Abstract
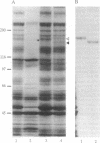
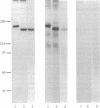
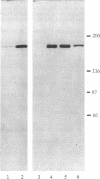
The postsynaptic density (PSD) is a specialization of the submembranous cytoskeleton that is visible in the electron microscope on the cytoplasmic face of the postsynaptic membrane. A subcellular fraction enriched in structures with the morphology of PSDs contains signal-transduction molecules thought to regulate receptor localization and function in the central nervous system. We have purified a prominent tyrosine-phosphorylated glycoprotein of apparent molecular mass 180 kDa, termed PSD-gp180, that is highly enriched in the rat forebrain PSD fraction. The sequences of four tryptic peptides generated from the protein reveal that it is the 2B subunit of the N-methyl-D-aspartate (NMDA) type glutamate receptor. We have confirmed the identity of PSD-gp180 by showing that it reacts with antibodies raised against a unique fragment of the 2B subunit of the NMDA receptor. We also show that the 2B subunit is the most prominently tyrosine-phosphorylated protein in the PSD fraction based upon recognition by an anti-phosphotyrosine antibody. Two types of NMDA receptor subunits have been identified by molecular cloning [Nakanishi, S. (1992) Science 258, 597-603]. The single type 1 subunit is expressed throughout the brain and is necessary for formation of the receptor channel. The four type 2 subunits (2A, 2B, 2C, and 2D) are expressed in discrete brain regions, contain unusually long unique C termini, and confer distinct kinetic properties on NMDA receptors that contain them. Our findings suggest that, in the forebrain, NMDA receptor subunit 2B may serve to anchor NMDA receptors at the postsynaptic membrane through its interaction with the PSD. The prominent presence of tyrosine phosphate further suggests that the NMDA receptor may be regulated by tyrosine phosphorylation or that it may participate in signaling through tyrosine phosphorylation and through its ion channel.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H., Greenberg M. E. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991 Aug 23;253(5022):912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Blackstone C. D., Moss S. J., Martin L. J., Levey A. I., Price D. L., Huganir R. L. Biochemical characterization and localization of a non-N-methyl-D-aspartate glutamate receptor in rat brain. J Neurochem. 1992 Mar;58(3):1118–1126. doi: 10.1111/j.1471-4159.1992.tb09370.x. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Cohen R. S., Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980 Sep;86(3):831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D. W., Stofko-Hahn R. E., Fraser I. D., Cone R. D., Scott J. D. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. J Biol Chem. 1992 Aug 25;267(24):16816–16823. [PubMed] [Google Scholar]
- Cho K. O., Hunt C. A., Kennedy M. B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992 Nov;9(5):929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Banker G., Churchill L., Taylor D. Isolation of postsynaptic densities from rat brain. J Cell Biol. 1974 Nov;63(2 Pt 1):441–455. doi: 10.1083/jcb.63.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore S. B., Gurd J. W. Postnatal age and protein tyrosine phosphorylation at synapses in the developing rat brain. J Neurochem. 1991 Oct;57(4):1240–1248. doi: 10.1111/j.1471-4159.1991.tb08285.x. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Weinberg R. A. The pathway to signal achievement. Nature. 1993 Oct 28;365(6449):781–783. doi: 10.1038/365781a0. [DOI] [PubMed] [Google Scholar]
- Ellis P. D., Bissoon N., Gurd J. W. Synaptic protein tyrosine kinase: partial characterization and identification of endogenous substrates. J Neurochem. 1988 Aug;51(2):611–620. doi: 10.1111/j.1471-4159.1988.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Matus A. Selective association of N-methyl aspartate and quisqualate types of L-glutamate receptor with brain postsynaptic densities. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6876–6880. doi: 10.1073/pnas.81.21.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J. R., McGuire J. S., Jr, DeLorenzo R. J. Identification of the major postsynaptic density protein as homologous with the major calmodulin-binding subunit of a calmodulin-dependent protein kinase. J Neurochem. 1984 Apr;42(4):1077–1084. doi: 10.1111/j.1471-4159.1984.tb12713.x. [DOI] [PubMed] [Google Scholar]
- Gurd J. W. Phosphorylation of the postsynaptic density glycoprotein gp180 by Ca2+/calmodulin-dependent protein kinase. J Neurochem. 1985 Oct;45(4):1128–1135. doi: 10.1111/j.1471-4159.1985.tb05532.x. [DOI] [PubMed] [Google Scholar]
- Gurd J. W. Phosphorylation of the postsynaptic density glycoprotein gp180 by endogenous tyrosine kinase. Brain Res. 1985 May 6;333(2):385–388. doi: 10.1016/0006-8993(85)91599-9. [DOI] [PubMed] [Google Scholar]
- Ishii T., Moriyoshi K., Sugihara H., Sakurada K., Kadotani H., Yokoi M., Akazawa C., Shigemoto R., Mizuno N., Masu M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993 Feb 5;268(4):2836–2843. [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988 Dec;1(10):911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., McGuinness T. L., Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Feb;81(3):945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., Montgomery P. R. Subcellular localization of the 52,000 molecular weight major postsynaptic density protein. Brain Res. 1982 Feb 11;233(2):265–286. doi: 10.1016/0006-8993(82)91202-1. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Bulleit R. F., Erondu N. E., Jennings V. R., Miller S. G., Molloy S. S., Patton B. L., Schenker L. J. Structure and regulation of type II calcium/calmodulin-dependent protein kinase in central nervous system neurons. Cold Spring Harb Symp Quant Biol. 1990;55:101–110. doi: 10.1101/sqb.1990.055.01.013. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B. The postsynaptic density. Curr Opin Neurobiol. 1993 Oct;3(5):732–737. doi: 10.1016/0959-4388(93)90145-o. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manabe T., Renner P., Nicoll R. A. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992 Jan 2;355(6355):50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E., Yamamoto H., Tan S. E., Brickey D. A., Soderling T. R. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993 Apr 15;362(6421):640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- Meguro H., Mori H., Araki K., Kushiya E., Kutsuwada T., Yamazaki M., Kumanishi T., Arakawa M., Sakimura K., Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992 May 7;357(6373):70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem. 1985 Jul 25;260(15):9039–9046. [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nitkin R. M., Smith M. A., Magill C., Fallon J. R., Yao Y. M., Wallace B. G., McMahan U. J. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J Cell Biol. 1987 Dec;105(6 Pt 1):2471–2478. doi: 10.1083/jcb.105.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S. K., Hwang J. M., Patterson P. H. A modified method for obtaining large amounts of high titer polyclonal ascites fluid. J Immunol Methods. 1993 Sep 27;165(1):75–80. doi: 10.1016/0022-1759(93)90108-j. [DOI] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity. Trends Neurosci. 1993 Apr;16(4):147–153. doi: 10.1016/0166-2236(93)90123-4. [DOI] [PubMed] [Google Scholar]
- Rogers S. W., Hughes T. E., Hollmann M., Gasic G. P., Deneris E. S., Heinemann S. The characterization and localization of the glutamate receptor subunit GluR1 in the rat brain. J Neurosci. 1991 Sep;11(9):2713–2724. doi: 10.1523/JNEUROSCI.11-09-02713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993 Sep;16(9):359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Tingley W. G., Roche K. W., Thompson A. K., Huganir R. L. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. 1993 Jul 1;364(6432):70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- Wallace B. G., Qu Z., Huganir R. L. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron. 1991 Jun;6(6):869–878. doi: 10.1016/0896-6273(91)90227-q. [DOI] [PubMed] [Google Scholar]
- Wang J. Y. Antibodies for phosphotyrosine: analytical and preparative tool for tyrosyl-phosphorylated proteins. Anal Biochem. 1988 Jul;172(1):1–7. doi: 10.1016/0003-2697(88)90403-4. [DOI] [PubMed] [Google Scholar]