean ages of controls was 41 ± 17 years. Thirty-three SLE patients (78.6%) and 35 subjects (79.5%) in control group were female. There were no significant differences between patient group with SLE and healthy controls in terms of age and gender distribution (p=0.628, p=0.912, respectively).
MPV was significantly lower in Group 1 (7.66±0.89fL) than in Grouand Group 3(8.62±1.11fL) (p<0.0001); however, there was no difference between group 2 and group 3 (p=0.772). MPV levels were not correlated with ESR and CRP.
The mean platelet volume and WBC count did not differ among the groups. CRP and ESR values were significantly higher in Group 1 than Group 2 (p<0.0001). All study parameters are presented in Table II and III.
Table II.
Relationship between blood parameters in Group 1 and Group 2
| Group 1 n=44 |
Group 2 n=44 |
P | |
| Platelet (x109/L) | 296.5±13.5 | 290.7±12.1 | 0.835 |
| MPV (fL) | 7.66±0.89 | 8.61±1.06 | 0.0001 |
| Hb (g/dL) | 11.9±1.4 | 12.3±1.6 | 0.356 |
| Hct | 36.35±3.70 | 36.96±4.69 | 0.503 |
| WBC (x109/L) | 7.72±4 | 8.29±3.26 | 0.493 |
| CRP (mg/L) | 71.4±22.3 | 5.1±6.1 | 0.0001 |
| ESR (mm/h) | 50.6±26.9 | 20.6±13.9 | 0.0001 |
Table III.
Relationship between blood parameters in Group 1 and Group 3
| Group 1 n=44 |
Group 3 n=44 |
P | |
| Platelet (x109/L) | 296.5±13.5 | 275.5±45.8 | 0.333 |
| MPV (fL) | 7.66±0.89 | 8.62±1.11 | 0.0001 |
| Hb (g/dL) | 11.9±1.4 | 12.2±1.7 | 0.563 |
| Hct | 36.35±3.70 | 36.93±1.79 | 0.786 |
| WBC (x109/L) | 7.72±4 | 7.68±1.80 | 0.931 |
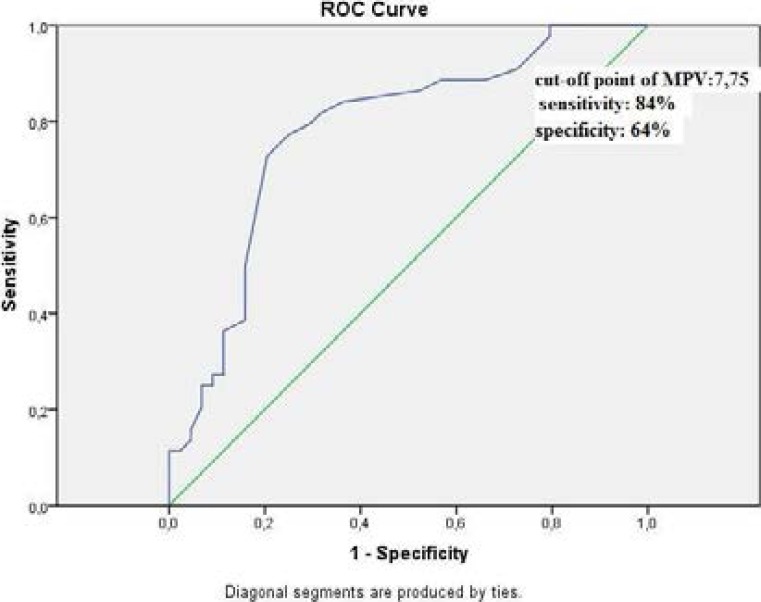
Correlation analysis showed that there was positive correlation between CRP (r= 0.534, p=0.001) and ESR (r=0.556, p=0.001) and negative correlation between MPV (r=−0.445, p=0.001) in activation period SLE patients with arthritis (Figure I).
Figure I.
Roc-curve analysis of MPV
ESR, CRP, MPV, platelet count, and WBC were entered in univariate analysis. CRP, ESR, and MPV were statistically important. These values were entered multivariate analyses to identify independent predictors of activation of arthritis in SLE patients.
Multivariate analysis showed that only MPV (odds ratio: 0,179, %CI: 0.048–0.671, p=0.011) and ESR (odds ratio: 1.134, %CI: 1.040–1.235, p=0.004) were independent predictors of arthritis activation in SLE patients (Table IV).
Table IV.
Univariate and multivariate analyses
| Univariate analysis | Multivariate analysis | |||||
| p | Odds ratio | CI(%) | p | Odds ratio | CI(%) | |
| MPV | 0.001 | 0.327 | 0.181–0.592 | 0.012 | 0.179 | 0.048–0.671 |
| ESR | 0.001 | 1.074 | 1.035–1.115 | 0.004 | 1.134 | 1.040–1.235 |
| CRP | 0.002 | 1.201 | 1.071–1.347 | 0.148 | 1.098 | 0.967–1.246 |
| Platelet count | 0.860 | 1.000 | 1.000–1.124 | |||
| WBC | 0.489 | 1.000 | 1.000–1.214 | |||
MPV: Mean platelet volume, WBC: White blood cell, CRP: C-reactive protein, ESR: Erythrocyte sedimentation rate
Discussion
In the present study, we demonstrated that MPV decreased in SLE patients during activation compared with remission and healthy controls. Low levels of MPV were associated with disease activity. We suggest its usefulness as a biomarker for global disease activity and clinical features. In patients with SLE, elevated ESR were found to be associated with disease activity 13, whereas serum CRP was only slightly elevated and was not correlated with disease activity3, 14. However, CRP and ESR may present some discordance in reflecting inflammation4. Recently, MPV has been considered a reliable marker for indicating platelet activation and inflammation. MPV is easily measured by automated cell counters under any storage conditions for blood samples. MPV is a marker of platelet function and activation, and it is also influenced by inflammation15.
To our knowledge, there is only one study in the literature about MPV levels in adult SLE patients. In this study, low MPV (<7.2 fl) was recorded by 2 different hematology analyzers in 5 (14.3%) and 23 (65.7%) patients with SLE, mostly at active stage of the disease (n=28, 80%).16 Results of this study, in which hematological parameters of SLE patients were studied, were in accordance with our results. A major difference of the present study from the ones in literature was that this is the first study evaluating MPV in SLE patients with arthritis in activation and remission periods.
Gasparyan et al.15 stated that high-grade inflammation accompanies a decrease of MPV in RA and SLE, possibly due to the increased consumption of large platelets at the sites of rheumatoid inflammation.
Kisacik et al.7 found that MPV was low in active ankylosing spondylitis and RA, and that MPV levels increased and then normalized with treatment. In another study lower MPV value of RA patients compared to controls were shown to increase after TNF-α treatment17. In the present study, lower MPV values of SLE patients with arthritis were normalized after treatment. There are two studies showing that MPV levels of FMF patients during the attack were significantly lower than those of healthy controls18,19. MPV has been reported to decrease in some inflammatory bowel diseases such as ulcerative colitis and it could be used for determination of disease activity20. This condition is thought to be related to the release of bioactive molecules of pro-inflammatory platelets in the presence of inflammation.
Two studies have suggested that SLE patients have increased platelet activation21, 22. Of these studies, Nagahama et al.22 showed that plasma platelet-derived microparticle levels were significantly higher in SLE patients than in healthy subjects. Furthermore, Nagahama et al stated22 that MPV levels were decreased in patients with arthritis of SLE activation as in our study. Several mechanisms for how complement components interact with platelets have been proposed, and most of these theories involve platelet activation.
Common trigger of platelets include immune complexes, which are frequently seen in SLE patients, shear stress due to atherosclerosis or inflammatory cytokines including interferon-alpha21, 23, 24.
Limitations
The results of this study are subjected to some limitations. First, this study was not based on longitudinal observations but was conducted with a retrospective design. Second, since the study is retrospective, clinical activation indices of SLE patients with arthritis (SLEDAI, BILAG) were not calculated. Third, it is a single center study with a relatively small sample size, which might underestimate or overestimate the relationship between MPV and inflammation in SLE patients presented with arthritis. Fourth, the fact that MPV values may be affected by many factors.
Conclusion
we suggest that MPV levels decrease in patients with arthritis of SLE activation when compared to same patients with remission and healthy controls. Decreased MPV levels were observed in patients with SLE and these results may be related to active inflammatory states. Namely, MPV is associated with disease activity. The results of our study further expand perspectives of use of MPV in conditions associated with SLE with arthritis for monitoring treatment. So, more specifically designed prospective studies are needed to externally cross-validate our findings in a larger cohort of APS patients.
Authors' contributions
Concept: SS; Data Collection and/or Processing: SS, AUU, SK; Interpretation: SS, TT, LA; Literature Review: SS, AUU, TT; Writer: SS, AUU, SK; Critical Review: SS. All authors read and approved the final manuscript.
Declaration of interest
The authors report no conflict of interest.
References
- 1.Hopkinson ND, Doherty M, Powell RJ. Clinical features and race-specific incidence/prevalence rates of systemic lupus erythematosus in a geographically complete cohort of patients. Annals of the rheumatic diseases. 1994;53:675–680. doi: 10.1136/ard.53.10.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osei-Bimpong A, Meek JH, Lewis SM. ESR or CRP? A comparison of their clinical utility. Hematology. 2007;12:353–357. doi: 10.1080/10245330701340734. [DOI] [PubMed] [Google Scholar]
- 3.Gaitonde S, Samols D, Kushner I. C-reactive protein and systemic lupus erythematosus. Arthritis and rheumatism. 2008;59:1814–1820. doi: 10.1002/art.24316. [DOI] [PubMed] [Google Scholar]
- 4.Pisetsky DS. Anti-DNA and autoantibodies. Current opinion in rheumatology. 2000;12:364–368. doi: 10.1097/00002281-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis and rheumatism. 1989;32:1107–1118. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 6.Petri M. Disease activity assessment in SLE: do we have the right instruments? Annals of the rheumatic diseases. 2007;66:61–64. doi: 10.1136/ard.2007.078477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson SR, Carter JM. Platelet volume: laboratory measurement and clinical application. Blood reviews. 1993;7:104–113. doi: 10.1016/s0268-960x(05)80020-7. [DOI] [PubMed] [Google Scholar]
- 8.Threatte GA. Usefulness of the mean platelet volume. Clinics in laboratory medicine. 1993;13:937–950. [PubMed] [Google Scholar]
- 9.Yuksel O, Helvaci K, Basar O, Koklu S, Caner S, Helvaci N, Abayli E, Altiparmak E. An overlooked indicator of disease activity in ulcerative colitis: mean platelet volume. Platelets. 2009;20:277–281. doi: 10.1080/09537100902856781. [DOI] [PubMed] [Google Scholar]
- 10.Kisacik B, Tufan A, Kalyoncu U, Karadag O, Akdogan A, Ozturk MA, Kiraz S, Ertenli I, Calguneri M. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint, bone, spine: revue du rhumatisme. 2008;75:291–294. doi: 10.1016/j.jbspin.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Endler G, Klimesch A, Sunder-Plassmann H, Schillinger M, Exner M, Mannhalter C, Jordanova N, Christ G, Thalhammer R, Huber K, Sunder-Plassmann R. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. British journal of hematology. 2002;117:399–404. doi: 10.1046/j.1365-2141.2002.03441.x. [DOI] [PubMed] [Google Scholar]
- 12.Bath P, Algert C, Chapman N, Neal B. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke; a journal of cerebral circulation. 2004;35:622–626. doi: 10.1161/01.STR.0000116105.26237.EC. [DOI] [PubMed] [Google Scholar]
- 13.Vilá LM, An G, McGwin G, Jr, Bastian HM, Fessler BJ, Reveille JD. Systemic lupus erythematosus in a multiethnic cohort (LUMINA): XXIX. Elevation of erythrocyte sedimentation rate is associated with disease activity and damage accrual. The Journal of rheumatology. 2005;32:2150–2155. [PubMed] [Google Scholar]
- 14.de Carvalho JF HB, Szyper-Kravitz M, Shoenfeld Y. C-reactive protein and its implications in systemic lupus erythematosus. Acta Reumatol Port. 2007;32:317–322. [PubMed] [Google Scholar]
- 15.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Current pharmaceutical design. 2011;17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 16.Turner-Stokes L, Jones D, Patterson KG, Todd-Pokropek A, Isenberg DA, Goldstone AH. Measurement of haematological indices of chronic rheumatic disease with two newer generation automated systems, the H1 and H6000 (Technicon) Annals of the rheumatic diseases. 1991;50:583–587. doi: 10.1136/ard.50.8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasparyan AY, Sandoo A, Stavropoulos-Kalinoglou A, Kitas GD. Mean platelet volume in patients with rheumatoid arthritis: the effect of anti-TNF-alpha therapy. Rheumatology international. 2010;30:1125–1129. doi: 10.1007/s00296-009-1345-1. [DOI] [PubMed] [Google Scholar]
- 18.Makay B, Turkyilmaz Z, Unsal E. Mean platelet volume in children with familial Mediterranean fever. Clinical rheumatology. 2009;28:975–978. doi: 10.1007/s10067-009-1148-5. [DOI] [PubMed] [Google Scholar]
- 19.Sahin S, Senel S, Ataseven H, Yalcin I. Does mean platelet volume influence the attack or attack-free period in the patients with Familial Mediterranean fever? Platelets. 2013;24:320–323. doi: 10.3109/09537104.2012.697591. [DOI] [PubMed] [Google Scholar]
- 20.Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG, Koutroubakis IE, Kouroumalis EA. Mean platelet volume: a useful marker of inflammatory bowel disease activity. The American journal of gastroenterology. 2001;96:776–781. doi: 10.1111/j.1572-0241.2001.03621.x. [DOI] [PubMed] [Google Scholar]
- 21.Lood C, Amisten S, Gullstrand B, Jonsen A, Allhorn M, Truedsson L, Sturfelt G, Erlinge D, Bengtsson AA. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up- regulation of the type I interferon system is strongly associated with vascular disease. Blood. 2010;116:1951–1957. doi: 10.1182/blood-2010-03-274605. [DOI] [PubMed] [Google Scholar]
- 22.Nagahama M, Nomura S, Ozaki Y, Yoshimura C, Kagawa H, Fukuhara S. Platelet activation markers and soluble adhesion molecules in patients with systemic lupus erythematosus. Autoimmunity. 2001;33:85–94. doi: 10.3109/08916930108995993. [DOI] [PubMed] [Google Scholar]
- 23.Shanmugavelayudam SK, Rubenstein DA, Yin W. Effects of physiologically relevant dynamic shear stress on platelet complement activation. Platelets. 2011;22:602–610. doi: 10.3109/09537104.2011.585257. [DOI] [PubMed] [Google Scholar]
- 24.Larsson A, Egberg N, Lindahl TL. Platelet activation and binding of complement components to platelets induced by immune complexes. Platelets. 1994;5:149–155. doi: 10.3109/09537109409005528. [DOI] [PubMed] [Google Scholar]